A nanoscale perspective on solid oxide and semiconductor membrane fuel cells: materials and technology
Abstract
Fuel cells could play an important role in the ongoing energy transition by providing clean and efficient energy conversion. Although the solid oxide fuel cell (SOFC) technology is a potential alternative for large-scale applications, its commercialization is limited by its electrolyte materials and has not yet been realized. Progress on new functional semiconductor-ionic materials (SIMs) and the fundamentals of SOFCs will provide new paths for their research and development. Herein, we discuss the nanoscale electrochemistry phenomena of SIMs in the context of new concepts for advanced SOFCs. A traditional SOFC consists of a three-layer anode/electrolyte/cathode structure, where the physically separated electrolyte layer is indispensable for ion transport to support the redox reaction and prevent the occurrence of short circuiting. A novel nano-SOFC concept is proposed to replace the traditional electrolyte by a SIM or semiconductor membrane and it can deliver superior performance, even at a lower temperature range (< 500 °C). The scientific basis and prospects of this new technological approach are presented and discussed.
Keywords
INTRODUCTION
Climate change mitigation will require a step change in the introduction of clean and efficient energy production in the coming decades. The Paris Climate Accords from December 2015 call for limiting the global temperature rise to 1.5 °C, which equates to reaching carbon neutrality around the middle of this century. Many countries, including China, the EU and the USA, have committed to these goals.
In these circumstances, the global energy transition requires a multitude of new technologies to be developed for utilizing existing fossil fuels in a clean and more environmental benign manner, as well as exploiting renewable energies. Fuel cells, which convert fuel to electricity with high efficiency through an electrochemical route, represent a highly promising technology and could be more appropriate for the hydrogen era compared to battery technology. Solid oxide fuel cells (SOFCs) deliver the highest efficiency when converting chemical energy to electricity among other types of fuel cells, but their wider use is still hampered by degradation and material reliability issues due to the relatively high operating temperatures (800-1000 °C). Lowering the temperature below 600 °C has therefore been an important goal in recent SOFC research. As noted by Goodenough[1], SOFC commercialization requires an oxide-ion conductor with sufficient conductivity at a lower temperature range to be technically and commercially useful.
In order to reduce the operating temperature of SOFCs, some previous research activities have been focused on thin-film fabrication technologies to reduce the thickness of the electrolyte layer to obtain better electrochemical performance at ~700 °C[2,3]. An alternative path considered was to replace the most commonly used yttria-stabilized zirconia (YSZ) with a better ionic conducting electrolyte at low operating temperatures. In addition, new scientific understanding of the charge transfer mechanisms at the nanoscale occurring within a new set of alternative materials, such as mixed semiconductor-ionic materials, is being considered to open up radically new opportunities for SOFC at even lower temperature, e.g., below 550 °C.
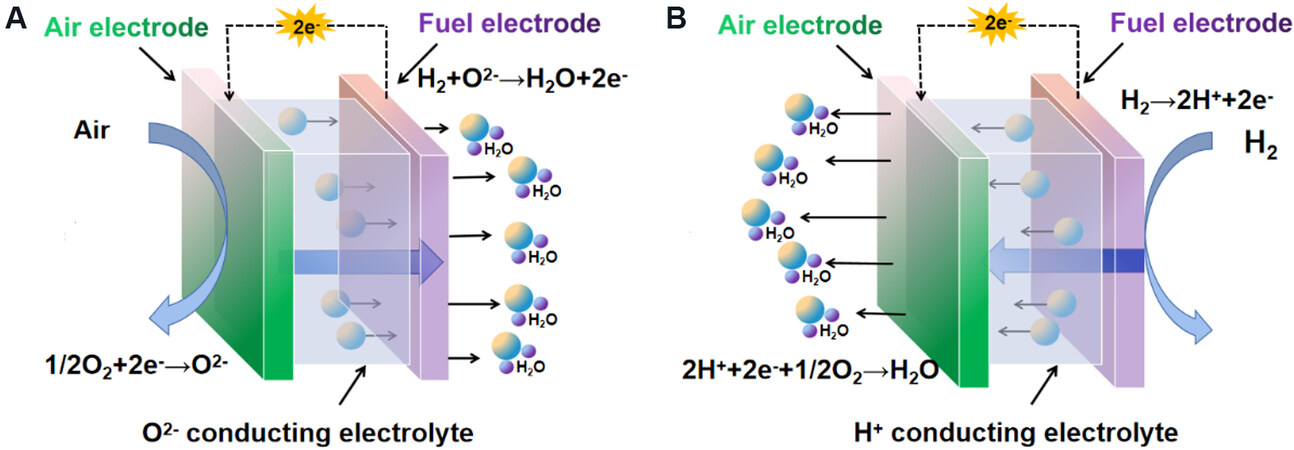
SOFCs are typically composed of a dense electrolyte layer assembled between two porous electrodes (an anode and a cathode)[4]. The basic working principle of the SOFC is based on oxygen-ion (O2-) conduction by oxygen reduced at the cathode. The oxygen ions are transported through the electrolyte layer, e.g., YSZ, which also acts as a separator to prevent a direct reaction between the fuel and oxidant. The free electrons created in the electrochemical oxidation of the fuels at the anode side travel through an external circuit to generate electric power [Figure 1A]. Using protons (H+) as the charge carriers instead of oxygen ions has also been well investigated, which converts the SOFC into a proton ceramic fuel cell (PCFC)[5,6]. In this case, hydrogen is oxidized at the anode to generate protons, which are transported through the dense electrolyte layer to react with O2 at the cathode side to complete the redox reaction for electric power generation [Figure 1B]. Compared to SOFCs, PCFCs can operate at lower temperatures as proton diffusion requires a lower activation energy to typically motivate the generation of power densities of up to 0.455 W·cm-2 at
STATE-OF-THE-ART AND CHALLENGES OF TRADITIONAL SOFCS
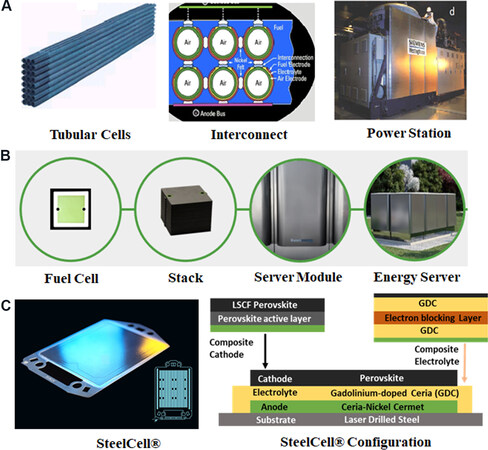
Commercial-scale SOFC systems capable of generating hundreds of kilowatts were already built at the end of the 1990s[7,8]. These included tubular SOFC stacks [Figure 2A] used in the Siemens/Westinghouse 220 kW SOFC power station, with a 3 MW power plant also planned. These tubular SOFCs used thick YSZ
Figure 2. (A) Tubular SOFC cells and a system delivering 220 kW from Siemens/Westinghouse[7,8]. (B) Bloom energy SOFC products from a single cell, stack to power systems. (C) Cross-sectional image of a metal-supported SOFC cell from Ceres Power Ltd. and an exemplary single cell from Ceres Power (Copyright from Ref.[11]).
Bloom Energy, which is deemed the most successful commercial SOFC manufacturer, has provided SOFC products in various system formats, including micro-grid/energy servers/power stations
Facing the commercialization challenges of macro-power plants, micro-tubular and micro-plate SOFC designs have been developed in parallel for portable and mobile applications due to their high thermal stability during rapid heat cycling and large volumetric active surface areas. These include 50-100 W micro-tubular SOFC stacks from the Japanese New Energy and Industrial Technology Development Organization (0.8-1.6 mm tube diameter) with an electrolyte thickness of < 5 μm, leading to improved power densities at reduced temperature (0.5 W·cm-2 at 550 °C)[13]. The Japanese Institute of Industrial Technology has further developed a “mini” stack working at 550 °C based on needle-type SOFC single cells, in which the diameter is reduced to the sub-millimeter scale (~0.8 mm) in order to increase the volumetric active electrode area. Such micro-SOFC cells are capable of rapid start-up and may present a cost reduction through their high volumetric power density, which is crucial for portable applications.
The electrolyte thickness can further be reduced through advanced vacuum deposition techniques, such as atomic layer deposition[14,15], inkjet printing[16], pulsed laser deposition[17] and chemical vapor deposition[18]. For example, pulsed laser deposition has been used to yield YSZ electrolyte thicknesses from 50 to a few hundred nanometers, so the cell could generate a power density of up to 90 mW·cm-2 at 400 °C[17]. Some adverse effects by utilizing thinner films have been identified, including mechanical failures due to intrinsic stress from the YSZ membrane growth mechanism and the extrinsic stress induced by the thermal expansion coefficient mismatch, which then deteriorate the long-term stability of micro-SOFCs. Overall, limited by the poor conductivity of the YSZ electrolyte at the desired temperature range, enabling sufficient ionic conduction in the electrolyte still remains one of the critical objectives currently hindering SOFC commercialization.
NOVEL SOFC CONCEPT
Recent developments in nanomaterials have played a crucial role in improving the electrocatalytic properties of redox reactions and correspondingly the electrochemical performance. However, the performance of SOFCs is affected by multiple factors, including the intrinsic properties of the basic components (anode, electrolyte and cathode) and the polarization losses at the electrolyte/electrode interface. In a traditional SOFC, the anode/electrolyte and cathode/electrolyte interfaces considerably affect the device performance, leading to severe polarization losses. In this case, two functional layers, also known as active layers, which are composed of a delicate mixture of electrode and electrolyte materials with fine structure, have been introduced to address this problem.
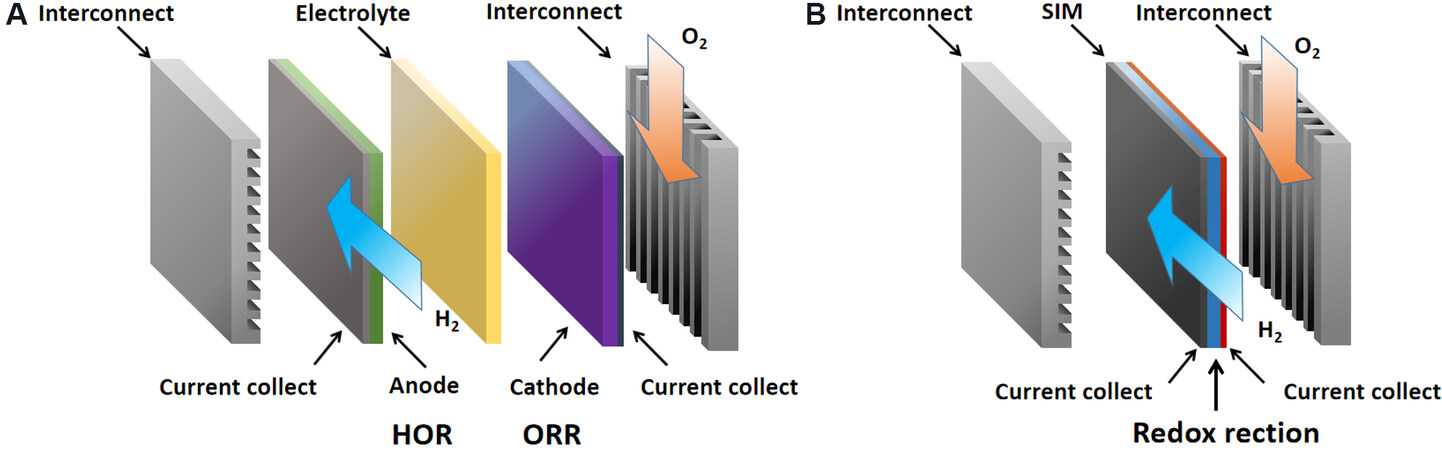
A newly developed novel SOFC has a distinguishable feature from its conventional counterpart that has electrolyte-separated electrode interfaces and a hydrogen oxidation reaction (HOR) and an oxygen reduction reaction (ORR) at the anode and cathode, as illustrated in Figures 3A and 4A, respectively. This new type of device does not have a physically separated electrolyte layer and is constructed using a semiconductor membrane (SM) or SIM to realize the fuel cell HOR and ORR in a single-layer design, as shown in Figure 3B, through a nano-redox mechanism[19,20]. The SM/SIM can be a mixture of a SOFC oxide cathode material with P-type semiconduction and an ionic electrolyte[21-23], whose composition is similar to the cathode component used in a SOFC[24,25]. On this basis, the interfacial polarization losses can be removed and the nanoscale redox phenomena of a complete fuel cell reaction can be scaled up into a single-layer device to form a macroscale generator that can function as a conventional SOFC, as illustrated in Figure 3B.
Figure 3. (A) A SOFC device with a three-layer anode/electrolyte/cathode structure. (B) Nano-SOFC with a single-layer SIM structure. HOR: Hydrogen oxidation reaction; ORR: oxygen reduction reaction.
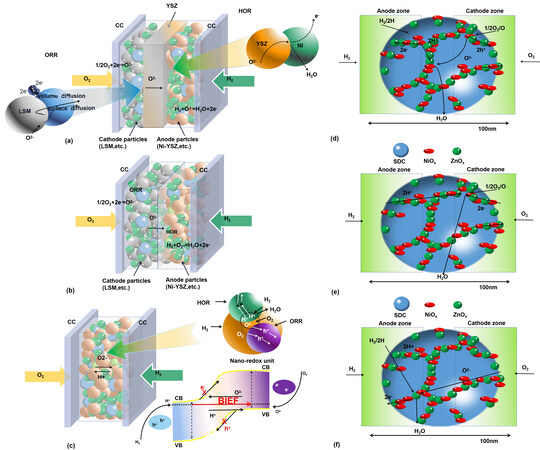
Figure 4. (A) Illustration of conventional anode/electrolyte/cathode SOFC from a physical-electrochemical view as a p-i-n structure. (B) A p-n junction double layer device from removing the ionic electrolyte layer. (C) A single-layer device based on the nano-redox reaction principle with a bulk p-n heterojunction and energy band alignment (CC). Schematic diagram of nano-redox reaction mechanisms based on (D) O2- and H+, (E) O and H+ and (F) O2- and H, respectively. Copyright from 2013 Nano Energy, including the text from (A), (B) and (C). CC: Current collector; CB: conduction band; VB: valence band; LSM: La0.85Sr0.15MnO3; YSZ: yttria-stabilized zirconia; HOR: hydrogen oxidation reaction; ORR: oxygen reduction reaction; SDC: samarium-doped ceria.
FUNDAMENTALS AND WORKING PRINCIPLES OF NANO-SOFCS BEYOND THE MACROSCALE
Singh et al.[26] proposed a new understanding of SOFCs, where the anode, electrolyte and cathode can be interpreted as n-type, ionic and p-type layers/zones, respectively, as shown in Figure 4A, where the electrolyte layer separates the HOR and ORR. If the middle electrolyte layer is removed (ionic zone), it is converted into a two-component (layer) device with n- (anode) and p (cathode)-type conduction, which is also known as a p-n junction device [Figure 4B]. By this means, the electronic short-circuiting risk can be prevented due to the charge separation function of the p-n junction, the same as the principle of a p-n solar cell. This suggests that a physically separated electrolyte layer may not actually be an indispensable component to run a SOFC. In fact, the feasibility of such a double-layer fuel cell constructed by the anode and cathode only has already been demonstrated[27].
Moreover, the anode, electrolyte and cathode can be further integrated at the nanoscale through nanoparticles consisting of n-type, ionic and p-type elements, which is known as a nano-redox unit, as illustrated in Figure 4C. Thus, a fuel cell redox reaction, as discussed above, can be realized at the nanoparticle scale, suggesting that a nano-SOFC can be constructed. A single-layer fuel cell (SLFC) is an up-scale device for the nano-redox or nano-SOFC units [Figure 4C]. These units form a bulk p-n heterostructure junction in an energy band alignment, so that the generated electrons are limited to pass through the cell only in the desired direction. For example, such a device was successfully built on a NiOx-ZnO homogeneous layer, as reported by Zhu et al.[19]. When a junction between two semiconductors is formed, the charge carriers (electrons in n-ZnO and holes in p-NiO) diffuse through the p-n interface, which causes the potential to increase until the flow flux caused by the potential and diffusion cancels out. This results in the Fermi levels aligning and the band edges of the semiconductor adjusting accordingly to reach thermal equilibrium and establish a space charge region with a built-in field directing from n-ZnO to p-NiO.
On this basis, when the generated electrons of the cell and the intrinsic electrons of the materials transport to the NiO/ZnO heterointerface region, they are easily modulated by the built-in field of the NiO/ZnO heterojunction, for instance, being suppressed by the electric field. Thus, the SIM layer could exhibit significantly reduced electronic conductivity through the energy band alignment, as shown below in Figure 4C. Using this feature, the electronic short-circuit risk of SIM fuel cells (SIMFCs) could be avoided. As reported in[19], such a single-layer device was built on p-n junction semiconductors, in which NiOx and ZnO formed a homogeneous nanocomposite to provide the necessary electrochemical reaction sites and charge transport paths for a fuel cell. These can be accomplished via tailoring the ionic and electronic (n and p types) conductivities and catalyst activities to enable redox reactions occurring on nanoparticles and ultimately accomplishing a fuel cell function. Bulk p-n heterojunctions are associated with the ionic conductor (e.g., samarium-doped ceria, SDC) that acts like an electrolyte layer to facilitate the charge transfer processes for ions (H+ and O2-) along with the electrons and holes among the involved p- and n-type and ionic particles to realize the nano-redox SOFC built on SIM materials.
In this single-layer SIM device, the working principle of the nano-SOFC is proposed from three different nano-redox processes involving protons, oxygen ions or both, as well as H/H2 and O/O2, which may result in different fuel cell processes below, as also illustrated in Figure 4D-F, respectively:
1. Completed directly by H+ and O2- [Figure 4D]:
Hydrogen side:
H2 → 2H+ + 2e- (1)
Air side:
1/2O2 + 2e- → O2- (2)
Total reaction:
H2 + 1/2O2 → H2O (3)
2. Completed by H+ and O atom (or O2) [Figure 4E]:
Hydrogen side:
H2 → 2H+ + 2e- (4)
Air side:
2H+ + 1/2O2 + 2e- → H2O (5)
Total reaction:
H2 + 1/2O2 → H2O (6)
3. Completed by O2- and H atom (or H2) [Figure 4F]:
Hydrogen side:
H2 + O2- → H2O + 2e- (7)
Air side:
1/2O2 + 2e- → O2- (8)
Total reaction:
H2 + 1/2O2 → H2O (9)
As there are several paths for realizing the nano-redox reactions discussed above, which also opens up more possibilities to find optimal materials and design the microstructure to realize nano-SOFCs under ongoing research and development. It is noteworthy that even though each individual reaction above has not yet been verified at the nanoscale due to experimental limitations, all possible reaction routes and redox pathways formulated in Equations (1)-(9) should theoretically be considered, since they lay a foundation for nano-SOFCs based on nano-redox processes and mechanisms.
MATERIALS AND TECHNOLOGIES
The aforementioned new type of nano-SOFC mainly relies on a single layer of SM/SIM to realize nano-redox processes to complete the fuel cell reaction, which is considerably different from that of conventional SOFCs and is thus indicative of a new material system and technology. Such a SLFC device was first demonstrated based on a homogeneous mixture of semiconducting oxides (NiO and ZnO) and ionic conducting oxides (SDC or GDC)[19,20]. It was regarded as a “three-in-one” device as the single layer can function simultaneously as an electrolyte for ion transport and as electrodes for HOR and ORR processes[28]. Inspired by these studies, a sequence of electrode materials, especially p-type semiconducting perovskite oxides, such as SrFeO3, SrFeMoO3, SrSmCoO3, LaSrCoFeO3-δ and La0.7Sr0.3Cr0.5Fe0.5O3-δ, was used to prepare the SIM or SMs with SDC/GDC and was demonstrated successfully in SLFCs[22,23,29-31]. In parallel, metal oxides with a layered structure were also applied in SLFCs individually or in heterostructure composite form, including the p-type semiconductors LiCoAlO2, NaFeNiO2 and Ni0.8Co0.15Al0.05LiO2-δ, which have shown promising fuel cell performance[32-34]. Further studies explored new SMs and SIMs with better fuel cell performances based on various common n- and p-type semiconductors, such as n-type ZnO[35,36], n-type TiO2[37], n-type SrTiO3, La-doped SrTiO3[38,39], n-type CeO2-δ[40,41] and p-type SiC[42]. Moreover, triple-charge (H+/O2-/e-) conducting oxide (TCO) materials, e.g., p-type BaCo0.4Fe0.4Zr0.1Y0.1O3-δ and Ba0.5Sr0.5Co0.1
We list the electrochemical performance of the cells fabricated from various SMs and SIMs in Table 1. All these works indicated the fact that SMs and SIMs can be used as new functional membranes to replace the conventional ionic electrolyte of SOFCs. These significant findings have stimulated a strong interest in the research and development of SM fuel cells (SMFCs) and SIMFCs. Moreover, studies on fundamental issues of SMFCs and SIMFCs have also been carried out with a focus on the avoidance of electronic short circuiting, which is known to be an inherent advantage introduced from using SMs/SIMs to replace the conventional electrolyte membrane. The underlying scientific mechanism and working principle for SMFCs and SIMFCs have been discovered in terms of junction effects and energy band alignments within the devices. For instance, bulk p-n, planar p-n and Schottky junctions have been considered as major factors that lead to a built-in-field for charge separation, which can block the electrons from passing internally through the devices and thus avoid short circuiting[20,21,46-48]. Based on these working principles, various junction-based fuel cells and relative technologies have emerged by means of interfacial modulation and energy band engineering[49].
A summary of performance studies of fuel cells fabricated from various SIMs and SMs
| No. | Configuration | Temperature (°C) | OCV | Pmax (mW/cm2) | Ref. |
| 1 | Ag/Li0.15Ni0.45Zn0.4-SDC/Ag/Ni | 550 | 1.0 | 600 | [19] |
| 2 | Ni-NCAL/LSCF-SCDC/Ni-NCAL | 550 | 1.06 | 1080 | [21] |
| 3 | Ni-NCAL/LSCrF-SDC/NCAL-Ni | 550 | 1.074 | 1059 | [22] |
| 4 | Ag/Ce0.8Sm0.2O2-δ (SDC)-Na2CO3-Sr2Fe1.5Mo0.5O6-δ (SFM)/Ag | 750 | 1.05 | 360 | [23] |
| 5 | Ni-NCAL/Ca0.04Ce0.96-xSmxO2-δ (x = 0, 0.09, 0.16, and 0.24) (SCDC)-La0.6Sr0.4 | 550 | 1.08 | 814 | [29] |
| 6 | Ni-NCAL/SrFeO3-Ce0.8Sm0.2O2-δ/NCAL-Ni | 550 | 1.08 | 780 | [30] |
| 7 | Ni-NCAL/SDC-SSC/NCAL-Ni | 550 | 1.1 | 742 | [31] |
| 8 | Ag/LiAl0.5Co0.5O2/Ag | 525 | 1 | 173 | [32] |
| 9 | Ni foam/LNF-SDC/Ag | 550 | 1 | 760 | [33] |
| 10 | Ni-NCAL/NSDC-NCAL/NCAL-Ni | 550 | 1 | 1072 | [34] |
| 11 | Ni-NCAL/ZnO/NCAL-Ni | 550 | 1.06 | 482 | [35] |
| 12 | Ni-NCAL/ZnO-LCP/NCAL-Ni | 550 | 1.04 | 1055 | [36] |
| 13 | Ni-NCAL/Film TiO2/NCAL-Ni | 550 | 1.1 | 364 | [37] |
| 14 | Ni-NCAL/SrTiO3(STO)/NCAL–Ni | 550 | 0.996 | 620 | [38] |
| 15 | Ni-NCAL/LST/NCAL-Ni | 550 | 1.128 | 908.2 | [39] |
| 16 | Ni-NCAL/CeO2/NCAL-Ni | 550 | 1.12 | 660 | [40] |
| 17 | Ni-NCAL/CeO/CeO2-δ/NCAL-Ni | 520 | 1.08 | 697 | [41] |
| 18 | Ni-NCAL/3C-SiC/ZnO/NCAL-Ni | 550 | 1.1 | 270 | [42] |
| 19 | Ni-NCAL/ BCFZY-ZnO/NCAL-Ni | 500 | 1.01 | 643 | [43] |
| 20 | Ni-NCAL/BCFCe0.2TZY/NCAL-Ni | 530 | 1.09 | 873 | [45] |
| 21 | Ni-LNCA/TDC-Co/Al | NA | NA | NA | [46] |
| 22 | Ni/LCN–NSDC/Ag | 550 | 0.8 | 500 | [47] |
| 23 | Ni-NCAL/LSCF-SCDC/NCAL-Ni | 550 | 1.08 | 1000 | [48] |
FUTURE RESEARCH AND DEVELOPMENT
Though SOFC technology has advanced over the last two decades, unleashing the potential of SOFCs requires more attention to be paid to the operation of the commonly used YSZ electrolyte material, which has limited ionic conductivity at lower temperatures. The nano-SOFC provides a new route to address this quest by taking advantage of nano-redox reaction principles and devices. A remarkable benefit from the new approach that eliminates a physically separated electrolyte layer deserves further investigations of nanomaterials based on SIMs and associated operational conditions in order to realize the excellent opportunities.
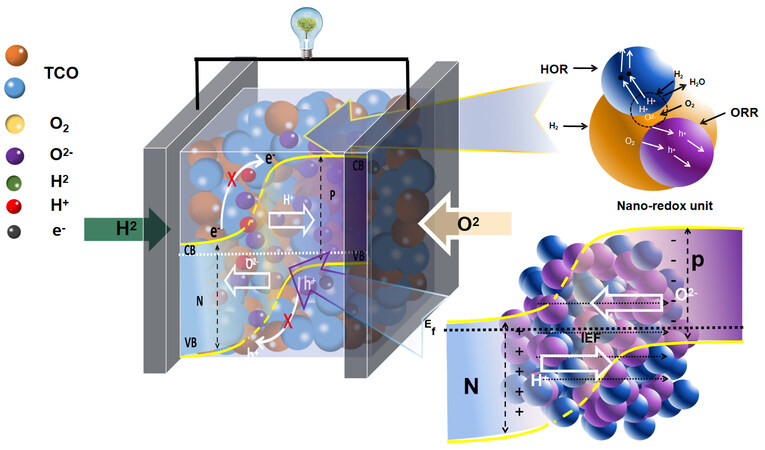
In this regard, a typical example can be proposed for an advanced technology and future development in nano-SOFCs may be a single-layer device made of TCOs[45]. TCOs have been frequently used as the electrodes in SOFCs in the form of simplex phases and heterostructure materials for enhancing the cell performance via facilitating the HOR and ORR activity. Based on the nano-SOFC principle, the device can be directly built on the TCO single layer, as presented in Figure 5. The TCO functions in this case like a membrane reactor, which can not only realize the HOR and ORR on sites within the TCO membrane, but also transport ions simultaneously to complete the fuel cell reactions. Due to the amphoteric semiconducting properties of the TCO, it displays either n- or p-type conductivity under a reducing or oxidizing environment, respectively, in the fuel cell hydrogen and air sides.
Figure 5. TCO SLFC built on the nano-redox unit/reactor (as illustrated above) and charge separation mechanism (as illustrated below). Ec: Energy of conduction band; Ev: energy of valence band; Ef: energy of Femi level; IEF: internal electric field; TCO: triple-charge conducting oxide; SLFC: single-layer fuel cell; HOR: hydrogen oxidation reaction; ORR: oxygen reduction reaction; CB: conduction band; VB: valence band.
Therefore, there is an in-situ formation of a spatial bulk n-p heterojunction to prevent the electron short circuiting problem, as illustrated in Figure 5, which enables a single-layer SOFC device composed of nano-redox generators. The HOR and ORR can occur at any site in the TCO membrane layer to realize the fuel cell function. Since the TCO itself is a SOFC electrode, when a TCO membrane device is in operation status, electrons are activated to the conduction band and the holes to the valence band, which can be transferred to the anode and cathode zones, as indicated in Figure 5; thus, further promoting the fuel cell HOR and ORR. Therefore, it can improve the electrocatalytic and redox reaction efficiency to endow the device with higher conversion efficiency and power output.
It is noteworthy that the spatial bulk p-n heterojunction of the TCO membrane device can provide several functions and contributions. The first is to block the electron flow in the TCO membrane avoiding electronic short-circuiting. Second, the direction of the bulk p-n heterojunction built-in or internal electric field (IEF) distributed over the TCO membrane, as illustrated in Figure 5, excels the transport of the ions, e.g., H+ and O2-, thus, increasing the ionic conductivity and output current/power. The TCO membrane itself is equivalent to the electrode available in traditional SOFCs, so it can attend and promote the fuel cell redox reaction simultaneously. The interfacial polarization is minimized or entirely removed, which is critical for traditional SOFCs. All these features support the uniqueness and advances of TCO membrane fuel cells.
The fuel cell device built on a TCO single layer can therefore reach a high efficiency and expected power output of > 1000 mW·cm-2 at ≤ 500 °C due to its efficient on-site redox reactions and charge transfer/transport, as well as the high ionic mobility accelerated by the IEF. Because of its rather simple structure, the low cost of materials and easy fabrication and manufacturing of the devices, the traditional SOFC’s associated multi-component structure, the complexity and the burden of matching chemical and physical compatibility, as well as the high fabrication cost problems can be eased or even totally avoided. Moreover, superior performance in the desired temperature range, e.g., 300-500 °C, can be expected for the realistic demonstration of low-temperature SOFC/PCFC operations. TCO-based nano-SOFCs therefore may offer a new research and development avenue for the commercialization of SOFCs and PCFCs.
At present, SMFCs and SIMFCs are still in the research stage, with constraints in terms of their engineering and financially supported technical projects, which limit the scope of the collected data for reviewing the technical and engineering issues. However, some important analysis can be performed with respect to the material and technological aspects to strengthen the viability of SMFC and SIMFC systems. Though current studies of SMFCs and SIMFCs have primarily focused on laboratory-scale single cells for new semiconductors or semiconductor-ionic materials for membrane applications, two major aspects can be availably evaluated: (1) that SMFCs and SIMFCs commonly deliver higher electrochemical performance compared to conventional SOFCs at low operating temperatures; and (2) the operation durability with 100-200 h of the SMFCs and SIMFCs have been successfully demonstrated, which have strongly proven the feasibility of the new working principle of SMFCs and SIMFCs, as discussed earlier. Table 2 lists the latest reported durability of SMFCs and SIMFCs for over 100 h. Moreover, the SMFC/SIMFC devices can be fabricated in a simplified structure to avoid the complex procedure for conventional anode/electrolyte/cathode assembly, even as a single-layer device. Therefore, both the material and manufacturing costs can be significantly reduced.
A summary of stability investigations of some typical SMFCs and SIMFCs
| Semiconductor membrane | Temperature (°C) | Current density (mA·cm-2) | Stability hours (h) | Years | Ref. |
| La0.6Sr0.4Co0.2Fe0.8O3-δ-SCDC | 550 | 179 | ~60 | 2017 | [50] |
| NiO-YSZ-La0.6Sr0.4Co0.2Fe0.8O3-δ | 550 | 115 | > 100 | 2018 | [51] |
| Core-shell CeO2/CeO2-δ | 520 | 100 | 200 | 2019 | [41] |
| BaCo0.2Fe0.1Ce0.2Tm0.1Zr0.3Y0.1O3-δ | 530 | 120 | ~100 | 2021 | [45] |
| Ba0.5Sr0.5Fe0.8Sb0.2O3-δ-SDC | 520 | 110 | > 100 | 2021 | [52] |
| CeO2-Na2CO3 coating layer | 500 | 100 | ~100 | 2021 | [53] |
From the material and technical aspects, compatibility issues still exist due to the lack of suitable electrode materials, which have good chemical and mechanical compatibilities with semiconductor-based membranes. This has actually been a long-existing issue in the conventional SOFC technology over several decades until the perovskite electrodes with proper compatibility with the YSZ electrolyte have been developed through suitable fabrication techniques. It is generally accepted that for these novel SMFCs and SIMFCs, significant technical and engineering bottlenecks still remain that prohibit the further scale-up of this fuel cell technology. However, there is enormous ongoing research to increase fuel cell performance by exploring new semiconductor or semiconductor ionic materials with the potential to be applied for industrial purposes.
Another distinct characteristic is the low-temperature (< 550 °C) operation superiority of SMFCs and SIMFCs over intermediate-temperature (> 600 °C) SOFCs or the commercial systems that typically operate above 700 °C, which results in knock-on benefits in terms of low-cost interconnect and the sealant to be adopted, lowering the investment while providing better durability. Therefore, the total cost of the cell is expected to be effectively reduced, making it more competitive in the energy conversion market. However, it is noteworthy that the development of SMFCs and SIMFCs is still in its early stage with material and technological restrictions, as frequent approaches for developing conventional SOFCs are not perfectly applicable for SMFCs and SIMFCs. Therefore, more efforts are required worldwide from academic researchers of various backgrounds and fields to elucidate the underlying science. In addition, input from the industrial stakeholders is urgently required and the policy supports from government are crucial to accelerate the pace of the technological transfer of SMFCs from the lab-scale to industrial pilot plant demonstration to achieve its early techno-economic target for commercialization.
DECLARATIONS
Authors’ contributionsConceived the idea, designed the manuscript: Zhu B, Xia C, Wang B, Kim JS, Lund P, Li T
Prepared most figures: Mi Y, Zhu B, Wang B
Wrote the paper: Zhu B, Xia C, Wang B, Kim JS, Lund P, Li T
All authors participated in the data analysis and results discussions, and commented on the manuscript.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNational Natural Science Foundation of China for providing financial support (Grant No.51772080, 51872080, 12004103), Jiangsu Provincial Innovation and Entrepreneurship Talent program support, Natural Science Foundation of Jiangsu Province for financial support (Grant No. BK20210252).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright©The Author(s) 2021.
REFERENCES
2. Nernst W. Electrolytic conductivity of solids at high temperatures. Zeitschrift fur Elektrochemie 1899;6:41-3.
3. Steele BCH, Heinzel A. Materials for fuel-cell technologies. Materials for Sustainable Energy. Co-Published with Macmillan Publishers Ltd, UK; 2010. p. 224-31.
4. Stambouli A, Traversa E. Solid oxide fuel cells (SOFCs): a review of an environmentally clean and efficient source of energy. Renew Sustain Energy Rev 2002;6:433-55.
5. Duan C, Tong J, Shang M, et al. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015;349:1321-6.
6. Duan C, Kee RJ, Zhu H, et al. Highly durable, coking and sulfur tolerant, fuel-flexible protonic ceramic fuel cells. Nature 2018;557:217-22.
7. Ray ER. Westinghouse tubular SOFC technology. Available from: https://www.osti.gov/servlets/purl/7128507. [Last accessed on 3 Sep 2021].
8. Bruijn F. The current status of fuel cell technology for mobile and stationary applications. Green Chem 2005;7:132.
9. Yu J, Ran R, Zhong Y, Zhou W, Ni M, Shao Z. Advances in porous perovskites: synthesis and electrocatalytic performance in fuel cells and metal-air batteries. Energy Environ Mater 2020;3:121-45.
10. Brett DJ, Atkinson A, Brandon NP, Skinner SJ. Intermediate temperature solid oxide fuel cells. Chem Soc Rev 2008;37:1568-78.
11. Leah RT, Bone A, Hammer E, et al. Development progress on the ceres power steel cell technology platform: further progress towards commercialization. ECS Trans 2017;78:87-95.
12. Barrett S. Ceres, Weichai Power develop first range-extender bus prototype. Fuel Cells Bulletin 2019;10:4.
13. Mizutani Y. Current State of R&D on Micro Tubular Solid Oxide Fuel Cells in Japan. In: Kakaç S, Pramuanjaroenkij A, Vasiliev L, editors. Mini-Micro Fuel Cells. Dordrecht: Springer Netherlands; 2008. p. 407-18.
14. Chao CC, Hsu CM, Cui Y, Prinz FB. Improved solid oxide fuel cell performance with nanostructured electrolytes. ACS Nano 2011;5:5692-6.
15. Swartwout R, Hoerantner MT, Bulović V. Scalable deposition methods for large-area production of perovskite thin films. Energy Environ Mater 2019;2:119-45.
16. Han GD, Bae K, Kang EH, Choi HJ, Shim JH. Inkjet printing for manufacturing solid oxide fuel cells. ACS Energy Letters 2020;5:1586-92.
17. Kwon C, Lee J, Kim K, Lee H, Lee J, Son J. The thermomechanical stability of micro-solid oxide fuel cells fabricated on anodized aluminum oxide membranes. J Power Sources 2012;210:178-83.
18. Schlupp MVF, Evans A, Martynczuk J, Prestat M. Micro-solid oxide fuel cell membranes prepared by aerosol-assisted chemical vapor deposition. Adv Energy Mater 2014;4:1301383.
19. Zhu B, Raza R, Abbas G, Singh M. An electrolyte-free fuel cell constructed from one homogenous layer with mixed conductivity. Adv Funct Mater 2011;21:2465-9.
20. Zhu B, Lund P, Raza R, et al. A new energy conversion technology based on nano-redox and nano-device processes. Nano Energy 2013;2:1179-85.
21. Zhu B, Huang Y, Fan L, et al. Novel fuel cell with nanocomposite functional layer designed by perovskite solar cell principle. Nano Energy 2016;19:156-64.
22. Meng Y, Mi Y, Xu F, et al. Low-temperature fuel cells using a composite of redox-stable perovskite oxide La0.7Sr0.3Cr0.5Fe0.5O3-δ and ionic conductor. J Power Sources 2017;366:259-64.
23. Dong X, Tian L, Li J, Zhao Y, Tian Y, Li Y. Single layer fuel cell based on a composite of Ce0.8Sm0.2O2-δ-Na2CO3 and a mixed ionic and electronic conductor Sr2Fe1.5Mo0.5O6-δ. J Power Sources 2014;249:270-6.
24. Leng Y, Chan S, Liu Q. Development of LSCF-GDC composite cathodes for low-temperature solid oxide fuel cells with thin film GDC electrolyte. Int J Hydrogen Energy 2008;33:3808-17.
25. Zhang Y, Knibbe R, Sunarso J, et al. Recent Progress on advanced materials for solid-oxide fuel cells operating below 500 °C. Adv Mater 2017;29:1700132.
26. Singh K, Nowotny J, Thangadurai V. Amphoteric oxide semiconductors for energy conversion devices: a tutorial review. Chem Soc Rev 2013;42:1961-72.
27. Zhu B, Raza R, Qin H, Liu Q, Fan L. Fuel cells based on electrolyte and non-electrolyte separators. Energy Environ Sci 2011;4:2986.
29. Wang B, Wang Y, Fan L, et al. Preparation and characterization of Sm and Ca co-doped ceria-La0.6Sr0.4Co0.2Fe0.8O3-δ semiconductor-ionic composites for electrolyte-layer-free fuel cells. J Mater Chem A 2016;4:15426-36.
30. Meng Y, Wang X, Xia C, et al. High-performance SOFC based on a novel semiconductor-ionic SrFeO3-δ-Ce0.8Sm0.2O2-δ membrane. Int J Hydrogen Energy 2018;43:12697-704.
31. Deng H, Zhang W, Wang X, et al. An ionic conductor Ce0.8Sm0.2O2-δ (SDC) and semiconductor Sm0.5Sr0.5CoO3 (SSC) composite for high performance electrolyte-free fuel cell. Int J Hydrogen Energy 2017;42:22228-34.
32. Lan R, Tao S. Novel proton conductors in the layered oxide material LixlAl0.5Co0.5O2. Adv Energy Mater 2014;4:1301683.
33. Zhu B, Fan L, Deng H, et al. Corrigendum to “LiNiFe-based layered structure oxide and composite for advanced single layer fuel cells” [J. Power Sources 316 (2016) 37–43]. J Power Sources 2016;316:37-43.
34. Zhang W, Cai Y, Wang B, et al. Mixed ionic-electronic conductor membrane based fuel cells by incorporating semiconductor Ni0.8Co0.15Al0.05LiO2-δ into the Ce0.8Sm0.2O2-δ-Na2CO3 electrolyte. Int J Hydrogen Energy 2016;41:15346-53.
35. Xia C, Qiao Z, Feng C, Kim JS, Wang B, Zhu B. Study on zinc oxide-based electrolytes in low-temperature solid oxide fuel cells. Materials (Basel) 2017;11:40.
36. Qiao Z, Xia C, Cai Y, et al. Electrochemical and electrical properties of doped CeO2-ZnO composite for low-temperature solid oxide fuel cell applications. J Power Sources 2018;392:33-40.
37. Dong W, Tong Y, Zhu B, et al. Semiconductor TiO2 thin film as an electrolyte for fuel cells. J Mater Chem A 2019;7:16728-34.
38. Chen G, Liu H, He Y, et al. Electrochemical mechanisms of an advanced low-temperature fuel cell with a SrTiO3 electrolyte. J Mater Chem A 2019;7:9638-45.
39. Chen G, Zhu B, Deng H, et al. Advanced fuel cell based on perovskite la-SrTiO3 semiconductor as the electrolyte with superoxide-ion conduction. ACS Appl Mater Interfaces 2018;10:33179-86.
40. Wang B, Zhu B, Yun S, et al. Fast ionic conduction in semiconductor CeO2-δ electrolyte fuel cells. NPG Asia Mater 2019:11.
41. Xing Y, Wu Y, Li L, et al. Proton shuttles in CeO2/CeO2-δ core-shell structure. ACS Energy Lett 2019;4:2601-7.
42. Xing Y, Hu E, Wang F, et al. Cubic silicon carbide/zinc oxide heterostructure fuel cells. Appl Phys Lett 2020;117:162105.
43. Xia C, Mi Y, Wang B, Lin B, Chen G, Zhu B. Shaping triple-conducting semiconductor BaCo0.4Fe0.4Zr0.1Y0.1O3-δ into an electrolyte for low-temperature solid oxide fuel cells. Nat Commun 2019;10:1707.
44. Rauf S, Zhu B, Yousaf Shah MA, et al. Application of a triple-conducting heterostructure electrolyte of Ba0.5Sr0.5Co0.1Fe0.7Zr0.1Y0.1O3-δ and Ca0.04Ce0.80Sm0.16O2-δ in a high-performance low-temperature solid oxide fuel cell. ACS Appl Mater Interfaces 2020;12:35071-80.
45. Rauf S, Zhu B, Shah MY, et al. Tailoring triple charge conduction in BaCo0.2Fe0.1Ce0.2Tm0.1Zr0.3Y0.1O3-δ semiconductor electrolyte for boosting solid oxide fuel cell performance. Renewable Energy 2021;172:336-49.
46. Wang G, Wu X, Cai Y, Ji Y, Yaqub A, Zhu B. Design, fabrication and characterization of a double layer solid oxide fuel cell (DLFC). J Power Sources 2016;332:8-15.
47. Zhu B, Lund PD, Raza R, et al. Schottky junction effect on high performance fuel cells based on nanocomposite materials. Adv Energy Mater 2015;5:1401895.
48. Zhu B, Wang B, Wang Y, et al. Charge separation and transport in La0.6Sr0.4Co0.2Fe0.8O3-δ and ion-doping ceria heterostructure material for new generation fuel cell. Nano Energy 2017;37:195-202.
49. Hu E, Jiang Z, Fan L, et al. Junction and energy band on novel semiconductor-based fuel cells. iScience 2021;24:102191.
50. Wang B, Cai Y, Xia C, et al. Semiconductor-ionic membrane of LaSrCoFe-oxide-doped ceria solid oxide fuel cells. Electrochimica Acta 2017;248:496-504.
51. Cai Y, Wang B, Wang Y, et al. Validating the technological feasibility of yttria-stabilized zirconia-based semiconducting-ionic composite in intermediate-temperature solid oxide fuel cells. J Power Sources 2018;384:318-27.
52. Mushtaq N, Lu Y, Xia C, et al. Promoted electrocatalytic activity and ionic transport simultaneously in dual functional Ba0.5Sr0.5Fe0.8
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zhu B, Mi Y, Xia C, Wang B, Kim JS, Lund P, Li T. A nanoscale perspective on solid oxide and semiconductor membrane fuel cells: materials and technology. Energy Mater 2021;1:100002. http://dx.doi.org/10.20517/energymater.2021.03
AMA Style
Zhu B, Mi Y, Xia C, Wang B, Kim JS, Lund P, Li T. A nanoscale perspective on solid oxide and semiconductor membrane fuel cells: materials and technology. Energy Materials. 2021; 1(1): 100002. http://dx.doi.org/10.20517/energymater.2021.03
Chicago/Turabian Style
Zhu, Bin, Youquan Mi, Chen Xia, Baoyuan Wang, Jung-Sik Kim, Peter Lund, Tao Li. 2021. "A nanoscale perspective on solid oxide and semiconductor membrane fuel cells: materials and technology" Energy Materials. 1, no.1: 100002. http://dx.doi.org/10.20517/energymater.2021.03
ACS Style
Zhu, B.; Mi Y.; Xia C.; Wang B.; Kim J.S.; Lund P.; Li T. A nanoscale perspective on solid oxide and semiconductor membrane fuel cells: materials and technology. Energy Mater. 2021, 1, 100002. http://dx.doi.org/10.20517/energymater.2021.03
About This Article
Copyright
Data & Comments
Data
 Cite This Article 58 clicks
Cite This Article 58 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.