Hierarchical Ni- and Co-based oxynitride nanoarrays with superior lithiophilicity for high-performance lithium metal anodes
Abstract
Lithium metal has emerged as the most prospective candidate for the realization of improved battery systems. However, notorious Li dendrite formation and the huge volume effect during cycling critically impair the further practical deployment of Li metal batteries. Herein, we propose hierarchical Ni- and Co-based oxynitride (NiCoO2/CoO/Ni3N) nanoarrays with superior lithiophilicity on a three-dimensional nickel foam (NiCoON/NF) as a host for highly stable Li metal anodes. The uniform nitrogen-infused nanorod-on-nanosheet arrays present improved electrical conductivity and an increased concentration of active sites with oxygen vacancies to enhance the surface lithiophilicity, which effectively facilitates homogeneous Li nucleation/growth. Moreover, the hyperbranched structure can induce a homogeneous distribution of Li-ion flux, owing to the enlarged surface area, thereby providing sufficient space to store deposited lithium and relieve the volume expansion. Consequently, the NiCoON/NF host delivers a high Coulombic efficiency (98.4% over 600 cycles) at 1 mA cm-2 and an ultralong lifespan (> 2000 h) under a high capacity of 3 mAh cm-2. Remarkably, a Li@NiCoON/NF-LiFePO4 full battery also reveals impressive electrochemical performance. This work demonstrates new insights into safe rechargeable Li metal batteries.
Keywords
INTRODUCTION
With the intensive demand for electric vehicles and a wide range of intelligent machines, high energy density storage technologies are attracting extensive interest, driven by their long cycle life and ultrahigh power[1-4]. Lithium metal is an excellent choice as an anode for next-generation batteries due its high specific capacity (3860 mAh g-1) and ultralow redox potential (-3.04 V vs. a standard hydrogen electrode)[5,6]. Nevertheless, the irregular Li dendrites and inevitable volume variation associated with lithium metal anodes result in internal short circuit and severe safety risks[7,8]. Meanwhile, the continuous breakdown/reconstruction of the solid electrolyte interphase (SEI) during repeated Li plating/stripping can cause poor Coulombic efficiency (CE) due to the constant consumption between the electrolyte and the newly exposed fresh Li[9]. All these problems deeply restrict the deployment of lithium metal batteries.
So far, multiple strategies have been reasonably put forward to address the above hurdles, including the construction of stable solid electrolyte interphase layers via electrolyte engineering[10-13], optimization of the functional separator and the design of solid-state or gel-polymer electrolytes[14-19]. Although clear achievements have been made regarding interfacial modification, the volume effect still limits the commercialization of lithium metal anodes. Notably, three-dimensional (3D) porous hosts with specific lithiophilic nanostructures have emerged as attractive alternatives to promote homogeneous Li nucleation/growth and accommodate the volume changes[20-23]. For example, Lu et al.[24] reported a lithiophilic NiO hexagonal plate on a Ni substrate to boost uniform lithium deposition. Wang et al.[25] developed hierarchical nanosheet arrays of metal oxides to effectively stimulate Li-ion redistribution.
Nitrogen doping can create a nitrogen-rich surface as an effective approach to boost conductivity and enable improved lithiophilicity of oxygen species and electrochemical performance[27,28]. Recently, plasma-enhanced strategies have been adopted to produce nitrogen-doped CuO nanosheets with high lithiophilicity on Cu foil and metal nitride-decorated Ni foam[29,30]. Ammonia calcination methods have also been used to prepare CuON and Co3N on 3D Cu/Ni foam to enhance the lithiophilicity and electronic conductivity to achieve even Li deposition[31,32]. Ni- and Co-based oxynitrides with certain levels of oxygen vacancies after the treatment with NH3 can produce enhanced electrical conductivity and increased active sites[33,34]. Therefore, the development of Ni- and Co-based oxynitrides as anodes for advanced lithium metal batteries is a highly promising approach.
Here, we present a hierarchical NiCoO2/CoO/Ni3N nanoarray (abbreviated as NiCoON)-decorated 3D Ni foam (NiCoON/NF) produced via a facile in situ hydrothermal and nitriding approach as a current collector for stable Li metal anodes. Compared with its NiCo2O4 counterpart, the newly obtained NiCoON/NF nanocomposite consisting of a significant level of oxygen vacancies shows significant improvements in electronic/ionic conductivity and more active sites to enhance surface lithiophilicity, which can reduce the nucleation barrier. Moreover, the hierarchical architecture, including vertical nanorod-on-nanosheet arrays, offers an enlarged surface area to ensure a uniform distribution of Li-ion flux. Therefore, the 3D NiCoON/NF host achieves a reduced overpotential of 22 mV, a high CE of 98.4% (600 cycles) and excellent cycling behavior (2000 h). Furthermore, the Li@NiCoON/NF-LiFePO4 full battery also presents a long lifespan for continuous 400 cycles at 1 C (160 mA g-1).
EXPERIMENTAL
Preparation of NiCoON/NF current collector
NiCoON/NF was prepared by a hydrothermal method and subsequent ammoniation. The surface oxides of the Ni foam were removed by immersion in hydrochloric acid, followed by ultrasonic cleaning with acetone, water and ethanol sequentially. 2.62 mmol Co(NO3)2·6H2O, 1.31 mmol Ni(NO3)2·6H2O,
Materials characterization
The surface and cross-sectional morphologies were observed using a field-emission S4800 instrument (Zeiss, Germany). The elemental mappings were captured using energy-dispersive X-ray spectroscopy. The high-resolution transmission electron microscopy (HRTEM) image was taken using a JEOL JEM-2100F. X-ray photoelectron spectroscopy (XPS) was carried out to obtain the surface chemical elements using a K-Alpha ESCALAB 250Xi system. All peaks were calibrated based on the C 1s peak (284.8 eV). X-ray diffraction (XRD) patterns were collected to analyze the phase composition on an XRD-6100 diffractometer coupled with Cu-Kα radiation (Shimadzu, Japan). The pore structure distributions and surface areas from the Brunauer-Emmett-Teller (BET) method were detected using an absorption analyzer (Micromeritics ASAP 2020) and N2 adsorption-desorption isotherm at 77 K.
Electrochemical measurements
CR2032-type coin cells were used to assemble lithium metal batteries with Li as the reference and NiCoON/NF, NiCoO/NF or NF as the working electrode, where the water and oxygen contents were required to be less than 0.5 ppm. 1 M LiTFSI dissolved in 1,3-dioxolane/1,2-dimethoxyethane (v:v = 1:1) with 2 wt.% LiNO3 and polypropylene (Celgard 2400) were selected as the electrolyte and separator, respectively. Before testing, all assembled batteries were activated for five cycles under a voltage window of 0.01-1.0 V to remove the contaminants at 50 μA and stabilize the SEI. To investigate the CE, a certain amount of lithium (1 mAh cm-2) was plated onto different working electrodes, followed by charging up to
For the performance test of the full battery, NiCoON/NF, NiCoO/NF and NF were used as substrates for pre-lithiation to obtain Li@NiCoON/NF, Li@NiCoO/NF and Li@NF as anodes, respectively. Commercial LiFePO4 was chosen as the stable cathode material. LiFePO4 powder, Super P and polyvinylidene difluoride at a mass ratio of 80:10:10 were mixed in a N-methyl-2-pyrolidone solvent, followed by continuous stirring overnight. The homogeneous slurry was coated onto clean aluminum foil and the solvent was removed in a vacuum environment at 80 °C for 12 h with an area loading of 3.5 mg cm-2. The corresponding full cells were charged and discharged between 2.4 and 4.2 V.
RESULTS AND DISCUSSION
Preparation and structure of NiCoON/NF
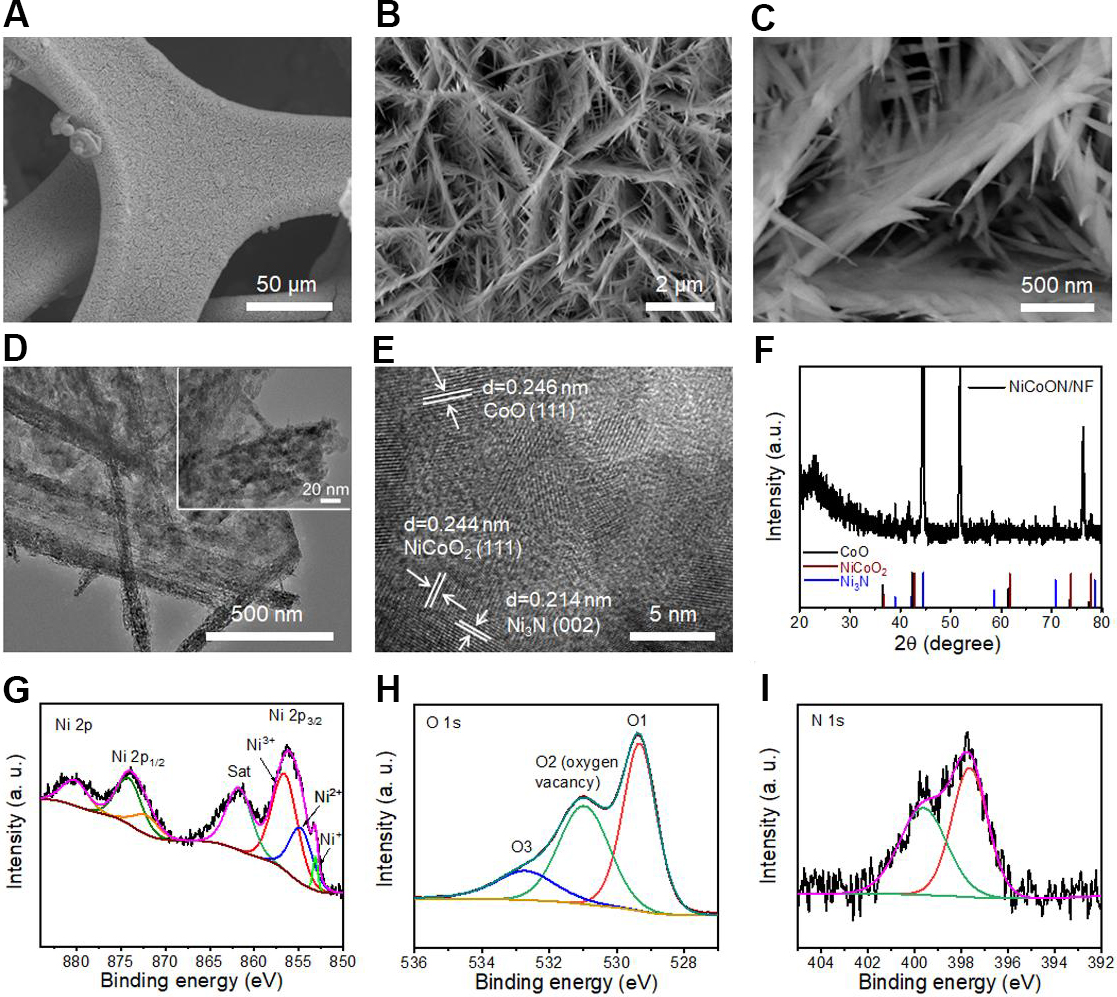
NiCoON/NF was obtained by a typical hydrothermal method and subsequent ammoniation [Figure 1]. The Ni-Co precursor was first in situ grown on Ni foam though a hydrothermal process. After the low-temperature ammoniating treatment, the hydroxide was converted into a NiCoO2/CoO/Ni3N nanocomposite (abbreviated as NiCoON). As shown in Supplementary Figure 1, the bare Ni foam shows a smooth and flat surface with an open porous structure. The XRD pattern in Supplementary Figure 2 displays characteristic peaks. An interesting architecture with nanorod-on-nanosheet arrays was uniformly deposited onto a 3D Ni skeleton [Supplementary Figure 3]. After ammoniation, the morphologies of the Ni-Co precursor were well inherited. As shown in Figure 2A, the NiCoON/NF exhibits a rough surface. The high-resolution scanning electron microscopy (SEM) images in Figure 2B and C show a well-maintained nanorod-on-nanosheet structure with a unique hyperbranched architecture, which can afford sufficient sites for lithium nucleation and a large electroactive surface area for Li plating/stripping. The corresponding EDS elemental mapping of NiCoON/NF confirms the homogeneous distribution of elemental Ni, Co, O and N throughout the nanoarray matrix, indicating the successful implantation of nitrogen species [Supplementary Figure 4].
Figure 2. (A-C) SEM images of NiCoON/NF host. (D) Low-magnification and (E) high-resolution TEM images of NiCoON/NF. (F) XRD pattern of NiCoON/NF. (G) Ni 2p, (H) O 1s and (I) N 1s XPS spectra of NiCoON/NF. SEM: Scanning electron microscopy; TEM: transmission electron microscopy; XRD: X-ray diffraction; XPS: X-ray photoelectron spectroscopy.
The TEM image in Figure 2D further reveals a typical piece of the nanorod-on-nanosheet structure. From the HRTEM [Figure 2E], three well-resolved lattice intervals of 0.244, 0.214 and 0.246 nm can be ascribed to the (111) plane of rock-salt NiCoO2, the (002) plane of hexagonal Ni3N and the (111) plane of cubic CoO, respectively, proving the co-existence of NiCoO2, CoO and Ni3N. The formation of the NiCoON nanocomposite was further confirmed by the XRD patterns in Figure 2F. The typical diffraction peaks of NiCoO2 (JCPDS 10-0188), CoO (JCPDS 48-1719) and Ni3N (JCPDS 10-0280) are also consistent with the TEM findings. For comparison, the SEM images of NiCoO/NF prepared by calcination under air at 350 °C reveal a similar hierarchical nanostructure and uniform elemental distribution [Supplementary Figure 5]. However, no nitride peak in the XRD pattern of NiCoO/NF is found, corresponding with the TEM findings [Supplementary Figure 6]. In addition, the measured BET surface area of the NiCoON nanoarrays is
XPS was performed to determine the composition and chemical states of the as-synthesized NiCoON/NF. The corresponding full-scan spectra contain elemental Ni, Co, O and N, consistent with the EDS results
Electrochemical performance of NiCoON/NF
To better evaluate the electrochemical features of NiCoON/NF, the CE was tested via the assembly of half cells using Li as the reference electrode. As depicted in Figure 3A, NiCoON/NF shows the lowest nucleation overpotential of only 22 mV, much lower than that of NiCoO/NF (32 mV) and bare NF (42 mV) at a current density of 1 mA cm-2. This clearly evidences that the strong lithiophilicity of NiCoON/NF is favorable for guiding the uniform Li nucleation. Figure 3B illustrates the CE comparison among the three samples. The NiCoON/NF host exhibits an inferior value of ~93.7% in the first cycle, which may be due to the conversion reaction between Li and NiCoON. This gradually rises to > 98% upon subsequent cycles and maintains stable cycling for 600 cycles. Compared with the published studies in Supplementary Table 1, such a CE is one of the best for 3D hierarchical hosts. In contrast, the CE of NiCoO/NF suffers from a sharp decline after 300 cycles, while bare NF shows a poor CE with huge fluctuations after 100 cycles due to the large consumption of electrolyte and lithium metal.
Figure 3. (A) Voltage profiles of nucleation overpotentials on different electrodes at 1 mA cm-2. Comparison of CEs on NF, NiCoO/NF and NiCoON/NF current collectors with a fixed capacity of 1 mAh cm-2 at (B) 1 mA cm-2, (C) 2 mA cm-2 and (F) 5 mA cm-2 and (G) CE at 2 mA cm-2 with 2 mAh cm-2. (D, E) Corresponding discharge-charge curves on NiCoON/NF and NF at 2 mA cm-2 with 1 mAh cm-2. (H) Electrochemical impedance spectroscopy (EIS) after 1st cycle at 1 mA cm-2. CE: Coulombic efficiency.
When the applied current density is increased to 2 mA cm-2, the NiCoON/NF electrode still shows stable values of > 98% after 400 cycles, while the NiCoO/NF and NF are still in a state of poor CE [Figure 3C]. In addition, the corresponding discharge-charge curves of the NiCoON/NF host fit well with the CE and show a low nucleation overpotential in the first cycle. The voltage hysteresis is smaller than that of bare NF in the subsequent cycles, indicating the excellent stability of NiCoON/NF [Figure 3D and E]. Furthermore, even at a higher current density of 5 mA cm-2, the NiCoON/NF still exhibits an excellent CE, while the NiCoO/NF and bare NF present severe fluctuations [Figure 3F]. Such excellent cycling stability of the NiCoON/NF electrode is mainly attributed to the formation of a stable SEI and the successful suppression of lithium dendrites.
Impressively, when the deposition capacity is substantially increased to 3 mAh cm-2, NiCoON/NF achieves a stable CE at 1 mA cm-2 for 160 cycles [Supplementary Figure 10]. Similarly, even at 2 mA cm-2 for
Next, galvanostatic cycling tests based on symmetrical cells were carried out to explore the long-term plating/stripping stability at different current densities and capacities. Figure 4A displays the voltage-time profiles of NiCoON/NF, NiCoO/NF and NF at 1 mA cm-2 with a fixed deposition capacity of 1 mAh cm-2. Although both NiCoON/NF and NiCoO/NF present ultrastable cycling, the voltage hysteresis value of NiCoON/NF (23 mV) is lower than that of NiCoO/NF (26 mV), running over 1000 h without obvious potential oscillation. As a control, bare NF undergoes larger voltage hysteresis (30 mV) and irregular potential fluctuation appears only for 400 h due to the emergence of Li dendrites. At a current density of
Lithium deposition behavior
The Li metal deposition morphology on NF, NiCoO/NF and NiCoON/NF is also systematically evaluated via ex-situ SEM. As shown in Figure 5A, after depositing 1 mAh cm-2 of Li onto the NF electrode, Li tends to sparsely and locally nucleate in the early stages. As the amount of Li deposition increases from 1 to 5 and 15 mAh cm-2, visible protuberances and cotton-like lithium dendrites accumulate along the NF skeleton
Figure 5. Top-view SEM images of Li deposition with 1, 5 and 15 mAh cm-2 at 1 mA cm-2: (A-C) NF; (E-G) NiCoO/NF; (I-K) NiCoON/NF. Cross-sectional SEM images of Li deposition with 15 mAh cm-2: (D) NF; (H) NiCoO/NF; (L) NiCoON/NF. SEM: Scanning electron microscopy.
Compared to the bare NF, Li is preferentially plated on the lithiophilic surface of NiCoO/NF as a result of the efficacious surface area of dense nanorod-on-nanosheet arrays and more Li nucleation sites. With increasing Li from 1 to 5 and 15 mAh cm-2, the growth of Li dendrites is largely controlled. However, inhomogeneous Li deposition and a small amount of dendrites are still unavoidable, which may be related to the low conductivity of the metal oxide [Figure 5E-G]. The cross-sectional SEM image in Figure 5H is consistent with this discussion. Li can enter the skeleton of NiCoO/NF, while there are still large chunks of Li deposits. Notably, the NiCoON/NF exhibits no visible lithium dendrites under various capacities of 1, 5 and 15 mAh cm-2 [Figure 5I-K]. After depositing Li at 1 mAh cm-2, Li uniformly enters the dense skeleton of NiCoON/NF, owing to the sufficient lithiophilic nucleation sites. Under deep plating conditions from 5 to 15 mAh cm-2, the wide internal space and ample pores of NiCoON/NF are gradually filled due to the cooperative action of enhanced surface lithophilicity and electrical conductivity, resulting in good Li nucleation and deposition. In particular, Li is uniformly deposited inside the 3D porous framework with the original thickness and no obvious lithium dendrites are found [Figure 5L]. These morphological results indicate that NiCoON/NF, as an ideal host, can provide enhanced surface lithiophilicity for inducing uniform nucleation, thereby promoting the formation of dense lithium deposition.
To clarify the underlying mechanism, XRD analysis of Li@NiCoON/NF obtained after depositing
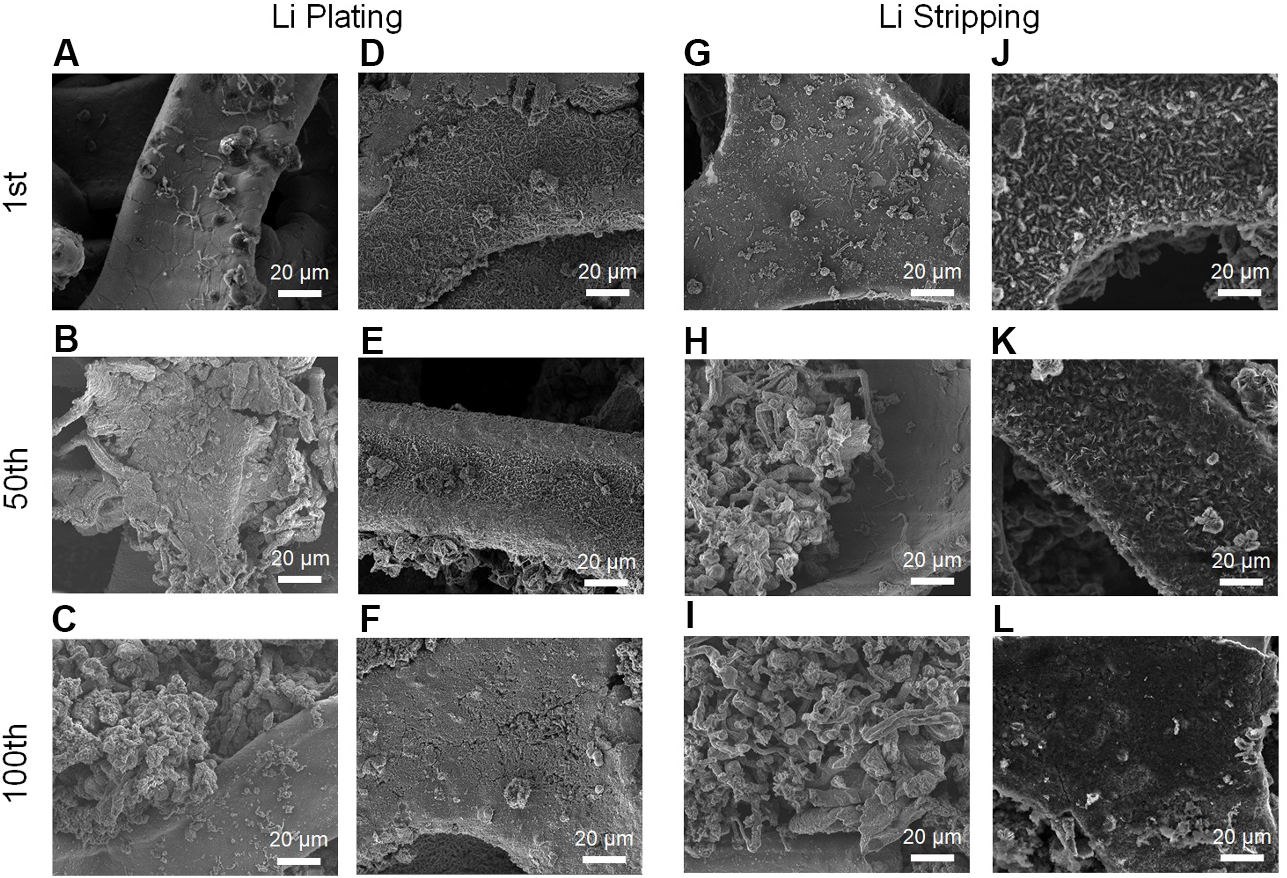
The morphological changes are also evaluated to realize the Li plating/stripping behavior on the NiCoON/NF and bare NF at 1 mA cm-2 under the areal capacity of 1 mAh cm-2. After the first electroplating, the surface of NF covers massive scattered dendritic lithium [Figure 6A], while metallic Li can enter the lithiophilic surface of NiCoON/NF [Figure 6D]. After stripping, moss-like Li remains on the NF surface [Figure 6G], while Li can be uniformly removed from NiCoON/NF [Figure 6J]. During the subsequent 50th plating/stripping, a significant amount of thick and loose Li is deposited on the NF
Electrochemical properties of full cells
To evaluate the practicality of the NiCoON/NF current collector, full cells were assembled and tested by pre-depositing Li onto NF, NiCoO/NF and NiCoON/NF to obtain Li@NF, Li@NiCoO/NF and Li@NiCoON/NF, respectively, as anodes with LiFePO4 as the cathode. Figure 7A illustrates the long-term cycling properties of full devices at 1 C (160 mA g-1). Li@NiCoON/NF-LFP achieves improved cycling behavior with a maximum reversible capacity of 119 mAh g-1 over 400 cycles, corresponding to a superior capacity retention of 85%. In comparison, Li@NiCoO/NF-LFP delivers a relatively reduced discharge capacity of 88.8 mAh g-1 over 400 cycles with a poor capacity retention of 66.8%. However, the Li@NF-LFP experiences faster capacity degradation over 100 cycles with a capacity retention of only 34.9%, which is mainly introduced by inhomogeneous Li plating and fast SEI depletion. According to the corresponding charge/discharge profiles of the three full cells at the 100th cycle, Li@NiCoON/NF-LFP exhibits the lowest voltage polarization, indicating its good ability to inhibit Li dendrites [Figure 7B]. Li@NiCoON/NF-LFP also exhibits a better rate performance and a high discharge capacity, especially at a high current density of
CONCLUSION
In summary, hierarchical Ni- and Co-based oxynitride nanoarrays with superior lithiophilicity have been constructed as stable hosts for uniform lithium deposition. The well-arranged nanoarrays with oxygen vacancies can offer high electronic conductivity and increased surface area to delocalize the practical current density and homogenize the Li-ion flux. Importantly, the in-situ formed Li3N by the conversion reaction of NiCoON (i.e., NiCoO2/CoO/Ni3N) and Li can enhance the electron/ion conductivity for the kinetic induction of the homogeneous deposition of Li. Consequently, a promising Coulombic efficiency of 98.4% up to 600 cycles at 1 mA cm-2 and an ultralong cycling life of 2000 h with a reduced voltage hysteresis under a high deposition capacity of 3 mAh cm-2 have been achieved. Furthermore, Li@NiCoON/NF-LFP full cells also exhibit extraordinary electrochemical performance. This work illustrates the potential advantages of Ni- and Co-based oxynitrides in the preparation of stable and dendrite-free lithium anodes to achieve safe and high-energy batteries.
DECLARATIONS
AcknowledgmentsThe authors thank M. Braun Intergas Systems Co., Ltd., Munich, Germany and Nanjing Mojiesi Energy Tech. Co., Ltd. for providing glove boxes and electrolyte, respectively.
Authors’ contributionsMade substantial contributions to conception and design of the study and performed data analysis and interpretation: Wang Y, Zhu J, Duan X
Performed data acquisition, as well as provided administrative, technical, and material support: Xu H, Zhong J, Wang T, Lu B
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was financially supported by National Natural Science Foundation of China (No. 52074113; No. 22005091; No. 22005092), the Fundamental Research Funds of the Central Universities (No. 531107051048). We also acknowledge the support from the Hunan Key Laboratory of Two-Dimensional Materials (No. 2018TP1010), the Innovative Research Groups of Hunan Province (Grant 2020JJ1001), the Natural Science Foundation of Hunan Province, China (No. 2021JJ40046).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
Supplementary Materials
REFERENCES
1. Zhang Y, Ang EH, Yang Y, Ye M, Du W, Li CC. Interlayer chemistry of layered electrode materials in energy storage devices. Adv Funct Mater 2021;31:2007358.
2. He W, Liu P, Qu B, et al. Uniform Na+ doping-induced defects in Li- and Mn-rich cathodes for high-performance lithium-ion batteries. Adv Sci (Weinh) 2019;6:1802114.
3. Yang Y, Zhu H, Xiao J, et al. Achieving ultrahigh-rate and high-safety Li+ storage based on interconnected tunnel structure in micro-size niobium tungsten oxides. Adv Mater 2020;32:e1905295.
4. Ou X, Zhang G, Zhang S, Tong X, Tang Y. Simultaneously pre-alloying and artificial solid electrolyte interface towards highly stable aluminum anode for high-performance Li hybrid capacitor. Energy Storage Mater 2020;28:357-63.
5. Wu C, Huang H, Lu W, et al. Mg doped Li-LiB alloy with in situ formed lithiophilic LiB skeleton for lithium metal batteries. Adv Sci (Weinh) 2020;7:1902643.
6. Guo J, Zhao S, Yang H, Zhang F, Liu J. Electron regulation enabled selective lithium deposition for stable anodes of lithium-metal batteries. J Mater Chem A 2019;7:2184-91.
7. Kang H, Song M, Yang M, Lee J. Lithium metal anode with lithium borate layer for enhanced cycling stability of lithium metal batteries. J Power Sources 2021;485:229286.
8. Liu S, Wang X, Xie D, et al. Recent development in lithium metal anodes of liquid-state rechargeable batteries. J Alloys Compd 2018;730:135-49.
9. Shi Y, Wan J, Liu GX, et al. Interfacial evolution of lithium dendrites and their solid electrolyte interphase shells of quasi-solid-state lithium-metal batteries. Angew Chem Int Ed Engl 2020;59:18120-5.
10. Tikekar MD, Choudhury S, Tu Z, Archer LA. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat Energy 2016:1.
11. Hou G, Ci C, Salpekar D, et al. Stable lithium metal anode enabled by an artificial multi-phase composite protective film. J Power Sources 2020;448:227547.
12. Wang G, Xiong X, Xie D, et al. Suppressing dendrite growth by a functional electrolyte additive for robust Li metal anodes. Energy Storage Mater 2019;23:701-6.
13. Chu F, Hu J, Wu C, et al. Metal-organic frameworks as electrolyte additives to enable ultrastable plating/stripping of Li anode with dendrite inhibition. ACS Appl Mater Interfaces 2019;11:3869-79.
14. Liang J, Chen Q, Liao X, et al. A nano-shield design for separators to resist dendrite formation in lithium-metal batteries. Angew Chem Int Ed Engl 2020;59:6561-6.
15. Ren W, Zheng Y, Cui Z, Tao Y, Li B, Wang W. Recent progress of functional separators in dendrite inhibition for lithium metal batteries. Energy Storage Mater 2021;35:157-68.
16. Hatzell KB, Chen XC, Cobb CL, et al. Challenges in lithium metal anodes for solid-state batteries. ACS Energy Lett 2020;5:922-34.
17. Krauskopf T, Richter FH, Zeier WG, Janek J. Physicochemical concepts of the lithium metal anode in solid-state batteries. Chem Rev 2020;120:7745-94.
18. Dong T, Zhang J, Xu G, et al. A multifunctional polymer electrolyte enables ultra-long cycle-life in a high-voltage lithium metal battery. Energy Environ Sci 2018;11:1197-203.
19. Lin Z, Guo X, Wang Z, et al. A wide-temperature superior ionic conductive polymer electrolyte for lithium metal battery. Nano Energy 2020;73:104786.
20. Zhang R, Li NW, Cheng XB, Yin YX, Zhang Q, Guo YG. Advanced micro/nanostructures for lithium metal anodes. Adv Sci (Weinh) 2017;4:1600445.
21. Park S, Jin HJ, Yun YS. Advances in the design of 3D-structured electrode materials for lithium-metal anodes. Adv Mater 2020;32:e2002193.
22. Li K, Hu Z, Ma J, Chen S, Mu D, Zhang J. A 3D and stable lithium anode for high-performance lithium-iodine batteries. Adv Mater 2019;31:e1902399.
23. Zhang J, Zhou Y, Tu F, et al. In situ constructing lithiophilic and Ion/Electron dual-regulated current collector for highly stable lithium metal batteries. Chem Eng J 2022;428:132510.
24. Lu W, Wu C, Wei W, Ma J, Chen L, Chen Y. Lithiophilic NiO hexagonal plates decorated Ni collector guiding uniform lithium plating for stable lithium metal anode. J Mater Chem A 2019;7:24262-70.
25. Wang Z, Cheng D, He H, Zhou K. Hierarchical nanosheet arrays of metal oxides guide uniform deposition for lithium anodes. ACS Sustainable Chem Eng 2020;8:102-10.
26. Huang X, Feng X, Zhang B, et al. Lithiated NiCo2O4 nanorods anchored on 3D nickel foam enable homogeneous li plating/stripping for high-power dendrite-free lithium metal anode. ACS Appl Mater Interfaces 2019;11:31824-31.
27. Chang X, Zhou X, Ou X, Lee C, Zhou J, Tang Y. Ultrahigh nitrogen doping of carbon nanosheets for high capacity and long cycling potassium ion storage. Adv Energy Mater 2019;9:1902672.
28. Park H, Kwon J, Song T, Paik U. Lithiophilic surface treatment of metal- and metallic compound-based frameworks by gas nitriding for lithium metal batteries. J Power Sources 2020;477:228776.
29. Luan J, Zhang Q, Yuan H, et al. Plasma-strengthened lithiophilicity of copper oxide nanosheet-decorated Cu foil for stable lithium metal anode. Adv Sci (Weinh) 2019;6:1901433.
30. Zhu J, Chen J, Luo Y, et al. Lithiophilic metallic nitrides modified nickel foam by plasma for stable lithium metal anode. Energy Storage Mater 2019;23:539-46.
31. Lei M, You Z, Ren L, Liu X, Wang J. Construction of copper oxynitride nanoarrays with enhanced lithiophilicity toward stable lithium metal anodes. J Power Sources 2020;463:228191.
32. Lei M, Wang JG, Ren L, et al. Highly lithiophilic cobalt nitride nanobrush as a stable host for high-performance lithium metal anodes. ACS Appl Mater Interfaces 2019;11:30992-8.
33. Li Y, Hu L, Zheng W, et al. Ni/Co-based nanosheet arrays for efficient oxygen evolution reaction. Nano Energy 2018;52:360-8.
34. Zhao D, Dai M, Liu H, et al. Sulfur-induced interface engineering of hybrid NiCo2O4@NiMo2S4 structure for overall water splitting and flexible hybrid energy storage. Adv Mater Interfaces 2019;6:1901308.
35. Wang H, Hsu Y, Chen R, Chan T, Chen HM, Liu B. Ni3+-induced formation of active NiOOH on the spinel Ni-Co oxide surface for efficient oxygen evolution reaction. Adv Energy Mater 2015;5:1500091.
36. Xu K, Chen P, Li X, et al. Metallic nickel nitride nanosheets realizing enhanced electrochemical water oxidation. J Am Chem Soc 2015;137:4119-25.
37. Bao J, Zhang X, Fan B, et al. Ultrathin spinel-structured nanosheets rich in oxygen deficiencies for enhanced electrocatalytic water oxidation. Angew Chem 2015;127:7507-12.
38. Cai Z, Bi Y, Hu E, et al. Single-crystalline ultrathin Co3O4 nanosheets with massive vacancy defects for enhanced electrocatalysis. Adv Energy Mater 2018;8:1701694.
39. Xu L, Jiang Q, Xiao Z, et al. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew Chem Int Ed Engl 2016;55:5277-81.
40. Park K, Goodenough JB. Dendrite-suppressed lithium plating from a liquid electrolyte via wetting of Li3N. Adv Energy Mater 2017;7:1700732.
41. Wang Y, Zhang W, Qi Y, et al. Uniform titanium nitride decorated Cu foams by electrophoretic deposition for stable lithium metal anodes. J Alloys Compd 2021;874:159916.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Wang Y, Xu H, Zhong J, Wang T, Lu B, Zhu J, Duan X. Hierarchical Ni- and Co-based oxynitride nanoarrays with superior lithiophilicity for high-performance lithium metal anodes. Energy Mater 2021;1:100012. http://dx.doi.org/10.20517/energymater.2021.18
AMA Style
Wang Y, Xu H, Zhong J, Wang T, Lu B, Zhu J, Duan X. Hierarchical Ni- and Co-based oxynitride nanoarrays with superior lithiophilicity for high-performance lithium metal anodes. Energy Materials. 2021; 1(2): 100012. http://dx.doi.org/10.20517/energymater.2021.18
Chicago/Turabian Style
Wang, Yaya, Hanjiao Xu, Jiang Zhong, Tao Wang, Bingan Lu, Jian Zhu, Xidong Duan. 2021. "Hierarchical Ni- and Co-based oxynitride nanoarrays with superior lithiophilicity for high-performance lithium metal anodes" Energy Materials. 1, no.2: 100012. http://dx.doi.org/10.20517/energymater.2021.18
ACS Style
Wang, Y.; Xu H.; Zhong J.; Wang T.; Lu B.; Zhu J.; Duan X. Hierarchical Ni- and Co-based oxynitride nanoarrays with superior lithiophilicity for high-performance lithium metal anodes. Energy Mater. 2021, 1, 100012. http://dx.doi.org/10.20517/energymater.2021.18
About This Article
Copyright
Data & Comments
Data
 Cite This Article 9 clicks
Cite This Article 9 clicks


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.