Recent developments in advanced anode materials for lithium-ion batteries
Abstract
The rapid expansion of electric vehicles and mobile electronic devices is the main driver for the improvement of advanced high-performance lithium-ion batteries (LIBs). The electrochemical performance of LIBs depends on the specific capacity, rate performance and cycle stability of the electrode materials. In terms of the enhancement of LIB performance, the improvement of the anode material is significant compared with the cathode material. There are still some challenges in producing an industrial anode material that is superior to commercial graphite. Based on the different electrochemical reaction mechanisms of anode materials for LIBs during charge and discharge, the advantages/disadvantages and electrochemical reaction mechanisms of intercalation-, conversion- and alloying-type anode materials are summarized in detail here. The methods and strategies for improving the electrochemical performance of different types of anode materials are described in detail. Finally, challenges for the future development of LIBs are also considered. This review offers a meaningful reference for the construction and performance optimization of anode materials for LIBs.
Keywords
INTRODUCTION
With the decline of oil and other traditional energy sources, the development and utilization of renewable energy sources, such as solar, wind and tidal power, have become critical problems to be solved in the new era[1-3]. However, these new energy supplies are unstable and cannot be used continuously, so they need to be converted into electricity before being exported[4]. Research into rechargeable batteries has therefore become especially important[5-7]. Traditional lead-acid, nickel-cadmium and nickel-metal hydride batteries have some disadvantages, such as short service life, low energy density and environmental pollution, which greatly limit their large-scale application[8-11]. The development of rechargeable batteries to replace these traditional batteries has been the main priority of the battery industry in recent decades[12-14]. Therefore, there is an urgent need to develop non-toxic and pollution-free battery electrode materials and separators.
Compared with traditional batteries, Li-ion batteries (LIBs) already dominate the development of electronic products and show superior development prospects due to their small size, lightweight, high working voltage, high energy density, good cycling performance, lack of memory effect and environmental friendliness[15-17]. LIBs have been widely used in smartphones, laptops and other portable electronic devices since the 20th century[18-20]. Currently, LIBs are used as the main power supply of electric vehicles but they still require higher energy density, lower costs and enhanced environmental performance[21-23]. The lithium-ion shuttle between the anode and the cathode forms the basis of a LIB as the power supply, and the Li+ insertion ability of the anode is the main factor that determines its performance[24-27]. In order to maintain the development of advanced anode materials, it is becoming ever more necessary to develop high-capacity anode materials to improve the performance of the overall LIB[28-31].
Graphite has been the main LIB anode material since its commercialization by Sony in 1991[32,33]. The reason for this is that graphite has numerous advantages, such as low cost, absolute abundance, non-toxicity and structural stability[19,34-36]. However, the theoretical capacity of graphite is low, which limits its feasibility for high-power applications[37-39]. It is also found that the delithiation/lithiation in the cycle process cause a volume change in the material, which produces stress on the electrode and is not conducive to cycling stability[40-42]. In addition, the graphite electrode may react with the electrolyte at low operating voltages, resulting in lithium deposition[43-45]. This phenomenon not only reduces battery performance, but also poses a serious safety hazard. Therefore, the study of alternative anode materials to improve the electrochemical properties of LIBs, such as specific capacity, cycle stability and safety, is essential. After decades of continuous research, a variety of different types of anode materials have been discovered and improved. So far, according to the different reaction mechanisms, LIB anode materials can be divided into the following three types: intercalation (e.g., graphite[46,47] and lithium titanate[48,49]), conversion (e.g., transition metal oxides[50,51] and sulfides[52]) and alloying anode materials (e.g., silicon[53], tin[54], germanium[55] and
The earliest anode material considered for LIBs was lithium metal. However, lithium dendrites can be formed during the reaction process, thereby limiting its commercialization. In the review, recent advances in the different electrochemical reaction mechanisms of LIB anode materials are summarized, as summarized in Scheme 1. We provide a comprehensive report and in-depth discussion of the electrochemical reactions of anode materials, as well as the advantages and challenges of each type of anode material. The corresponding optimization strategies are also described in detail. This review represents an important reference for the construction and optimization of high-performance anode materials.
INTERCALATION-TYPE ANODE MATERIALS
The reaction mechanism for intercalation-type anode materials is based on the intercalation and deintercalation of lithium ions in the crystal lattice of the host material. Such LIBs are also known as rocking-chair batteries[57-59]. As a layered carbon material, graphite was the first commercialized LIB anode material and is also the most well-known[60,61]. Layered LiC6 can be formed by intercalating lithium ions. The discharge plateau of LiC6 is below 0.2 V (vs. Li+/Li) and it has outstanding dynamic performance for the intercalation of lithium[5,62,63]. However, because of the slow diffusion rate of lithium ions, the rate performance of graphite is not ideal. The intercalated lithium potential is similar to the stripping potential of lithium metal and therefore lithium dendrites and solid electrolyte interphase (SEI) films can easily form[64,65]. In addition, the miscibility of the solution with an electrolyte makes the organic solvent and lithium insert into the graphite layer, causing the graphite to peel off gradually. These problems are not favorable in practical applications. There are two main methods to improve the electrochemical performance of graphite anodes, namely, accelerating the diffusion of lithium ions into graphite and the entire electrode and enhancing the interfacial reaction between graphite and electrolyte to form a thinner and more stable SEI layer.
Cheng et al.[61] prepared a multichannel graphite anode with channels etched into the graphite surface, which enabled the rapid entry of lithium ions into graphite particles for the rapid charging of LIBs. This structure can improve the accessibility of these ions inside the graphite and has good coulombic efficiency. Their results showed that the multichannel graphite anode exhibits excellent charge rate capacities of 83% and 73% at 6 C and 10 C, respectively, which are much better than the pristine graphite anode. In addition, multichannel graphite anodes exhibit a higher enhanced discharge rate capability than pristine graphite. In addition, it has excellent cycling stability with a capacity retention rate of 85% after 3000 cycles at 6 C without any additives.
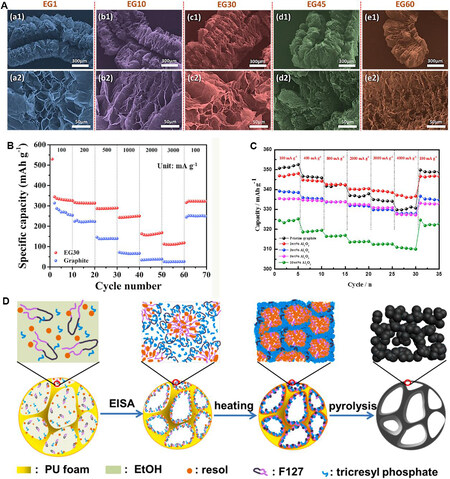
Son et al.[63] systematically studied the thermal exfoliation of expandable graphite (EG) in order to increase its interlayer spacing distance and to determine the optimal temperature for volume expansion. As shown in Figure 1A, all the EG samples exhibit a worm-like morphology with rich open/semi-open inner pore structures. In particular, at the heat treatment temperature of 600 °C for a period of 30 min (EG30), there is a maximum shrinkage of 3.37 Å of d-spacing and a crystallite size of 20.96 nm, while maintaining a similar long range-ordered graphite layer/sheet. In addition, EG30 shows excellent performance in LIBs, with a very high average reversible specific capacity of 338 mAh·g-1 at 100 m·Ag-1 and a high rate capacity of
Figure 1. (A) SEM micrographs of EG samples at different magnifications. (B) Rate performance of EG30 and graphite[63]. (C) Rate capabilities of Al2O3@graphite electrode[65]. (D) Schematic illustration of the synthesis of P-doped mesoporous carbons[73]. Reproduced from Refs.[63,65,73] with permission from Elsevier. EG: Expandable graphite; EG30: the thermal exfoliation process of EG maintains 30 min; PU foam: polyurethane foam; EISA: evaporation induced self-assembly.
Surface modification is an effective method to improve the rapid charging ability of graphite anode materials. Kim et al.[65] improved the rapid charging ability of graphite anode materials by modifying Al2O3 on the surface of graphite. As shown in Figure 1C, the 1 wt.% Al2O3@graphite electrode retains a reversible capacity of ~337.1 mAh·g-1 at a high charge rate of 4000 mA g-1, which corresponds to 97.2% of the capacity obtained at 100 mA g-1. The full battery test with a LiCoO2 cathode and Al2O3-coated graphite anode proves that the introduction of amorphous Al2O3 improves the fast charging ability of the graphite anode material. This method is a practical way of enhancing the fast charging ability of graphite anode materials for high-power LIBs.
Similar to graphite, hard carbon (non-graphitized carbon) materials also belong to the intercalation type of anode material. Their layer spacing is generally greater than 3.8 Å, more than twice the diameter of Li+
Ai et al.[72] prepared N and S co-doped graphene (NS-G). The initial discharge capacity of the obtained material is 1636 mAh·g-1. After 500 cycles, the electrode still offers a reversible capacity of 1090 mAh·g-1. As the number of cycles increases, the capacity of the NS-G anode increases gradually; this can be ascribed to the enhancement of the sample properties of lithium ions. The difference in electronegativity and size between the doped and carbon atoms results in changes in the internal structure (the formation of topological defects) and charge density of the graphene, thereby enhancing the electrochemical performance of the material.
Wang et al.[73] synthesized P-doped mesoporous carbon with a high P content and large pore size through evaporation-induced self-assembly. Tricresyl phosphate was used as a phosphorus precursor, a phenolic solution was used as a carbon precursor, the triblock copolymer F127 was used as a soft template and polyurethane foam was used as a sacrificial template [Figure 1D]. The obtained P-doped mesoporous carbon with a high P content (up to 1.90%) consists of small collaterals and interconnected nanoparticles (10-20 nm), showing large adjustable mesopore dimensions (6.6-14.2 nm) and high surface areas (338-630 m2 g-1). When used as anodes for LIBs, these materials have excellent electrochemical storage performance. The P-doped mesoporous carbon shows a high reversible capacity of 500 mA h g-1 after 200 cycles at 0.5 C, outstanding rate performance and cycling stability after 100 cycles at 10 A g-1.
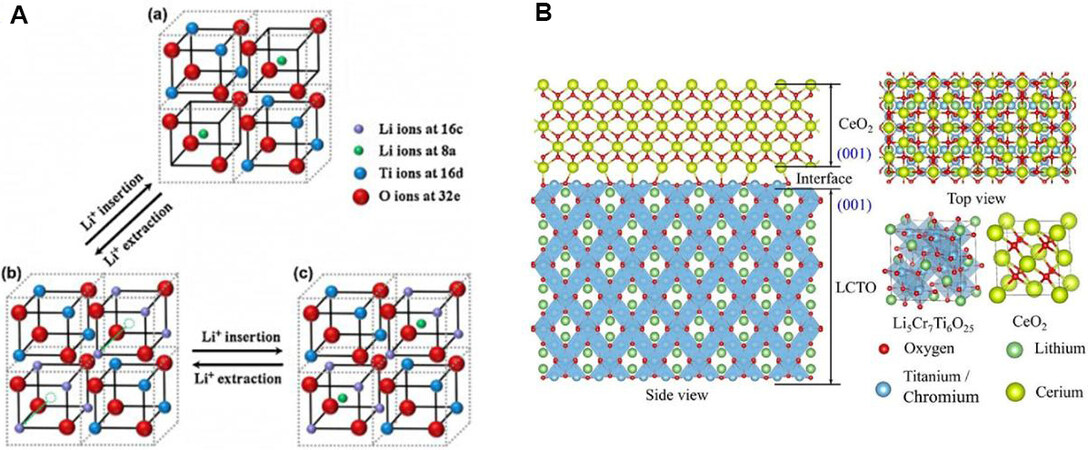
Furthermore, lithium titanium oxide (Li4Ti5O12) is also a typical intercalation-type anode material and exhibits high safety and excellent cycling stability[79,80]. At room temperature, Li+ (1/6) and Ti4+ (5/6) ions are randomly dispersed at the 16d site of the octahedron while the O atoms occupy all 32e sites. The structure can be represented by [Li3]8a[Ti5Li]16d[O12]32e[81,82]. The redox mechanism of Li4Ti5O12 consists of two stages. In the first stage, Li4Ti5O12 can be embedded with up to 3 moL Li+ at ~1.5 V (vs. Li/Li+) to produce Li7Ti5O12. In the second stage, Li7Ti5O12 is inserted below 1 V (vs. Li/Li+) by 2 moL Li+ to obtain Li9Ti5O12. In addition, the reaction equation can be expressed as[83,84]:
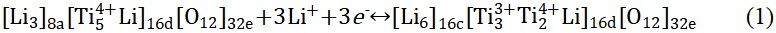
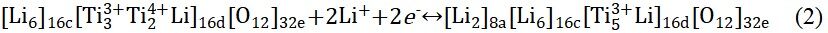
Surprisingly, the structure of Li4Ti5O12 changes only slightly during charging and discharging. In addition, Figure 2A shows the delithiation/lithiation process of Li4Ti5O12 at 3, 1-3 and 0.01-1 V, respectively[83]. However, due to the low conductivity of Li4Ti5O12 (10-13 S cm-1)[5], it is difficult to transfer electrons from the Li4Ti5O12 electrode to the external circuit, resulting in high electrode polarization during the continuous cycling process, especially at high current density, which seriously limits its practical application. Researchers have utilized many strategies to enhance the electronic conductivity of Li4Ti5O12 anodes and enhance their electrochemical performance. Surface modification and doping can improve the ion diffusion and conductivity, which can improve the high rate performance of the material[85-88].
Figure 2. (A) Schematic illustration of the Li+ interaction and deintercalation from the spinel Li4Ti5O12 structure at (a) 3, (b) 1-3 and (c) 0.01-1 V[83]. Reproduced from Ref.[83] with permission from Elsevier. (B) Interface model between Li5Cr7Ti6O25 and CeO2[90]. Reproduced from Ref.[90] with permission from the American Chemical Society.
Ion doping usually reduces the theoretical capacity of spinel Li4Ti5O12 due to the decrease in active Ti or Li. Hence, the development of new Li4Ti5O12-based materials is imperative in the field of high-power LIBs. In addition, considering the cost of Li, it is vital to exploit low Li intercalation-type Ti-based anode materials. Lately, our group constructed a new Cr,Ti-based complex, Li5Cr7Ti6O25, with a low content of Li. According to the one-electron transfer of Ti4+/3+ and Cr2+/3+ ions, the theoretical capacity of Li5Cr7Ti6O25 is ~320 mAh·g-1 when cycled between 3 and 0 V. According to the one-electron transfer of Ti4+/3+, the theoretical capacity of Li5Cr7Ti6O25 is ~147 mAh·g-1 when cycled between 3 and 1 V. Therefore, Li5Cr7Ti6O25 as a promising anode material has also received significant attention[89]. Mei et al.[90] prepared Li5Cr7Ti6O25@CeO2 composite electrode materials by a simple high-temperature solid phase method and studied the effects of different coating amounts of CeO2 on the electrochemical properties of electrode materials. The results show that Li5Cr7Ti6O25 has the best electrochemical performance when the coating amount of CeO2 is 3 wt.%. In particular, the reversible capacity is 101 mAh·g-1 when cycled between 3 and 1 V after 100 cycles at 5 C. According to the TEM image, the existence of a coating layer can be clearly seen, indicating that there is an interface between Li5Cr7Ti6O25 and CeO2. Figure 2B shows the interface model. According to first-principles calculations, the crystal plane mismatch of the two materials is only 8%, which theoretically explains how the CeO2 coating can effectively enhance the cycling performance of the materials.
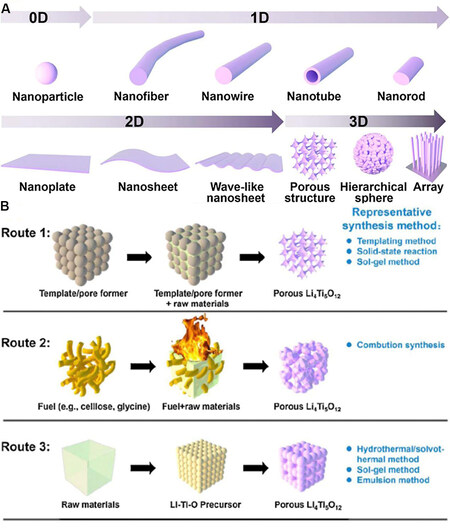
Figure 3A shows a schematic of Li4Ti5O12 with various 0D, 1D, 2D and 3D nano/microstructures[80]. The morphology control of Li4Ti5O12 can be utilized to reduce the diffusion distance of Li+[91,92]. In addition, the nanostructures increase the surface area that can be used to reduce interfacial charge transfer, thus substantially improving the electrochemical activity. Therefore, nanostructured electrodes generally have higher power densities than coarse powder electrodes. The addition of micrometer particles to the holes is another effective method for increasing the rate properties of electrode materials. Micron-sized porous electrodes have the advantage of being easy to assemble and are used for short diffusion pathways of lithium ions. The porous structure can increase the surface area of the electrode material, thus exposing more active sites for lithium-ion insertion into the electrode material, while the nanometer thick wall shortens the diffusion distance of lithium. The nanostructures help to reduce electrode polarization. Microparticles with a 3D structure can also ensure contact between the particles, thus increasing the stability of the electrode and inhibiting the capacity loss. The three common preparation pathways of porous Li4Ti5O12 are summarized in Figure 3B. The first route uses a template or hole front to fabricate pores in the sample. The pore size and porosity can be easily adjusted by using various templates or pore formers. The second method is combustion synthesis. The formation of pores is mainly through the diffusion of gas in the reaction process. The process of this method is relatively simple but the controllability is poor. The third method is self-assembly synthesis. Pores arise from voids produced from a particular structure and sites left by the removal of some organic groups.
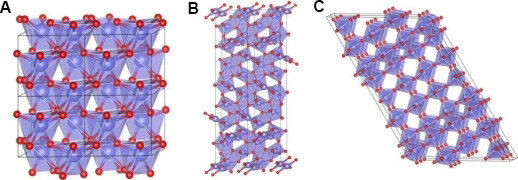
In recent years, a series of research results on Nb-based oxide materials as anode materials have shown significant advantages in the performance of batteries[93,94]. Nb-based oxides have a high potential window (> 1 V vs. Li/Li+), similar to Li4Ti5O12, which effectively prevents the formation of lithium dendrites and SEI films. The high specific capacity of Nb-based oxides is due to two Li+ insertion and extraction during the charge-discharge process, corresponding to Nb5+/Nb4+ and Nb4+/Nb3+, respectively[95-97]. Nb-based oxides are a large family, mainly including the following two types: the Nb-O type represented by Nb2O5 and the M-Nb-O type represented by TiNbxO2+2.5x. Nb-O compounds include NbO (2+), Nb2O3 (3+), NbO2 (4+) and Nb2O5 (5+). Nb2O5 is the most common and stable, as well as one of the most studied[98]. As shown in Figure 4, Nb2O5 mainly includes pseudo-hexagonal (TT-Nb2O5), orthogonal (T-Nb2O5) and monoclinic crystal structures (M-Nb2O5)[99]. Although Li+ deintercalation may occur in each crystal structure, there are some differences in their electrochemical behavior and performance. Notably, orthogonal T-Nb2O5 has attracted increasing attention due to its pseudocapacitivity[100]. In addition, its (001) lattice spacing (3.90 Å) is about twice the diameter of Li+ (1.52 Å). Such large lattice spacing accelerates the diffusion of Li+. In addition, the volume change during the insertion/extraction process of Li+ is very small, ensuring a long service life[101].
Compared with Nb2O5, M-Nb-O materials show higher specific capacity. For example, the Ti-Nb-O group has three redox electric pairs of Ti4+/Ti3+, Nb5+/Nb4+ and Nb4+/Nb3+, which have a large theoretical capacity[102]. In addition, most of the Ti-Nb-O groups can be represented by the chemical formula
C = 403 - 5441/(133x + 80) (3)
Thus, the theoretical capacities of TiNb2O7, Ti2Nb10O29, TiNb6O17 and TiNb24O62 are 388, 396, 397 and 401 mAh·g-1, respectively, which are about 1.2 times the theoretical capacity of Li4Ti5O12 (0-3 V) and even higher than that of graphite. However, they all have the inherent problem of poor electrical conductivity and their theoretical capacity is relatively low relative to alloying and conversion-type anode materials. So far, researchers have conducted a series of studies on these existing problems and made remarkable achievements. There are many strategies for improving M-Nb-O materials, mainly involving structural engineering[104,105], doping[106,107] and conductive phase modification[108,109]. Conductive phase modification is considered to be a direct and effective method for improving their electrical conductivity.
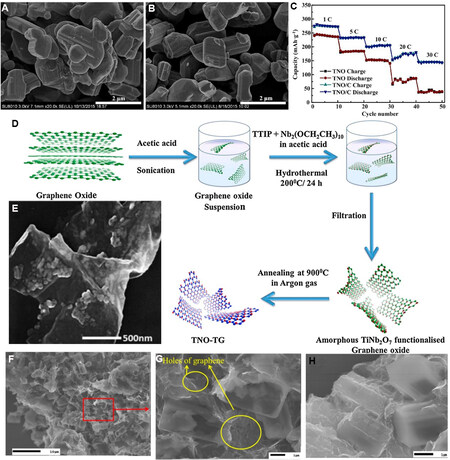
Carbonaceous materials are the most common modified conductive phase because of their variety, low cost and good electrical conductivity. Liu et al.[110] synthesized Ti2Nb10O29/C composites by a simple high-temperature solid phase method. As shown in Figures 5A and B, the size and morphology of the two samples are almost the same and the average particle size is ~1 μm. Compared with pure Ti2Nb10O29,
Figure 5. SEM images of (A) Ti2Nb10O29 and (B) Ti2Nb10O29/C composites. (C) Rate performance of Ti2Nb10O29 and Ti2Nb10O29/C[110]. (D) Schematic illustration and (E) TEM image of TiNb2O7/graphene electrodes[111]. (F-H) FESEM micrographs of LVO/G[112]. Reproduced from Refs.[110,111] with permission from Elsevier. Reproduced from Ref.[112] with permission from the American Chemical Society.
In addition to the above electrode materials, Li3VO4 is another competitive intercalation-type anode material because of its suitable voltage platform (~1.5 V) and high theoretical capacity (394 mAh·g-1).
CONVERSION-TYPE ANODE MATERIALS
Conversion-type anode materials (CTAMs) mainly refer to transition metal oxides[114-116], sulfides[26], phosphides[117] and nitrogen compounds[118] (M = Co, Ni, Fe or Mn). The transformation reaction of metal oxides and sulfides with Li+ is as follows[119]:
MxOy + 2yLi+ + 2ye-↔xM + yLi2O (4)
MxSy + 2yLi+ + 2ye-↔xM + yLi2S (5)
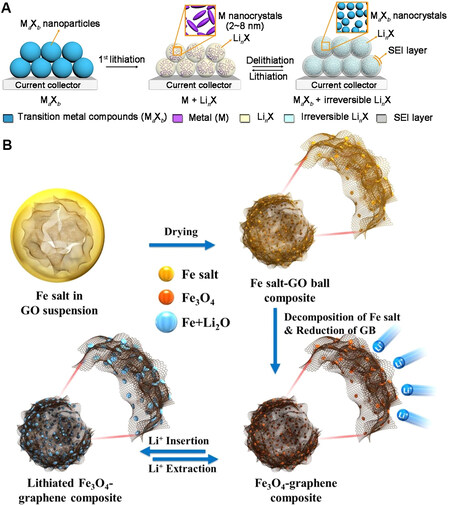
Because there is no position for Li+ insertion and extraction in the spatial structure of CTAMs, it is generally believed that the reaction with Li at room temperature is irreversible. CTAMs possess some advantages, such as composition diversity and high theoretical capacity. A schematic of the lithium storage mechanism of typical CTAMs is depicted in Figure 6A[120]. Wang et al.[121] obtained a NiO nanooctahedron with a unique structure by pyrolysis of hexagonal nickel nanoplate microspheres. Such a structure not only offers a large surface area for rapid diffusion of Li+ between the anode and the electrolyte, but the exposed active surface can also effectively improve charge transport motion mechanics and lithium-ion diffusion. The reversible specific capacity of NiO can reach 792 mAh·g-1 after 200 cycles at 0.2 C, which fully indicates that NiO has outstanding cycle performance as an anode material for LIBs. Choi et al.[122] prepared Fe3O4-decorated hollow graphene spherical composites according to spray pyrolysis. Figure 6B exhibits a detailed schematic of the formation process of hollow Fe3O4/graphene spheres and the electrochemical reaction mechanism. The structure integrity of the Fe3O4-decorated hollow graphene spheres composites can be well maintained during the deintercalation of lithium, indicating that the hollow Fe3O4/graphene spheres have good structural stability and the cycling performance of the hollow Fe3O4/graphene spheres is improved after long cycling at high current density. Fe3O4 was still uniformly dispersed on the graphene spheres after a long period of cycling without aggregation.
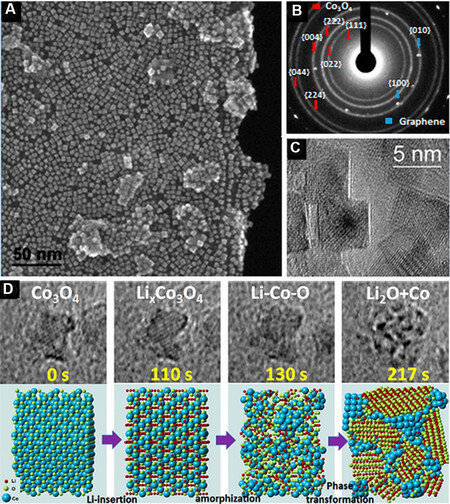
Luo et al.[114] used SEM, selected electron diffraction and high-resolution transmission electron microscopy to study the transformation process of Co3O4 nanocrystals with a particle size of ~5 nm [Figure 7A-C] and the reaction mechanism of Co3O4 as a LIB anode. As shown in Figure 7D, lithium ions enter into the phase of Co3O4 (i.e., LixCo3O4) in the process of lithiation, which is composed of nano-Co-Li-O clusters and is regarded as the intermediate product of the transformation reaction. In the final stage of lithiation, the
Figure 7. (A) SEM image; (B) SAED pattern; and (C) HRTEM image of Co3O4 nanocubes. (D) In situ HRTEM pattern and schematic atomistic models of the lithiation process of a single Co3O4 nanocube[114]. Reproduced from Ref.[114] with permission from the American Chemical Society. SAED: Selected electron diffraction; HRTEM: high-resolution transmission electron microscopy.
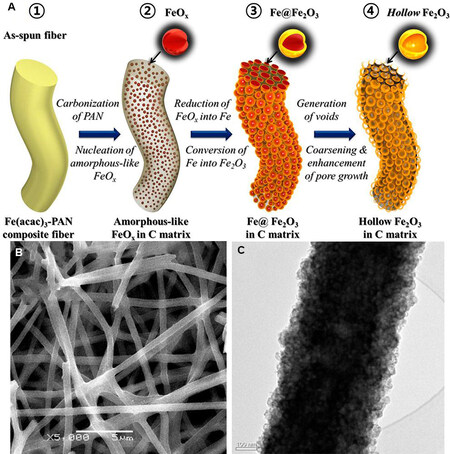
Cho et al.[115] prepared a 1D nanorod-like Fe2O3/C electrode material using a simple and general electrospinning method by the Kirkendall effect and studied the formation mechanism of the nanobubble structure in detail by tracking and observing each step of the nanobubble formation process [Figure 8A]. According to SEM [Figure 8B] and TEM [Figure 8C] images, the nanofiber consists of hollow Fe2O3 spheres evenly dispersed in an amorphous carbon matrix, which has the advantages of a large specific surface area, short diffusion path of lithium ions and direct and efficient electron transport channels. This enables the material to interact with Li+ more effectively, thus giving it with excellent rate capability. In addition, its special bubble-like structure also allows the Fe2O3 bubble void and surrounding carbon atoms to effectively regulate the mechanical stress generated in the process of charging and discharging. Hence, the cycle life of the material is improved. The Fe2O3/C nanorods exhibited excellent cycling performance when used as an anode electrode of LIBs. During the first thirty cycles, the specific capacity of Fe2O3/C nanorods has a slight decrease, but almost no decrease from the 31st to the 300th cycles, reaching a maximum of 824 mAh·g-1 at 1 A g-1. When the measured current density gradually rises from 0.5 to 5 A g-1 and then backs to 0.5 A g-1, the capacity of the material only slightly decreases. When the current density reaches the maximum, the specific capacity remains at 491 mAh·g-1, which fully demonstrates the excellent structure.
However, this transformation mechanism was first intercalated to form highly electroactive M nanoparticles and a LinX matrix surrounded by a SEI film. Due to a large amount of structural rearrangement after lithiation, the voltage lag of CATMs during discharge and charge leads to low energy density and internal thermal evolution. The voltage lag is closely related to the properties of anions, so the hysteresis is most obvious for fluorides, followed by oxides, sulfides, nitrides and phosphides[120]. In addition, due to its low inherent conductivity and the powder pulverization problem in the repeated cycling process, the rate capacity is poor and the capacity decay is fast[123]. Therefore, significant progress must be made before CATMs become practical electrode materials.
ALLOYING-TYPE ANODE MATERIALS
Alloyed anode materials primarily belong to the IVA and VA groups, which include Si, Ge, Sn, Pb, P, As, Sb and Bi. The lithium storage mechanism is an alloying reaction with lithium to form a LiwM alloy[124]. The corresponding alloying reaction, taking Si as an example, is as follows[125]:
Si + 4.4Li+ + 4.4e-↔Li4.4Si (6)
Due to the atoms of any alloy-type material theoretically holding 4.4 or 3 Li+[5], they exhibit very high specific capacities. For example, silicon (4200 mAh·g-1) is ten times higher than graphite in theoretical capacity[126]. It has the highest theoretical capacity of any anode material (with the exception of hydrogen). However, the volume changes greatly during the lithiation reaction, resulting in the formation of a significant outward stress inside the material, which causes the electrode material to be crushed and separated from the collector in serious cases[5]. The SEI film also ruptures, exposing a new surface and requiring the continued consumption of lithium to reform the SEI film. Thus, the cycling performance is relatively low[127,128].
In response to the above problems, researchers have found that for a single Si particle, there is a critical size of 150 nm, below which it does not break after lithiation[129]. Therefore, by reducing the size of Si particles, the inside stress of the electrode material can be released, which reduces the possibility of peeling off from the collector in the reaction process. The pores in porous Si also alleviate the large volume expansion. Various Si nanostructures have been designed to improve their cycling stability, such as nanoparticles (0D)[128,130], 1D structures (nanowires[131,132], nanorods[133,134], and nanotubes[135]), 2D thin films[136] and 3D porous structures[137]. In addition, the reasonable design of Si/C nanocomposites with special structures is also considered to be an effective method to reduce the capacity attenuation caused by the volume change of Si anodes[138].
Gu et al.[139] synthesized a Si-CNF composite structure via an electrospinning method and further studied the lithiation process with in situ TEM. The Si particles do not break whether embedded or attached to the carbon nanofibers. Simultaneously, compared with the Si particles attached to the surface, the particles embedded in the CNF show delayed lithiation, which limits the rate capacity of the battery. In addition, the lithiation of particles embedded in CNF produce a high stress field, resulting in cracking of CNF. Therefore, the spatial correlation between Si nanoparticles and carbon nanocomposites is very important when designing carbon-based Si nanoparticle composites.
Ge is a common semiconductor material that belongs to the same main group as Si and has similar chemical properties. Ge has better conductivity and a faster lithium-ion diffusion rate than silicon. The surface oxide layer is thinner and Ge-based anode materials generally have higher coulomb efficiency. The volume expansion of Ge is isotropic and the anode material is subjected to uniform stress, avoiding the problem of electrode material cracking caused by stress concentration[140]. As a rare metal, Ge has a relatively high cost. During battery cycling, Ge and Li ions form an alloy, which can form a Li-rich local region, which explains the high lithium storage performance based on the Ge-based anode electrode. Similar to Si, nanoscale and composite materials are effective improvement measures to solve the inherent shortcomings of Ge.
Yan et al.[141] obtained silver-embedded 3D nanoporous Ge (Ag-np-Ge) by a melt spinning and one-step dealloying method. It was found that when Ge is used as the active material, ensuring a high theoretical capability, the porous network can provide sufficient space for volume expansion and contraction of the material. In addition, the embedded Ag nanoparticles can promote the electron transport rate. Ag-np-Ge presents a high capacity of 953 mAh·g-1 after 100 cycles at 100 mA·g-1 and an excellent reversible capacity of 522 mAh·g-1 even at 1000 mA·g-1.
Sn metal is one of the most studied anode materials due to its excellent theoretical capacity (994 mAh·g-1) calculated from the final lithium product of Li4.4Sn[142]. However, the large ~300% volume expansion during the lithium process leads to the fracture of the anode material, electrode pulverization, electrical contact failure between the anode materials and conductive additives and an unstable SEI, which limit its commercial application. Jin et al.[143] prepared a novel 3D structured Sn anode material by a simple method. First, the nanosized SnO2 spheres were heat-treated in a tube furnace (C2H2/Ar flow mixing at 400 ℃). After heat treatment, the nanosized SnO2 sphere was transformed into a pure Sn bulk material (~20 μm), which consisted of Sn nanowires (diameter of ~50 nm and several microns in length). The obtained samples have a unique 3D structure with rich voids between the nanowires, which reduce the volume expansion of the Sn bulk material and ensure good electrical contact between the anode material and the conductive additive. The 3D structured Sn anode material shows a specific reversible capacity of 600 mAh·g-1, with no significant capacity degradation at 0.2 C (compared to the 20th cycle).
The theoretical specific capacity of P is 2596 mAh·g-1, which possesses a similar electrochemical reaction mechanism to Si[144], as follows[145]:
P + 3Li+ + 3e-↔Li3P (7)
There are four allotropes of phosphorus, among which white phosphorus is highly toxic and volatile and is therefore not suitable to be used as an electrode material. In recent decades, there has been little research on violet phosphorus. In contrast, red and black phosphorus have good chemical stability at room temperature and atmospheric pressure, so they are often used as electrode materials[146,147]. However, P and Si share the same challenges. So far, the modification of phosphorus anode materials using carbon materials has become the main route to solving these problems[148,149].
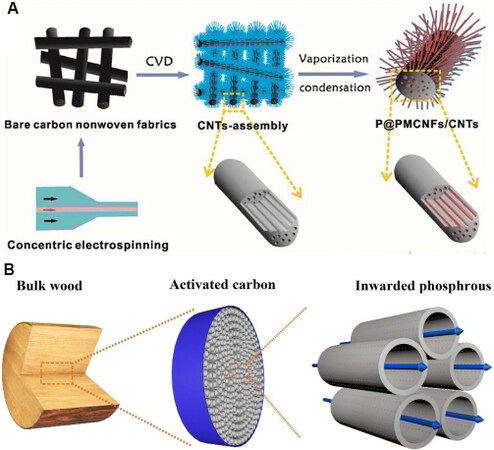
Liang et al.[148] synthesized a free-standing flexible P/C electrode by encapsulating phosphorous in a dual-conducting network of porous multichannel carbon nanofibers and in situ carbon nanotubes (P@PMCNFs/CNTs) [Figure 9A]. The PMCNF/CNT electrode exhibited an outstanding rate performance (601 mAh·g-1 at 3 A g-1) and good cycling ability (802.3 mAh·g-1 at 1 A g-1 after 500 cycles). Yan et al.[149] explored a P@rGO-ACW electrode by constructing phosphorus directly from 3D wood-derived carbon and confining it to a 3D micro-channel carbon matrix [Figure 9B]. This structure not only buffers the volume expansion of phosphorus in the alloying process, but also shortens the transport distance of lithium ions and improves the conductivity of electrons and ions. Therefore, it has good electrochemical behavior. In addition, Sun et al.[150] proposed a novel P-TiO2@CNT composite material. The red phosphorus was modified through the synergistic effect of titanium dioxide and CNTs. The modification not only improved the capacity of P but also regulated the stress during the expansion process of red phosphorus and avoided structural damage.
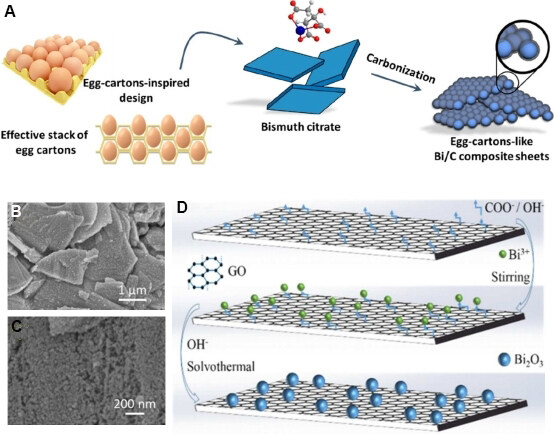
As an important alloy-type anode material, Bi metal has a pseudo-layer structure that is similar to graphite. The volume expansion is 74% due to the formation of a Li3Bi alloy, which is significantly smaller than that of Li4.4Si (~400%) and Li4.25Sn (~257%)[151-153]. Yuan et al.[151] designed an egg-carton-like Bi/C nanocomposite structure [Figure 10A]. From Figure 10B and C, the Bi nanoparticles with a diameter of ~20 nm are placed on micron-sized carbon sheets. The obtained Bi/C fractional complex not only inherits the high electrochemical activity of the Bi nanoparticles but also gains additional advantages from the compact micron-size two-dimensional carbon framework, such as high compaction density, medium specific surface area and strong mechanical protection.
In addition, Bi-based transition metal oxides, as potential anode materials for LIBs, have not been extensively studied. Like all other alloy-type anode materials, volume expansion occur during the repeated reaction, which eventually crushes the active material and separates it from the collector, thus reducing the cycling stability[152,153]. Liang et al.[153] developed a Bi2O2.33/rGO composite material as an anode material for LIBs. The material has large contact area and unique flexibility. After 600 cycles at 10 C, the capacity was still 346 mAh·g-1. Deng et al.[152] prepared a Bi2O3@rGO nanocomposite by a solvothermal method and introduced chemical bonds [Figure 10D]. The capacity of the anode material is 79% after 100 times of continuous charge and discharge at 0.1 C. Even at 10 C, the capacity can reach 270 mAh·g-1. The application of the three typical anode material types in the field of LIBs is summarized in Table 1.
Electrochemical performance of three typical anode material types
| Type of anode material | Initial capacity | Cycling stability | Ref. | |
| Intercalation-type anode materials | Multichannel graphite | 365 mAh·g-1 at 0.1C | 85% retention at 6 C after 3000 cycles | [61] |
| Expanded graphite | 338 mAh·g-1 (0.1 A g-1) | 93 % after 500 cycles (1 A g-1) | [63] | |
| Al2O3@graphite | 344.85 mAh·g-1 (100 mA g-1) | 335mAh·g-1 after 100 cycles (100 mA g-1) | [65] | |
| N, S-doped graphene | 1636 mAh·g-1 (200 mA g-1) | 1090 mAh·g-1 after 500 cycles (200 mA g-1) | [72] | |
| P-doped mesoporous C | 622 mAh·g-1 (0.2 C) | 500 mAh·g-1 after 200 cycles (0.5 C) | [73] | |
| Li5Cr7Ti6O25@CeO2 | 107.5 mAh·g-1 (5 C) | 100.5 mAh·g-1 after 100 cycles (5 C) | [90] | |
| Ti2Nb10O29/C | 204 mAh·g-1 (10 C) | 194 mAh·g-1 after 100 cycles (10 C) | [110] | |
| Conversion-type anode material | NiO | 1219 mAh·g-1 (0.2 C) | 792 mAh·g-1 after 200 cycles (0.2 C) | [121] |
| Fe3O4 | 1374 mAh·g-1 (2 A g-1) | 1050 mAh·g-1 after 300 cycles (2 A g-1) | [122] | |
| CoP-NPPCS | 837.5 mAh·g-1 (0.2 A g-1) | 640 mAh·g-1 after 200 cycles (0.2 A g-1) | [117] | |
| Fe2O3/C | 957 mAh·g-1 (1 A g-1) | 812 mAh·g-1 after 300 cycles (1 A g-1) | [115] | |
| Mn2O3/graphene | 1350 mAh·g-1 (50 mA g-1) | 1180 mAh·g-1 after 250 cycles (0.5 A g-1) | [116] | |
| Alloying-type anode materials | HF-etching Si | 1700 mAh·g-1 (0.2 A g-1) | 959 mAh·g-1 after 300 cycles (0.2 A g-1) | [130] |
| Ag-np-Ge | 1854 mAh·g-1 (100 mA g-1) | 953 mAh·g-1 after 100 cycles (100 mA g-1) | [141] | |
| P@PMCNFs/CNTs | 601 mAh·g-1 (3 A g-1) | 802.3 mAh·g-1 after 500 cycles (1 A g-1) | [148] | |
| Bi/C | 1057 mAh·g-1 (100 mA g-1) | 523 mAh·g-1 after 100 cycles (100 mA g-1) | [151] |
CONCLUSIONS AND PERSPECTIVE
In this work, intercalation, conversion and alloying anode materials with different electrochemical reaction mechanisms for LIBs are reviewed. The advantages and problems of each type of anode material and the corresponding optimization strategies are described in detail. Intercalation anode materials, such as
In general, alloying anode materials represented by Si and Sn have become the most attractive anode materials because of their high capacity, but their large volume variation during cycling is the main factor that impedes their large-scale application. To solve this problem, the most successful method at present is to conduct nanoscale design and future research should focus on the following aspects. The first is a quantitative understanding of nanoscale design, such as the size dependence of nanostructure properties and the development of effective methods for encapsulating nanostructured materials into electrodes. Second are the factors affecting the first coulombic efficiency, such as the formation and properties of the SEI film. Third, in terms of structural design, it is necessary to further develop advanced in situ and non-in situ characterization techniques, combined with first-principles calculations, to reveal the microstructural changes occurring in the process of lithiation/delithiation and understand the inherent electrochemical mechanism and structural advantages. This will allow the Li storage performance to be further optimized.
Similar to alloying anode materials, conversion anode materials also have the problems of easy pulverization, instability of SEI film and large volume change during cycling. To improve the cycling stability, it is necessary to design nanomaterials to realize the mutual conversion of multiple solid phases. In addition, another challenge of conversion-type anode materials is the large voltage hysteresis (~1 V) between charge and discharge. An effective method to solve the problem is to select appropriate electrode design and electrolyte type, such as coating a conductive polymer or lithium-ion conductive solid electrolyte layer on the surface of the anode material.
Anode materials cannot be blindly pursued for high capacity. The synergistic effect of cathode and anode can maximize battery performance. Researchers must design LIB electrodes for overall battery structural stability and high performance and do not need to be limited to current commercial cathode materials. Cathode and anode materials need to be developed together to withstand higher operating voltages and capacities.
Safety is a major consideration in anode design for commercial batteries. Actual battery conditions are often more complex than laboratory test conditions. The structural stability and safety of the battery cannot be ignored. Simultaneously, the selection of materials should avoid toxic and harmful substances. The use of environmentally friendly compounds and composites should be preferred, not only to protect the users but also to reduce the threat to the environment, which is good for recycling.
DECLARATIONS
Authors’ contributionsConceptualization, data curation, writing - original draft: Chang H
Conceptualization, data curation, writing - editing: Wu YR
Data curation: Han X
Writing - review and editing, funding acquisition, supervision: Yi TF
Availability of data and materialsNot applicable.
Financial support and sponsorshipNational Natural Science Foundation of China (No. U1960107), the “333” Talent Project of Hebei Province (No. A202005018), and the Fundamental Research Funds for the Central Universities (No. N2123001).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright©The Author(s) 2021.
REFERENCES
1. Cheng XB, Zhang R, Zhao CZ, Zhang Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem Rev 2017;117:10403-73.
2. Albertus P, Babinec S, Litzelman S, Newman A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat Energy 2018;3:16-21.
3. Chen H, Ling M, Hencz L, et al. Exploring chemical, mechanical, and electrical functionalities of binders for advanced energy-storage devices. Chem Rev 2018;118:8936-82.
5. Zhou L, Zhang K, Hu Z, et al. Recent Developments on and prospects for electrode materials with hierarchical structures for lithium-ion batteries. Adv Energy Mater 2018;8:1701415.
7. Zhang W, Nie J, Li F, Wang ZL, Sun C. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 2018;45:413-9.
8. Tran MK, Rodrigues MF, Kato K, Babu G, Ajayan PM. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat Energy 2019;4:339-45.
9. Zou C, Zhang L, Hu X, Wang Z, Wik T, Pecht M. A review of fractional-order techniques applied to lithium-ion batteries, lead-acid batteries, and supercapacitors. J Power Sources 2018;390:286-96.
10. Winslow KM, Laux SJ, Townsend TG. A review on the growing concern and potential management strategies of waste lithium-ion batteries. Resour Conserv Recy 2018;129:263-77.
11. Wu H, Gong Y, Yu Y, Huang K, Wang L. Superior "green" electrode materials for secondary batteries: through the footprint family indicators to analyze their environmental friendliness. Environ Sci Pollut Res Int 2019;26:36538-57.
12. Yu L, Yu XY, Lou XWD. The design and synthesis of hollow micro-/nanostructures: present and future trends. Adv Mater 2018;30:e1800939.
13. Young C, Wang J, Kim J, Sugahara Y, Henzie J, Yamauchi Y. Controlled chemical vapor deposition for synthesis of nanowire arrays of metal-organic frameworks and their thermal conversion to carbon/metal oxide hybrid materials. Chem Mater 2018;30:3379-86.
14. Yang C, Chen J, Ji X, et al. Aqueous Li-ion battery enabled by halogen conversion-intercalation chemistry in graphite. Nature 2019;569:245-50.
15. Xu W, Wang J, Ding F, et al. Lithium metal anodes for rechargeable batteries. Energy Environ Sci 2014;7:513-37.
16. Wu F, Maier J, Yu Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem Soc Rev 2020;49:1569-614.
17. Wei Z, Wang L, Zhuo M, Ni W, Wang H, Ma J. Layered tin sulfide and selenide anode materials for Li- and Na-ion batteries. J Mater Chem A 2018;6:12185-214.
18. Schmuch R, Wagner R, Hörpel G, Placke T, Winter M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat Energy 2018;3:267-78.
19. Liu Y, Zhu Y, Cui Y. Challenges and opportunities towards fast-charging battery materials. Nat Energy 2019;4:540-50.
20. Liu J, Bao Z, Cui Y, et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat Energy 2019;4:180-6.
21. Yan W, Gao X, Jin X, et al. Nonporous Gel electrolytes enable long cycling at high current density for lithium-metal anodes. ACS Appl Mater Interfaces 2021;13:14258-66.
23. Li L, Zheng Y, Zhang S, Yang J, Shao Z, Guo Z. Recent progress on sodium ion batteries: potential high-performance anodes. Energy Environ Sci 2018;11:2310-40.
24. Hwang J, Myung S, Sun Y. Recent progress in rechargeable potassium batteries. Adv Funct Mater 2018;28:1802938.
25. Han F, Westover AS, Yue J, et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat Energy 2019;4:187-96.
26. Geng P, Zheng S, Tang H, et al. Transition metal sulfides based on graphene for electrochemical energy storage. Adv Energy Mater 2018;8:1703259.
27. Gao Y, Yan Z, Gray JL, et al. Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat Mater 2019;18:384-9.
28. Fang R, Chen K, Yin L, Sun Z, Li F, Cheng HM. The regulating role of carbon nanotubes and graphene in lithium-ion and lithium-sulfur batteries. Adv Mater 2019;31:e1800863.
29. Famprikis T, Canepa P, Dawson JA, Islam MS, Masquelier C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat Mater 2019;18:1278-91.
30. Ji L, Lin Z, Alcoutlabi M, Zhang X. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ Sci 2011;4:2682.
31. Kim S, Seo D, Ma X, Ceder G, Kang K. Electrode materials for rechargeable sodium-ion batteries: potential alternatives to current lithium-ion batteries. Adv Energy Mater 2012;2:710-21.
32. Didier C, Pang WK, Guo Z, Schmid S, Peterson VK. Phase evolution and intermittent disorder in electrochemically lithiated graphite determined using in operando neutron diffraction. Chem Mater 2020;32:2518-31.
33. Li Y, Lu Y, Adelhelm P, Titirici MM, Hu YS. Intercalation chemistry of graphite: alkali metal ions and beyond. Chem Soc Rev 2019;48:4655-87.
34. Kumar R, Sahoo S, Joanni E, et al. Recent progress in the synthesis of graphene and derived materials for next generation electrodes of high performance lithium ion batteries. Prog Energy Combust Sci 2019;75:100786.
35. Luo Y, Yan Y, Zheng S, Xue H, Pang H. Graphitic carbon nitride based materials for electrochemical energy storage. J Mater Chem A 2019;7:901-24.
36. Zhang W. A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J Power Sources 2011;196:13-24.
37. Verma P, Maire P, Novák P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochimica Acta 2010;55:6332-41.
38. Reddy AL, Srivastava A, Gowda SR, Gullapalli H, Dubey M, Ajayan PM. Synthesis of nitrogen-doped graphene films for lithium battery application. ACS Nano 2010;4:6337-42.
39. Marom R, Amalraj SF, Leifer N, Jacob D, Aurbach D. A review of advanced and practical lithium battery materials. J Mater Chem 2011;21:9938.
40. Ding J, Hu W, Paek E, Mitlin D. Review of hybrid ion capacitors: from aqueous to lithium to sodium. Chem Rev 2018;118:6457-98.
42. Nguyen BH, Nguyen VH. Promising applications of graphene and graphene-based nanostructures. Adv Nat Sci: Nanosci Nanotechnol 2016;7:023002.
43. Kleiner K, Ehrenberg H. Challenges considering the degradation of cell components in commercial lithium-ion cells: a review and evaluation of present systems. Top Curr Chem (Cham) 2017;375:54.
44. Senyshyn A, Mühlbauer M, Dolotko O, Ehrenberg H. Low-temperature performance of Li-ion batteries: the behavior of lithiated graphite. J Power Sources 2015;282:235-40.
45. Persson K, Sethuraman VA, Hardwick LJ, et al. Lithium diffusion in graphitic carbon. J Phys Chem Lett 2010;1:1176-80.
46. Chen M, Wang Z, Wang A, et al. Novel self-assembled natural graphite based composite anodes with improved kinetic properties in lithium-ion batteries. J Mater Chem A 2016;4:9865-72.
47. Zhang C, Cai X, Chen W, et al. 3D porous silicon/N-doped carbon composite derived from bamboo charcoal as high-performance anode material for lithium-Ion batteries. ACS Sustainable Chem Eng 2018;6:9930-9.
48. Nuroniah I, Priyono S, Subhan A, Prihandoko B, Suhandi A, Sohib A. Synthesis and characterization of Al-doped Li4Ti5O12 with Sol Gel method for anode material lithium ion battery. Materials Today: Proceedings 2019;13:65-70.
49. Yin P, Peng H, Xiao Y, Lin T, Lin J. Facile synthesis of an Al-doped carbon-coated Li4Ti5O12 anode for high-rate lithium-ion batteries. RSC Adv 2016;6:77151-60.
50. Yi T, Peng P, Han X, Zhu Y, Luo S. Interconnected Co3O4@CoNiO2@PPy nanorod and nanosheet composite grown on nickel foam as binder-free electrodes for Li-ion batteries. Solid State Ionics 2019;329:131-9.
51. Dou Q, Li Y, Ming Ng K. CoO/CoFe2O4 core/shell nanoparticles assembled in carbon sheets as anode materials for lithium ion battery. J Alloys Compds 2019;808:151691.
52. Xiang G, Meng Y, Qu G, et al. Dual-functional NiCo2S4 polyhedral architecture with superior electrochemical performance for supercapacitors and lithium-ion batteries. Sci Bull 2020;65:443-51.
53. Lv G, Zhu B, Li X, et al. Simultaneous perforation and doping of Si nanoparticles for lithium-ion battery anode. ACS Appl Mater Interfaces 2017;9:44452-7.
54. Jiang Q, Li J, Yuan N, Wu Z, Tang J. Black phosphorus with superior lithium ion batteries performance directly synthesized by the efficient thermal-vaporization method. Electrochimica Acta 2018;263:272-6.
55. Youn DH, Patterson NA, Park H, Heller A, Mullins CB. Facile synthesis of Ge/N-doped carbon spheres with varying nitrogen content for lithium ion battery anodes. ACS Appl Mater Interfaces 2016;8:27788-94.
56. Liu Z, Song T, Paik U. Sb-based electrode materials for rechargeable batteries. J Mater Chem A 2018;6:8159-93.
57. Zhang H, Eshetu GG, Judez X, Li C, Rodriguez-Martínez LM, Armand M. Electrolyte additives for lithium metal anodes and rechargeable lithium metal batteries: progress and perspectives. Angew Chem Int Ed Engl 2018;57:15002-27.
58. Zhang X, Cheng X, Chen X, Yan C, Zhang Q. Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries. Adv Funct Mater 2017;27:1605989.
59. Schipper F, Aurbach D. A brief review: Past, present and future of lithium ion batteries. Russ J Electrochem 2016;52:1095-121.
60. Xu J, Dou Y, Wei Z, et al. Recent progress in graphite intercalation compounds for rechargeable metal (Li, Na, K, Al)-Ion batteries. Adv Sci (Weinh) 2017;4:1700146.
61. Cheng Q, Zhang Y. Multi-channel graphite for high-rate lithium ion battery. J Electrochem Soc 2018;165:A1104-9.
62. Wang Z, Selbach SM, Grande T. Van der Waals density functional study of the energetics of alkali metal intercalation in graphite. RSC Adv 2014;4:3973-83.
63. Son D, Kim J, Raj MR, Lee G. Elucidating the structural redox behaviors of nanostructured expanded graphite anodes toward fast-charging and high-performance lithium-ion batteries. Carbon 2021;175:187-201.
64. Goodenough JB, Park KS. The Li-ion rechargeable battery: a perspective. J Am Chem Soc 2013;135:1167-76.
65. Kim DS, Kim YE, Kim H. Improved fast charging capability of graphite anodes via amorphous Al2O3 coating for high power lithium ion batteries. J Power Sources 2019;422:18-24.
66. Zheng H, Qu Q, Zhang L, Liu G, Battaglia VS. Hard carbon: a promising lithium-ion battery anode for high temperature applications with ionic electrolyte. RSC Adv 2012;2:4904.
67. Liu J, Xue D. Hollow nanostructured anode materials for Li-ion batteries. Nanoscale Res Lett 2010;5:1525-34.
68. Malgras V, Tang J, Wang J, et al. Fabrication of nanoporous carbon materials with hard- and soft-templating approaches: a review. J Nanosci Nanotechnol 2019;19:3673-85.
69. Huang S, Li Z, Wang B, et al. N-doping and defective nanographitic domain coupled hard carbon nanoshells for high performance lithium/sodium storage. Adv Funct Mater 2018;28:1706294.
70. Singh V, Joung D, Zhai L, Das S, Khondaker SI, Seal S. Graphene based materials: past, present and future. Progress in Materials Science 2011;56:1178-271.
71. Huang X, Yin Z, Wu S, et al. Graphene-based materials: synthesis, characterization, properties, and applications. Small 2011;7:1876-902.
72. Ai W, Luo Z, Jiang J, et al. Nitrogen and sulfur codoped graphene: multifunctional electrode materials for high-performance li-ion batteries and oxygen reduction reaction. Adv Mater 2014;26:6186-92.
73. Wang J, Xia Y, Liu Y, Li W, Zhao D. Mass production of large-pore phosphorus-doped mesoporous carbon for fast-rechargeable lithium-ion batteries. Energy Storage Materials 2019;22:147-53.
74. Zhang B, Kang F, Tarascon J, Kim J. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog Mater Sci Science 2016;76:319-80.
75. Qie L, Chen WM, Wang ZH, et al. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv Mater 2012;24:2047-50.
76. Wen L, Li F, Cheng HM. Carbon Nanotubes and graphene for flexible electrochemical energy storage: from materials to devices. Adv Mater 2016;28:4306-37.
77. Zhao MQ, Ren CE, Ling Z, et al. Flexible MXene/carbon nanotube composite paper with high volumetric capacitance. Adv Mater 2015;27:339-45.
78. Yu D, Goh K, Wang H, et al. Scalable synthesis of hierarchically structured carbon nanotube-graphene fibres for capacitive energy storage. Nat Nanotechnol 2014;9:555-62.
79. Zhu G, Liu H, Zhuang J, Wang C, Wang Y, Xia Y. Carbon-coated nano-sized Li4Ti5O12 nanoporous micro-sphere as anode material for high-rate lithium-ion batteries. Energy Environ Sci 2011;4:4016.
80. Zhao B, Ran R, Liu M, Shao Z. A comprehensive review of Li4Ti5O12-based electrodes for lithium-ion batteries: the latest advancements and future perspectives. Mater Sci Eng Rep 2015;98:1-71.
81. Yi T, Yang S, Xie Y. Recent advances of Li4Ti5O12 as a promising next generation anode material for high power lithium-ion batteries. J Mater Chem A 2015;3:5750-77.
82. Sun X, Radovanovic PV, Cui B. Advances in spinel Li4Ti5O12 anode materials for lithium-ion batteries. New J Chem 2015;39:38-63.
83. Hsieh C, Chen I, Jiang Y, Lin J. Synthesis of spinel lithium titanate anodes incorporated with rutile titania nanocrystallites by spray drying followed by calcination. Solid State Ionics 2011;201:60-7.
84. Ge H, Li N, Li D, Dai C, Wang D. Study on the theoretical capacity of spinel lithium titanate induced by low-potential intercalation. J Phys Chem C 2009;113:6324-6.
85. Zhu Z, Cheng F, Chen J. Investigation of effects of carbon coating on the electrochemical performance of Li4Ti5O12/C nanocomposites. J Mater Chem A 2013;1:9484.
86. Liu J, Song K, van Aken PA, Maier J, Yu Y. Self-supported Li4Ti5O12-C nanotube arrays as high-rate and long-life anode materials for flexible Li-ion batteries. Nano Lett 2014;14:2597-603.
87. Liu Y, Zhao M, Xu H, Chen J. Fabrication of continuous conductive network for Li4Ti5O12 anode by Cu-doping and graphene wrapping to boost lithium storage. J Alloys Compds 2019;780:1-7.
88. Khan F, Oh M, Kim JH. N-functionalized graphene quantum dots: Charge transporting layer for high-rate and durable Li4Ti5O12-based Li-ion battery. Chem Eng J 2019;369:1024-33.
89. Yan L, Qian S, Yu H, et al. Carbon-enhanced electrochemical performance for spinel Li5Cr7Ti6O25 as a lithium host material. ACS Sustainable Chem Eng 2017;5:957-64.
90. Mei J, Yi TF, Li XY, Zhu YR, Xie Y, Zhang CF. Robust strategy for crafting Li5Cr7Ti6O25@CeO2 composites as high-performance anode material for lithium-ion battery. ACS Appl Mater Interfaces 2017;9:23662-71.
91. Wang Y, Zhu W. Micro/nano-structured Li4Ti5O12 as high rate anode material for lithium ion batteries. Solid State Ionics 2020;349:115297.
92. Chen C, Guo H, Zhao Z, et al. A robust strategy for engineering Li4Ti5O12 hollow micro-cube as superior rate anode for lithium ion batteries. Electrochimica Acta 2019;293:141-8.
93. Han J, Huang Y, Goodenough JB. New anode framework for rechargeable lithium batteries. Chem Mater 2011;23:2027-9.
94. Han J, Goodenough JB. 3-V Full cell performance of anode framework TiNb2O7/Spinel LiNi0.5Mn1.5O4. Chem Mater 2011;23:3404-7.
95. Deng Q, Fu Y, Zhu C, Yu Y. Niobium-based oxides toward advanced electrochemical energy storage: recent advances and challenges. Small 2019;15:e1804884.
96. Yan L, Rui X, Chen G, Xu W, Zou G, Luo H. Recent advances in nanostructured Nb-based oxides for electrochemical energy storage. Nanoscale 2016;8:8443-65.
97. Shen P, Zhang B, Wang Y, et al. Nanoscale niobium oxides anode for electrochemical lithium and sodium storage: a review of recent improvements. J Nanostruct Chem 2021;11:33-68.
98. Lim E, Jo C, Kim H, et al. Facile synthesis of Nb2O5@Carbon core-shell nanocrystals with controlled crystalline structure for high-power anodes in hybrid supercapacitors. ACS Nano 2015;9:7497-505.
99. Yi T, Sari HMK, Li X, et al. A review of niobium oxides based nanocomposites for lithium-ion batteries, sodium-ion batteries and supercapacitors. Nano Energy 2021;85:105955.
100. Zhang C(, Maloney R, Lukatskaya MR, et al. Synthesis and electrochemical properties of niobium pentoxide deposited on layered carbide-derived carbon. J Power Sources 2015;274:121-9.
101. Lou S, Cheng X, Wang L, et al. High-rate capability of three-dimensionally ordered macroporous T-Nb2O5 through Li+ intercalation pseudocapacitance. J Power Sources 2017;361:80-6.
102. Hu L, Luo L, Tang L, Lin C, Li R, Chen Y. Ti2Nb2xO4+5x anode materials for lithium-ion batteries: a comprehensive review. J Mater Chem A 2018;6:9799-815.
103. Lin C, Wang G, Lin S, Li J, Lu L. TiNb6O17: a new electrode material for lithium-ion batteries. Chem Commun (Camb) 2015;51:8970-3.
104. Li H, Zhang Y, Tang Y, et al. TiNb2O7 nanowires with high electrochemical performances as anodes for lithium ion batteries. Appl Surf Sci 2019;475:942-6.
105. Li H, Shen L, Wang J, et al. Three-dimensionally ordered porous TiNb2O7 nanotubes: a superior anode material for next generation hybrid supercapacitors. J Mater Chem A 2015;3:16785-90.
106. Gao J, Cheng X, Lou S, et al. Self-doping Ti1-Nb2+O7 anode material for lithium-ion battery and its electrochemical performance. J Alloys Compds 2017;728:534-40.
107. Yu Z, Waclawik ER, Wang Z, Gu X, Yuan Y, Zheng Z. Dual modification of TiNb2O7 with nitrogen dopants and oxygen vacancies for selective aerobic oxidation of benzylamine to imine under green light. J Mater Chem A 2017;5:4607-15.
108. Wan G, Yang L, Shi S, Tang Y, Xu X, Wang G. Ti2Nb10O29 microspheres coated with ultrathin N-doped carbon layers by atomic layer deposition for enhanced lithium storage. Chem Commun (Camb) 2019;55:517-20.
109. Lyu H, Li J, Wang T, et al. Carbon coated porous titanium niobium oxides as anode materials of lithium-ion batteries for extreme fast charge applications. ACS Appl Energy Mater 2020;3:5657-65.
110. Liu G, Jin B, Zhang R, et al. Synthesis of Ti2Nb10O29/C composite as an anode material for lithium-ion batteries. Int J Hydrogen Energy 2016;41:14807-12.
111. Ashish A, Arunkumar P, Babu B, Manikandan P, Sarang S, Shaijumon M. TiNb2O7/Graphene hybrid material as high performance anode for lithium-ion batteries. Electrochimica Acta 2015;176:285-92.
112. Shi Y, Wang JZ, Chou SL, et al. Hollow structured Li3VO4 wrapped with graphene nanosheets in situ prepared by a one-pot template-free method as an anode for lithium-ion batteries. Nano Lett 2013;13:4715-20.
113. Shi Y, Gao J, Abruña HD, et al. The mechanism of the one-step synthesis of hollow-structured Li(3)VO(4) as an anode for lithium-ion batteries. Chemistry 2014;20:5608-12.
114. Luo L, Wu J, Xu J, Dravid VP. Atomic resolution study of reversible conversion reaction in metal oxide electrodes for lithium-ion battery. ACS Nano 2014;8:11560-6.
115. Cho JS, Hong YJ, Kang YC. Design and synthesis of bubble-nanorod-structured Fe2O3-carbon nanofibers as advanced anode material for li-ion batteries. ACS Nano 2015;9:4026-35.
116. Yuan S, Chen W, Zhang L, et al. Nitrogen-doped graphene-buffered Mn2O3 nanocomposite anodes for fast charging and high discharge capacity lithium-ion batteries. Small 2019;15:1903311.
117. Bai J, Xi B, Mao H, et al. One-step construction of N,P-codoped porous carbon sheets/CoP hybrids with enhanced lithium and potassium storage. Adv Mater 2018;30:e1802310.
118. Dong Y, Li Y, Shi H, et al. Graphene encapsulated iron nitrides confined in 3D carbon nanosheet frameworks for high-rate lithium ion batteries. Carbon 2020;159:213-20.
119. Cabana J, Monconduit L, Larcher D, Palacín MR. Beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Adv Mater 2010;22:E170-92.
120. Lu Y, Yu L, Lou XW. Nanostructured conversion-type anode materials for advanced lithium-ion batteries. Chem 2018;4:972-96.
121. Wang C, Zhao Y, Su D, et al. Synthesis of NiO Nano octahedron aggregates as high-performance anode materials for lithium ion batteries. Electrochimica Acta 2017;231:272-8.
122. Choi SH, Kang YC. Fe3O4-decorated hollow graphene balls prepared by spray pyrolysis process for ultrafast and long cycle-life lithium ion batteries. Carbon 2014;79:58-66.
123. Zhang J, Chu R, Chen Y, et al. MOF-derived transition metal oxide encapsulated in carbon layer as stable lithium ion battery anodes. J Alloys Compds 2019;797:83-91.
124. Yu X, Yu L, Lou XWD. Metal sulfide hollow nanostructures for electrochemical energy storage. Adv Energy Mater 2016;6:1501333.
125. Kummer M, Badillo JP, Schmitz A, et al. Silicon/polyaniline nanocomposites as anode material for lithium ion batteries. J Electrochem Soc 2013;161:A40-5.
126. Luo Z, Xiao Q, Lei G, Li Z, Tang C. Si nanoparticles/graphene composite membrane for high performance silicon anode in lithium ion batteries. Carbon 2016;98:373-80.
127. Kim H, Lee E, Sun Y. Recent advances in the Si-based nanocomposite materials as high capacity anode materials for lithium ion batteries. Mater Today 2014;17:285-97.
128. Su X, Wu Q, Li J, et al. Silicon-based nanomaterials for lithium-ion batteries: a review. Adv Energy Mater 2014;4:1300882.
129. Liu XH, Zhong L, Huang S, Mao SX, Zhu T, Huang JY. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 2012;6:1522-31.
130. Zuo X, Xia Y, Ji Q, et al. Self-templating construction of 3D hierarchical macro-/mesoporous silicon from 0D silica nanoparticles. ACS Nano 2017;11:889-99.
131. Chan CK, Peng H, Liu G, et al. High-performance lithium battery anodes using silicon nanowires. Nat Nanotechnol 2008;3:31-5.
132. Cui LF, Ruffo R, Chan CK, Peng H, Cui Y. Crystalline-amorphous core-shell silicon nanowires for high capacity and high current battery electrodes. Nano Lett 2009;9:491-5.
133. Tao H, Fan L, Qu X. Facile synthesis of ordered porous Si@C nanorods as anode materials for Li-ion batteries. Electrochimica Acta 2012;71:194-200.
134. Wang J, Meng X, Fan X, Zhang W, Zhang H, Wang C. Scalable synthesis of defect abundant si nanorods for high-performance li-ion battery anodes. ACS Nano 2015;9:6576-86.
136. Bensalah N, Kamand FZ, Zaghou M, Dawoud HD, Tahtamouni TA. Silicon nanofilms as anode materials for flexible lithium ion batteries. Thin Solid Films 2019;690:137516.
137. McDowell MT, Lee SW, Nix WD, Cui Y. 25th anniversary article: understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv Mater 2013;25:4966-85.
138. Terranova ML, Orlanducci S, Tamburri E, Guglielmotti V, Rossi M. Si/C hybrid nanostructures for Li-ion anodes: an overview. J Power Sources 2014;246:167-77.
139. Gu M, Li Y, Li X, et al. In situ TEM study of lithiation behavior of silicon nanoparticles attached to and embedded in a carbon matrix. ACS Nano 2012;6:8439-47.
140. Cheng H, Shapter JG, Li Y, Gao G. Recent progress of advanced anode materials of lithium-ion batteries. J Energy Chem 2021;57:451-68.
141. Yan Y, Liu Y, Zhang Y, Qin C, Bakenov Z, Wang Z. Improving the cycling stability of three-dimensional nanoporous Ge anode by embedding Ag nanoparticles for high-performance lithium-ion battery. J Colloid Interface Sci 2021;592:103-15.
142. Wang X, Zhu S, Dong X, Huang H, Qi M. Ionic liquid assisted electrospinning synthesis for ultra-uniform Sn@ mesoporous carbon nanofibers as a flexible self-standing anode for lithium ion batteries. J Alloys Compds 2021;866:158984.
143. Jin Z, Ben L, Yu H, Zhao W, Huang X. A facile method to synthesize 3D structured Sn anode material with excellent electrochemical performance for lithium-ion batteries. Progress in Natural Science: Materials International 2020;30:456-60.
144. Zhang C, Wang X, Liang Q, et al. Amorphous phosphorus/nitrogen-doped graphene paper for ultrastable sodium-ion batteries. Nano Lett 2016;16:2054-60.
145. Chen X, Qiu J, Wang Y, et al. Cactus-like iron diphosphide@carbon nanotubes composites as advanced anode materials for lithium-ion batteries. Electrochimica Acta 2018;259:321-8.
146. Li X, Wang X, Sun J. Recent progress in the carbon-based frameworks for high specific capacity anodes/cathode in lithium/sodium ion batteries. New Carbon Materials 2021;36:106-16.
147. Liu W, Zhi H, Yu X. Recent progress in phosphorus based anode materials for lithium/sodium ion batteries. Energy Storage Materials 2019;16:290-322.
148. Liang S, Pei X, Jiang W, et al. Free-standing dual-network red phosphorus@porous multichannel carbon nanofibers/carbon nanotubes as a stable anode for lithium-ion batteries. Electrochimica Acta 2019;322:134696.
149. Yan Y, Xu H, Peng C, Zhang P, Yang J, Zheng S. 3D phosphorus-carbon electrode with aligned nanochannels promise high-areal-capacity and cyclability in lithium-ion battery. Appl Surf Sci 2019;489:734-40.
150. Sun L, Zhang Y, Si H, et al. TiO2-modified red phosphorus nanosheets entangled in carbon nanotubes for high performance lithium ion batteries. Electrochimica Acta 2019;297:319-27.
151. Yuan H, Jin Y, Chen X, Lan J, Yu Y, Yang X. Large-scale fabrication of egg-carton-inspired Bi/C composite toward high volumetric capacity and long-life lithium ion batteries. ACS Sustainable Chem Eng 2019;7:6033-42.
152. Deng Z, Liu T, Chen T, et al. Enhanced electrochemical performances of Bi2O3/rGO nanocomposite via chemical bonding as anode materials for lithium ion batteries. ACS Appl Mater Interfaces 2017;9:12469-77.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Chang H, Wu YR, Han X, Yi TF. Recent developments in advanced anode materials for lithium-ion batteries. Energy Mater 2021;1:100003. http://dx.doi.org/10.20517/energymater.2021.02
AMA Style
Chang H, Wu YR, Han X, Yi TF. Recent developments in advanced anode materials for lithium-ion batteries. Energy Materials. 2021; 1(1): 100003. http://dx.doi.org/10.20517/energymater.2021.02
Chicago/Turabian Style
Chang, Hui, Yu-Rong Wu, Xiao Han, Ting-Feng Yi. 2021. "Recent developments in advanced anode materials for lithium-ion batteries" Energy Materials. 1, no.1: 100003. http://dx.doi.org/10.20517/energymater.2021.02
ACS Style
Chang, H.; Wu Y.R.; Han X.; Yi T.F. Recent developments in advanced anode materials for lithium-ion batteries. Energy Mater. 2021, 1, 100003. http://dx.doi.org/10.20517/energymater.2021.02
About This Article
Copyright
Data & Comments
Data
 Cite This Article 92 clicks
Cite This Article 92 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.