Design of Zn anode protection materials for mild aqueous Zn-ion batteries
Abstract
Rechargeable aqueous Zn-ion batteries (AZIBs) are considered alternative stationary storage systems for large-scale applications due to their high safety, low cost, and high power density. However, Zn anode issues including dendrite formation and side reactions greatly hinder the practical application of AZIBs. To solve the Zn anode issues, various strategies based on material designs have been developed. It is necessary to analyze and classify these strategies according to different materials, because different properties of materials determine the underlying mechanisms. In this review, we briefly introduce the fundamental issues in Zn anodes. Furthermore, this review highlights the material designs for the protection of Zn anodes in mild AZIBs. Finally, we also offer insight into potential directions in the material designs to promote the development of AZIBs in the future.
Keywords
INTRODUCTION
Rechargeable aqueous Zn-ion batteries (AZIBs) are regarded as a promising candidate for next-generation energy storage systems due to their remarkable advantages[1-6]. Common AZIBs are composed of Zn2+ storage cathodes, Zn metal anodes, and aqueous electrolytes containing Zn2+ salt. Among them, the typical cathode materials can be mainly divided into manganese-based oxides, vanadium-based oxides, Prussian blue analogs, and organic compounds[7-15]. Mild AZIBs usually refer to the pH range of their electrolytes of ca. 4-6[16]. There are several advantages of AZIBs. For instance, aqueous batteries are safe, cheap, and can be assembled in air. Moreover, higher ion conductivity can be obtained in aqueous electrolytes than the case in organic electrolytes[17]. As a result, aqueous batteries often possess high power density[18]. Besides, compared with alkaline metals and Ca/Mg/Al, Zn has suitable equilibrium electrode potential [-0.76 V vs. standard hydrogen electrode (SHE)] and high hydrogen evolution reaction (HER) overpotential in aqueous electrolytes. As a result, Zn metal can be directly used as the anode in aqueous electrolytes. Furthermore, Zn anodes with two-electron transfer characteristics possess high theoretical capacity (5855 mAh cm-3 and 820 mAh g-1), leading to high energy density. These advantages make AZIBs have great prospects in the field of large-scale energy storage systems[19-20].
The electrochemical performance of AZIBs mainly depends on the design of cathodes and anodes. Normally, Zn metal can directly serve as the anodes in AZIBs. However, Zn anodes suffer from two main issues: dendrite growth and side reactions[21-23]. Similar to the Li dendrites in lithium-ion batteries[24-25], the unlimited growth of Zn dendrites on Zn anodes is also a fatal hazard for AZIBs due to the nonuniform Zn nucleation and deposition. The dendrites may pierce the separator and eventually cause the batteries to fail. In addition, the side reactions such as HER, corrosion, and passivation, driven by the contact between Zn anodes and aqueous electrolytes, are also regarded as big threats to AZIBs. The side reactions not only greatly increase the polarization but also reduce the Coulombic efficiency (CE) of the cell. The two issues of Zn anodes exist simultaneously and promote each other, which seriously affects the reversibility of Zn chemistry and the electrochemical performance of AZIBs.
Therefore, some strategies have been developed to stabilize the Zn anodes[26-29], including surface modification, structural design, and electrolyte regulation, as presented in Figure 1. These strategies effectively suppress the Zn dendrite growth and/or side reactions, thus being beneficial for enhancing the electrochemical performance of AZIBs. However, a comprehensive review of strategies focusing on material designs for stabilizing the Zn anodes is still absent. In this review, we describe the origins and hazards of dendrite formation and side reactions of Zn anodes in mild AZIBs. Subsequently, we focus on the material design strategies for the protection of Zn anodes and the high performance of AZIBs. The characteristics and functions of these materials on Zn anodes are discussed in detail. Finally, the challenges and further prospects of material designs for Zn anodes are put forward.
ZN ANODE ISSUES
In general, Zn metal is directly deployed as the anodes of AZIBs. The AZIBs with Zn metal anodes have great potential for large-scale energy storage due to their safety and low cost. However, their practical performances are still not as good as expected, which are primarily impeded by the anode-electrolyte interface issues. These issues mainly include dendrite formation and side reactions on the surface of Zn anodes and are analyzed as follows.
Dendrite formation
In the mild aqueous electrolyte, the reaction mechanism of a Zn anode can be summarized as follows:
Zn2 + (aq) + 2e- ↔ Zn(s) (1)
During electrodeposition, Zn2+ typically undergoes four processes: adsorption, diffusion, nucleation, and growth. These processes are susceptible to the surface microenvironment of Zn anodes. Specifically, the surface of Zn anodes is not atomically smooth, which could result in uneven electric field distribution, heterogeneous ion flux distribution, and different nucleation barrier sites on Zn anodes. As a result, Zn2+ is more likely to adsorb and aggregate on the higher active sites under the unrestricted 2D Zn2+ diffusion [Figure 2A][30]. Then, the Zn2+ would nucleate on these sites to form Zn atomic clusters. The formed Zn atomic clusters disperse heterogeneously on the surface of Zn, which would further exacerbate the uneven field distribution in turn. These clusters can also serve as tiny protrusions with larger curvature and induce Zn dendrite growth due to the tip effect [Figure 2B and C][31]. The growing Zn dendrites would bring several hazards. Owing to the loose and porous 3D structure of Zn dendrites, more of the fresh Zn could contact with aqueous electrolytes, leading to more reaction sites for side reactions. In addition, the dendrites are prone to breaking away from Zn substrate and then becoming “dead Zn” due to the bad connection between the dendrites and anodes. The inactive “dead Zn” with the insulating byproduct layer increases the internal resistance and polarization of the battery. In addition to “dead Zn”, some dendrites could continually grow along with the separator until they pierce it and thus cause a short circuit.
Figure 2. (A) Schematic diagram of Zn atomic clusters formation under unrestricted 2D Zn2+ diffusion[30]. (B) Schematic diagram illustrating the tip effect. (C) The formation of dendrites[31]. (D) Pourbaix diagram of the Zn/H2O system containing 10-4 M Zn2+[32]. (E) The gas evolution of Zn symmetric cell after resting different times in 3 M ZnSO4 electrolyte. (F) The surface morphology of Zn anode after immersing in 3 M ZnSO4 electrolyte for 30 days[33]. Reproduced from Ref.[30], Ref.[31], and Refs.[32-33] with permission from Wiley-VCH, Springer Nature, and Elsevier, respectively.
Side reactions
In addition to the dendrite growth, other detrimental problems of Zn anodes are side reactions, including HER, corrosion, and passivation. Among them, HER is the primary problem, which is described as follows:
2H2O + 2e- → 2OH- + H2↑ (2)
Specifically, Zn2+/Zn has a lower equilibrium potential than that of H2O/H2 in the entire pH range, according to the Pourbaix diagram [Figure 2D][32]. Therefore, HER tends to occur on Zn metal anode surface by chemical or electrochemical processes due to the thermodynamic activity of Zn in an aqueous solution [Figure 2E][33]. As a result, an accompanying Zn corrosion process would exist, and HER competition with the Zn plating would occur during the charging process of AZIBs. HER increases the internal pressure of the battery, which could further cause extremely increased polarization, swell, and even burst the battery. In addition, HER also results in an increased pH at the anode surface due to the accumulated OH-. The continuously increased OH- would further react with Zn2+ and anion of Zn salts to form byproducts with limited solubility [Figure 2F][33], such as Zn(OH)2, Zn4SO4(OH)6·xH2O (ZHS), etc. These byproducts are electrical insulators and passivate the Zn surface to block the sites for further Zn plating/stripping. In the meantime, they are loose in the structure; thus, they cannot avoid the further HER and Zn corrosion on the Zn anodes. Therefore, Zn and electrolytes around anodes are constantly consumed, leading to an inferior CE. Moreover, the rough and uneven surface caused by corrosion and passivation could further accelerate the growth of Zn dendrites.
MATERIAL DESIGNS FOR SURFACE MODIFICATION ON ZN METAL
Surface modification on Zn metal is recognized as an attractive and effective strategy to solve the serious issues on Zn anode-electrolyte interfaces, such as dendrite growth and side reactions. Surface modified layers can be obtained through doctor-blade coating, pre-reaction, physical vapor deposition (such as magnetron sputtering, ion beam sputtering, and thermal evaporation), chemical vapor deposition [CVD, such as atomic layer deposition (ALD)], etc. In general, the modified layers have two functions: (1) as a barrier layer to isolate the Zn anodes from the water of aqueous electrolytes; and (2) as a guiding layer to assist the uniform Zn deposition. To realize their functions, the ideal modified layers need to meet the following requirements: (1) high Zn2+ conductivity, facilitating fast ion diffusion and good deposition kinetics; (2) high adhesion to the Zn surface, high mechanical stability, and high dynamic adaptability, ensuring effective protection of Zn under the drama volume changes while Zn plating/stripping; (3) light weight and thin thickness, avoiding the remarkable degradation of energy and power densities of the batteries; and (4) cheap materials and simple preparation process. Many materials have been used as Zn surface modification thus far, which can be classified into carbon-based materials, inorganic compounds, organic compounds, metal-based materials, and composite materials.
Carbon-based materials
Carbon materials with outstanding electrical conductivity have been extensively utilized as modified layers of Zn anodes to inhibit the formation of Zn dendrites in AZIBs. As described above, under an uneven electric field, Zn dendrites are inclined to form on the anodes. The high electrical conductivity and large specific surface area of carbon materials are beneficial to uniform the interfacial electric field distribution and avoid charge accumulation on the Zn anode surface, thus effectively avoiding dendrite proliferation and stabilizing the Zn anodes. Commercially available carbon materials (activated carbon[34], graphite[35], etc.) with convenient manufacturing processes can be directly assembled on the anode surface to protect the Zn anodes. For example, Du et al.[36] mixed carbon fibers, acetylene black, and polyvinylidene fluoride (PVDF) to prepare a slurry, which was then sprayed onto the surface of Zn foil to form a carbon fiber micron film (CFMF) [Figure 3A]. By virtue of its high conductivity and surface area, CFMF could improve Zn electrodeposition kinetics and homogenize the electrical field. The porous scaffold structure of CFMF could also accommodate the Zn anode volume changes during cycling, thus endowing a stable cycling performance of 2500 h at 1 mA cm−2 and 1 mAh cm−2 in the symmetric cells[36]. It is worth noting that such a strategy could be extended to Li metal anodes for inducing the enhanced electrochemical performance[36].
Figure 3. (A) Surface morphology of CFMF coated on Zn anode surface[36]. (B) Current distribution and electric field distribution of separator with VG (3D scaffold), and electric field distribution of pristine separator (2D planar structure)[49]. (C) Schematic diagram of CG separator inducing preferential orientation deposition of Zn. (D) The galvanostatic charge/discharge profile of Zn symmetric cells with CG or cellulose separator[50]. (E) Schematic illustration of the sub-Ångström ion tunnel of HsGDY[55]. (F) The effect of different separators on interface pH change around Zn anode. (G) Schematic diagram illustrating stabilization of interface pH and suppression of Zn dendrites using the CS@NGDY[56]. Reproduced from Ref.[36] and Refs.[49-50,55-56] with permission from Wiley-VCH and Elsevier, respectively.
Compared with commercial carbon materials, carbon nanomaterials have received more attention since they can adjust the Zn deposition by their larger specific surface area and nanoscale size. The common carbon nanomaterials that are utilized in protecting Zn anodes mainly include CNT[37], graphene[38-40], mesoporous hollow carbon spheres[41], and various other carbon nanomaterials[42-44]. In particular, graphene-based materials usually exhibit a small lattice misfit (no larger than 25% as an empirical value) with Zn (002); thus, they can induce reversible lamella-nanostructured Zn (002) epitaxial electrodeposition, which presents a non-dendritic Zn deposition[38,45]. Inspired by this, Zhang et al.[46] designed a cellulose nanowhisker-graphene (CNG) membrane on a Zn anode surface. The CNG membrane induced a redirected Zn (0002) deposition due to the graphene component. Moreover, the CNG could restrain the contact between Zn anode and water molecules through an enhanced [Zn(H2O)6]2+ desolvation process, which alleviated HER and Zn corrosion. Simultaneously, the CNG could shield anions due to the deanionization shock from surface negative charges. Therefore, the inert byproducts were effectively inhibited. Benefitting from the suppressed Zn dendrites and side reactions, the CNG-modified Zn anodes achieved stable cycles for 5500 h at 0.25 mA cm−2 and 0.5 mAh cm−2. In addition to modifying the Zn anode surface, graphene-based materials can also be utilized on separators to stabilize the Zn anodes[47-48]. For instance, a vertical graphene (VG) carpet[49] growing on one side of separators could effectively homogenize the current distribution, smooth the electric field distribution, and lower the local current density [Figure 3B]. Therefore, it can regulate Zn2+ transport behavior and induce a dendrite-free deposition. Furthermore, when mixing graphene-based materials with other materials to serve as separators, regulated (002) Zn deposition behavior can also be achieved. Inspired by this, Cao et al.[50] designed a cellulose/GO (CG) composite separator. Benefitting from the in-plane and dendrite-free Zn deposition [Figure 3C], the CG induced stable cycles for 1750 h at 2 mA cm−2 and 1 mAh cm−2, which was far more than the 35 h achieved by the commercial glass fiber separator [Figure 3D]. In addition to the regulation of the electric field and Zn2+ ions, some functionalized carbon-based materials can offer lots of zincophilic active sites for Zn nucleation and deposition[51]. These sites could capture and redistribute the Zn2+ and result in lower nucleation overpotential, which is also conducive to dendrite-free Zn deposition.
Graphdiyne is also a typical 2D carbon material similar to graphene[52-54]. Graphdiyne can redistribute Zn2+ concentration field through special ion tunnels. For instance, Yang et al.[55] synthesized hydrogen-substituted graphdiyne (HsGDY) as Zn surface protection by in situ growth. They found that HsGDY could promote a homogeneously dispersed Zn2+ around the Zn surface, benefitting from its sub-Ångström level ion tunnels [Figure 3E]. Based on HsGDY, the modified Zn exhibited a long lifetime over 2400 h in symmetric cells and stable 10,000 cycles in the full batteries coupled with a high 22.95 mg cm−2 loading mass of N-doped porous carbon cathode. Furthermore, they also found that a stable Zn-electrolyte interface could be obtained when the graphdiyne was used to modify the separators. To confirm this, an N-modification graphdiyne interface (NGDY) on a cellulose separator (CS@NGDY) was constructed[56]. NGDY could stabilize interface pH by accelerating the desolvation process of hydrated Zn2+ [Figure 3F]. This could be explained by the fact that the N atoms of NGDY would interact with coordinated H2O of hydrated Zn2+ and capture electrons from the coordinated H2O [Figure 3G], which would further reduce activation energy and avoid the weakening of O-H bonds. Therefore, the CS@NGDY suppressed HER and the formation of ZHS, increasing the lifespan of symmetric cells by 116 times at 10 mA cm−2 and 1 mAh cm−2.
Inorganic compounds
Inorganic compounds usually have excellent electrochemically and chemical stability in mild electrolytes. As a result, they can serve as a stable barrier layer to isolate the Zn metal to form bulky electrolytes, which is conducive to mitigating the HER and Zn corrosion. Various inorganic compounds have been reported as Zn anode coating materials so far, such as metal oxides, metal sulfides, metal nitrides, inorganic salts, MXene, etc.
Metal oxides have been broadly utilized to inhibit dendrites and side reactions. Different metal oxides possess different properties; thus, they can affect the Zn anode-electrolyte interface in various ways when they decorate the Zn anode surface. For example, ZrO2[57-58] and Sc2O3[59] have a high dielectric constant. When they are used as coating layers to modify the anode-electrolyte interface, they can induce controllable nucleation sites for Zn2+ as well as fast Zn2+ transport due to the Maxwell-Wagner polarization between the Zn anodes and the layers. In addition, Al-doped ZnO (AZO) prepared by magnetron sputtering possesses strong adsorption energy to Zn2+ due to the doped Al [Figure 4A][60]. Therefore, AZO can provide abundant sites to attract Zn2+, facilitating the desolvation of hydrated Zn2+ and regulating the Zn2+ flux [Figure 4B]. It is noteworthy that a coating with low Zn affinity can also adjust the Zn2+ flux. Because of its relatively low Zn affinity [Figure 4C], the F-TiO2 protective layer with a highly exposed (001) facet can repulse Zn2+ to the Zn-layer interface [Figure 4D], thus leading to increased interfacial Zn2+ concentration and the subsequent uniform nucleation and lateral growth[61]. Moreover, some metal oxides (TiO2 (4.4-5.0 eV), WO3 (4.3-4.8 eV), MoO3 (6.2-6.7 eV), and CeO2 (4.3-4.7 eV)) have a higher work function than Zn (3.6-3.8 eV), which means that the electrons can flow from Zn to the metal oxide to build an Ohmic contact interface when using these metal oxides as Zn anode protective layers [Figure 4E][62]. The Ohmic contact interface would further induce an anti-blocking layer at the interfaced metal oxides [Figure 4E]. The anti-blocking layer can not only improve Zn2+ diffusion but also reduce the Zn2+ nucleation barrier, thus regulating the homogeneous Zn deposition behavior. In addition to the inherent properties, a 3D nanoporous structure of ZnO can also accelerate the desolvation of hydrated Zn2+ and thus relieve the side reactions[63]. However, most metal oxides have good surface wettability. When the metal oxides layers have a large pore structure[64-65], the layers cannot completely inhibit the HER despite their effective suppression for Zn dendrites. Therefore, entire coverage and conformal deposition techniques are needed and should be considered, such as ALD[66-67].
Figure 4. (A) Binding energies of a foreign Zn2+ with the surfaces of bare Zn, ZnO coating, and AZO coating. (B) Schematic illustrating the effect of AZO coating on Zn deposition process[60]. (C) Binding energies between Zn atom and different facets of TiO2 and Zn. (D) Schematic illustration of the Zn plating process on Zn foil (ZF), commercial TiO2-coated ZF (ZF@C-TiO2), and faceted TiO2-coated ZF (ZF@F-TiO2)[61]. (E) Schematic diagram of the formation of an Ohmic contact interface and the anti-blocking layer[62]. (F) The slice of the electron density difference map illustrating the unbalanced charge distribution[68]. (G) Effect of different facets of TiN on the growth pattern of byproduct[71]. (H) Cycling stability of AZIBs with 850 mAh at 0.2 C[73]. (I) Self-assembly process of MXene layer on the Zn anode surface and the regulation of MXene layer on Zn deposition[90]. (J) Schematic illustrating the lattice matching degree between Zn anode and MXene[92]. Reproduced from Refs.[60,62,68,73,90], Ref.[61], and Refs.[71,92] with permission from Wiley-VCH, Springer Nature, and American Chemical Society, respectively.
In addition to metal oxides, other metal compounds have also been designed as protective layers of the Zn surface, such as sulfides (ZnS[68] and MoS2[69]), nitrides (N-Zn[70], TiN[71], and Cu3N[72]), fluorides (ZnF2[73-75] and gradient fluorinated alloy[76]), phosphides (ZnP[77] and ZrP[78]), etc. These metal compounds contain S, N, F, or P atoms that exhibit a good affinity for Zn. Therefore, they can redistribute the Zn2+ flux, drive fast Zn2+ diffusion, and tightly adhere to the Zn anode surface. For instance, S atoms could bond with the Zn atoms at the Zn metal and ZnS interphase, which would induce an unbalanced charge distribution [Figure 4F] and further accelerate the Zn2+ diffusion through ZnS layer[68]. Moreover, a ZnS layer with poor electronic conductivity was in situ obtained on the Zn anodes by a vapor-solid strategy. Therefore, the ZnS layer was dense and robust, which exhibited good suppression for Zn dendrite growth and side reactions[68]. In addition to inhibiting the Zn dendrite, the metal compounds can also regulate the growth pattern of the byproduct through tuning their specific orientations. Taking TiN as an example, the (200) crystal facet of TiN on Zn anode was conducive for parallel ZHS, while the (111) one could lead to the vertical growth of ZHS [Figure 4G][71]. By virtue of uniform Zn deposition and alleviated side reaction, these metal compounds exhibit excellent cycle stability even under harsh conditions. For instance, a ZnP layer was designed on the Zn surface using electrodeposition. The ZnP layer could induce stable symmetric cells with over 100 h cycling at a large depth of discharge (DOD ~ 82%, 48 mAh cm−2) and a high current density of 15 mA cm−2[77]. In addition, a ZnF2 layer formed on the Zn anodes via an in situ ion metathesis method possessed a high Zn2+ transfer number of 0.65[73]. Based on the ZnF2 layer, an 850 mAh large-capacity battery was successfully obtained coupled with the MnO2 cathode. This battery could achieve 160 cycles with 93.17% capacity retention at 0.2 C [Figure 4H][73].
Inorganic salts have also been widely researched as modified materials on Zn anode surface. Some inorganic salts can act as fast Zn2+ conductors to facilitate Zn2+ diffusion at the interface between the anode and electrolyte, such as Zn-Mont[79], Mg-Al layered double hydroxide[80], NaTi2(PO4)3[81], etc. Usually, these inorganic salts possess large interlayer space[82] or voids[81], which can serve as Zn2+ channels or sites to lower Zn2+ migration barrier. Therefore, they can guarantee rapid Zn2+ transfer kinetics and regulate Zn2+ flux. Moreover, these inorganic salts can function as an inert shield to prevent electron tunneling. As a result, they can eliminate Zn dendrites and side reactions simultaneously. In addition, some inorganic salts have similar characteristics as metal oxides[83]. BaTiO3 (BTO), for instance, has a giant dielectric constant. When coated on the Zn surface, the BTO layer would induce an additional directional electric field on the Zn surface under an external field, thus regulating the interfacial electric field and further rendering an ordered and fast Zn2+ migration[84-85]. Furthermore, some metal salts can also offer abundant sites for the Zn2+ adsorption to induce uniform Zn nucleation and deposition, such as O-atom sites in ZnMoO4[86] and metal sites in NiCo layered double hydroxides[87].
As a kind of 2D transition metal carbides/nitrides[88-89], MXene is an attractive candidate for Zn anode protection. Owing to the good electrical conductivity, abundant surface functional groups (-OH, -O, and -F) and hydrophilicity, MXene can induce uniformly distributed electric field and Zn2+ flux when it is constructed on the Zn anode surface. Similar to rGO[40], MXene could be in situ assembled on the Zn surface [Figure 4I][90-91]. The in situ formed MXene layer had a tight combination with the Zn anodes, which was beneficial for fast Zn2+ transport and low Zn nucleation barrier. As a result, the MXene layer could induce a dendrite-free morphology and even a preferentially oriented Zn deposition. In addition, the surficial terminations of MXene have a significant influence on the Zn2+ deposition. Recently, Li et al.[92] studied Mxenes with different halogen functional groups (-Cl2, -Br2, and -I2) as Zn surface modified layers via changing the etchant. They found that halogen surficial termination could tune the Zn2+ distribution to tile the Zn2+ on the MXene substrate. Especially, the Cl termination could better regulate the Zn2+ than O/F, Br, and I due to its moderate Zn2+ adsorption and diffusion coefficient. Moreover, Ti3C2 matrix had high lattice matching (90%) with Zn [Figure 4J] and thus could induce Zn to deposit along the (0001) crystal plane[92]. With the synergetic effect, the modified Zn presented over 9000 cycles at 3 A g−1 in the full battery coupled with the Ti3C2I2 cathode[92].
Organic compounds
Unlike rigid inorganic compounds oxides, organic compounds, especially polymers, usually exhibit better flexibility to accommodate the large volume changes during cycles. More importantly, organic compounds have abundant and changeable functional groups, making their properties easy to regulate. The organic compounds designed for Zn anode modification mainly contain polymers and metal-organic frameworks (MOFs).
Polymers possess abundant polar functional groups and have been extensively studied to protect Zn anodes. The polymers have several functions: (1) serve as an ion regulator to Zn2+ migration through the adsorption or coordination of the functional groups[93], further redistributing the Zn2+ flux field and restricting Zn2+ 2D diffusion; (2) act as an electrostatic shield[94] to avoid Zn2+/electron aggregation and eliminate tip effects, thus suppressing the growth of Zn dendrites; and (3) function as a desolvation shield[95-96] or physical barrier layer[97-99] to reduce water molecules on the Zn surface, further inhibiting the side reactions and HER. It is worth mentioning that some polymers (such as PVDF[100] and its copolymers[101]) are ferroelectric materials and can regulate Zn2+ transport just as BTO discussed above does when they are coated on the surface of Zn anodes. At present, many polymers have been studied as the Zn anode surface modification, including PA[102-103], PAM/PVP[94], polymer glue[97,104], polysiloxane[105], PPy[106], PAN[107], polystyrene[99], g-C3N4[108], COFs[109-111], COPs-CMC[112], PI[113], gelatin[114], etc. Due to the advantages discussed above, polymers can induce improved electrochemical performance of Zn anodes. For instance, Zhao et al.[102] proposed a PA coating layer on Zn anode surface inspired by the brightener. The PA layer extended the lifespan of Zn anodes from 131 to 8000 h at 0.5 mA cm−2 and 0.25 mAh cm−2. This could be attributed to two points. First, PA possessed rich polar amide groups in its molecules. These groups could strongly coordinate with Zn2+ and form a unique H-bonding network. The strong interactions between PA and Zn2+ could alter the Zn2+ distribution and restrict Zn2+ 2D diffusion, which eventually led to uniform Zn deposition. The formed H-bonding network could constrain the water molecules to form solvated Zn2+, thus decreasing the water content on the Zn surface[102]. Second, the PA layer could directly block the water/oxygen to form bulky electrolytes to suppress the detrimental side reactions.
MOFs have also attracted great attention in recent investigations as surface modification materials for their adjustable pores and non-conduction. The porous structure of MOFs can significantly regulate the Zn2+ transport dynamics around the surface of Zn anodes. For instance, Liu et al.[115] explored the possibility of UIO-66 MOFs as modified materials on Zn metals. The microporous structure of MOFs facilitated a hydrophilic interface and a nano-level wetting effect with Zn [Figure 5A], which further adjusted the Zn2+ flux on the Zn anodes and induced a non-dendrite Zn deposition. Moreover, MOFs can serve as molecule sieves to block active water molecules from reaching the active sites. Yang et al.[116] developed a ZIF-7 coating to form a super-saturated electrolyte front surface on the Zn anodes [Figure 5B]. They found that the channel structure of the ZIF-7 could repel large-sized solvated Zn2+ complexes and partially desolvate the complex in advance under an electric field. Benefitting from this, a changed solvated structure and concentrated electrolyte were obtained in the channel, which further led to a homogeneous Zn deposition and decreased byproduct formation. In addition to the two merits discussed above, MOFs can also induce an electrokinetic effect [Figure 5C][117], leading to uniform shock electrodeposition. In the channel of ZIF-11, there were abundant zincophilic functional groups adsorbing the Zn2+, which contributed to forming an EDL. Under an applied electric field, electrokinetic effects such as electro-osmosis, electrophoresis, and surface conduction generated by the EDL[118] could guide the ZIF-11 with an even Zn2+ distribution and a uniform Zn deposition. As a result, the ZIF-11@Cu-Zn-based symmetric cells exhibited increased reversibility and could achieve a stable cycle for 1800 h at 0.5 mA cm−2 and 0.25 mAh cm−2[117].
Figure 5. (A) Schematic diagram of Zn anode–electrolyte interface without or with UIO-66 MOFs layer[115]. (B) Schematic illustrating the formation of super-saturated front surface on the Zn anode by MOF layer[116]. (C) Schematic diagram of the electrokinetic effect in the MOF layer[117]. (D) Schematic illustrating the uniform Zn deposition by Au nanoparticle[132]. (E) Schematic illustration of the Zn plating process on bare Zn and Sb layer modified Zn anode. (F) The H adsorption Gibbs free energy of Sb (001) and Zn (001)[137]. (G) Schematic illustrating the dynamic micro-crosslinking of B-O bond. (H) The dynamic adaptability of PDMS/TiO2-x layer on the Zn anode surface[153]. Reproduced from Refs.[115,132], Refs.[116,137,153], and Ref.[117] with permission from American Chemical Society, Wiley-VCH, and Elsevier, respectively.
Researchers have found that the organic compounds applied to the separators presented similar and beneficial functions because of the meaningful zincophilic functional groups and tunable structure. First, directly employing polymers with zincophilic functional groups as separators is an effective method for stable Zn anodes. Nafion possesses -SO3− groups on its side chains, where the -SO3− groups can interact with Zn2+. As a result, the Nafion membrane separators formed by the casting method could function as a cation selective separator to guide planar ZHS SEI and Zn (002) deposition on the anodes[119]. Moreover, a Zn2+-substituted Nafion separator could simultaneously induce a uniform electrical field and Zn2+ concentration field due to the Donnan potential on the interface[120]. In addition to the -SO3− groups in Nafion, the -CN in PAN can also coordinate with Zn2+ and thus homogenize the Zn2+ distribution. The PAN could guide a preferred Zn (101) deposition when it was used as a nanofiber porous separator via the electrospinning method[121]. When introducing the Li2S3 into the PAN solution, the PAN would react with the Li2S3 to form added S-containing functional groups. The added groups would further result in selective Zn2+ transport[122]. Another significant method is to apply the organic compounds to modify the separators. The polymers used on the separator modification include collagen hydrolysate[123], g-C3N4[124], supramolecules[125], etc. These polymers own abundant zincophilic sites; thus, they can further inhibit the Zn2+ accumulation and result in a uniform Zn2+ distribution. In addition to polymers, the MOFs can also modify the separators. Benefitting from special ion tunnels in MOFs, the MOF-decorated separators can regulate uniform Zn2+ flux[126] and regulate the solvation structure of Zn2+[127]. Therefore, the MOF-modified separators can suppress dendrite formation and side reactions.
Metal-based materials
Metal-based materials often possess good Zn affinity and high conductivity. When they are used as Zn anode protective layers, they can not only provide nucleation sites for Zn deposition but also redistribute the electric field and Zn2+ flux on the anode surface[128-130]. Various metal-based materials have been used to modify Zn metal surface thus far. For instance, Ni5Zn21 was designed on the Zn anode surface via electrodeposition[131]. The Ni5Zn21 layer could more strongly bond with Zn atom compared to the bare Zn. Therefore, Zn preferentially nucleated around Ni5Zn21, further inhabiting the rambling dendrites growing on the Zn anodes. When the metal-based materials are nanoparticles and uniformly dispersed on the anode surface, they can also act as nanoscale “tips”. These tips have high curvature; thus, they can enlarge the local electric field to strongly attract Zn2+ and suppress the large Zn dendrite growth. Based on this, Cui et al.[132] constructed nano-Au particles (NA) on Zn anode surface via sputtering. The size of NA was about 100 nm, and these NA could serve as heterogeneous seeds [Figure 5D]. As a result, the NA decorated anodes achieved uniform Zn-flake-arrays deposition and an enhanced lifespan of 2000 h[132]. However, Au is expensive, and the effect of NA on rampant side reactions should be further explored.
Multifunctional metal-based protective layers on Zn anodes have been extensively developed[133]. They can not only regulate the uniform Zn deposition but also restrain the side reactions. In has been studied for its higher equilibrium electrode potential (-0.338 V vs. SHE) than Zn and high hydrogen evolution overpotential[134-135]. Therefore, In can in situ form on Zn surface by cation replacement reaction to inhibit the Zn corrosion and other side reactions[134-136]. In also possesses a higher Zn atoms adsorption energy than Zn substrate to guide Zn deposition preferentially on the In layer[135]. Similarly, an Sb metal layer was synthesized on Zn surface by replacement reaction very recently[137]. The Sb layer could provide abundant zincophilic sites for Zn nucleation and guide uniform Zn2+ deposition [Figure 5E]. Meanwhile, it could also homogenize the electric field distribution to avoid the dendrites’ formation. Moreover, the Sb metal had a higher H adsorption Gibbs free energy (ΔGH*) than Zn [Figure 5F][137], which indicated that the layer on Zn anodes could suppress the HER process. In addition to In and Sb, Cu[33], Ag[138-140], and liquid Ga-In alloy[141-143] are electrochemical-inert. When they are assembled on Zn anode surface, they can also decrease the side reactions due to their good anticorrosion ability. As discussed above, different metals have slightly different properties when they are used as Zn protective layers. For instance, Ag[138-140] and Cu[33,138,144-145] usually have high Zn affinity; thus, the Ag and Cu metal in protective layers can usually transform into a certain amount of alloy with Zn under cycling, which could further guide the uniform Zn deposition. Sn is not likely to form alloy phases with Zn[146-147], but Sn is conducive to inhibiting byproduct growth[148]. To better utilize the properties of different metals, a layer-by-layer anode (Sn/Cu/Zn) was constructed by Huang et al.[148] for long-life AZIBs.
Multimaterial composites
Multimaterial composites, such as organic-inorganic composites and inorganic-carbon composites, usually possess multifunctional and synergistic effects because they can inherit the merits of each ingredient. Therefore, they have been regarded as powerful and promising candidates for Zn anode protection recently. At present, series of organic-inorganic composites have been proposed as protective coatings to protect the Zn anodes, such as PVDF-TiO2[149], Nafion-Zn-X[150], alucone[151], PAN-Si3N4[152], PDMS/TiO2-x[153], Zn3(PO4)2-ZnF2-ZnS with organic outer layer[154], ZnF2/Zn3(PO4)2/CFX[155], etc. These organic-inorganic composites are obtained via mixing two different kinds of matrix to coat the anodes[149] or in situ built on the Zn anode surface by pre-cycling in organic electrolyte[154]. In general, the organic (polymer) matrixes have rich functional groups[150], excellent mechanical flexibility[149,152] and high dynamic adaptability[153]. Therefore, they can serve as an elastic constraint to relieve the side reactions. Additionally, the inorganic ingredients in composites can modulate the ion distribution[156], which would further tune the uniform Zn deposition. For example, the PDMS/TiO2-x[153] layer was designed by coating a slurry composed of PDMS and TiO2-x on the surface of the Zn plate. The PDMS/TiO2-x layer could adapt to the volume change of Zn anodes during cycles due to the dynamic micro-crosslinking of B-O bond in PDMS [Figure 5G and H]. Moreover, TiO2-x could induce rapid Zn2+ transport and uniform Zn deposition. Consequently, the PDMS/TiO2-x-coated anode could achieve stable cycles for 300 h at 10 mA cm−2 and 10 mAh cm−2 in symmetry cells[153].
In addition to the organic-inorganic composites, other composites are also studied as Zn surface protection layers recently, such as Zn4SO4(OH)6·5H2O/Cu2O[157], ZnF2-Cu[158], etc. These composites can simultaneously promote uniform Zn2+ deposition and inhibit side reactions on the Zn surface. Similar to organic-inorganic composites, these composite coatings also inherit the advantages of different materials. For instance, in the S/MXene@ZnS composite layer[159], doped S could facilitate the electrolyte to penetrate into the Zn anode surface. MXene could effectively homogenize electric field distribution and decrease local current density. Meanwhile, the ZnS could inhibit side reactions, promote uniform Zn2+ distribution, and accelerate Zn2+ migration. As a result, S/MXene@ZnS induced stable cycles for 1600 h at 0.5 mA cm−2 and 0.5 mAh cm−2 in symmetric cells[159]. Taking the ZnO/C hybrid layer[160] as another example, the hybrid layer had different advantages, eventually leading to a dendrite-free Zn deposition and suppressed HER: (1) ZnO featured a unique dielectric constant, which could induce controllable Zn2+ nucleation and deposition sites; and (2) carbon materials could help to avoid the charge accumulation and buffer structure change during cycling. Benefitting from their merits, the ZnO/C coated Zn symmetric cells achieved stable cycles over 2000 h at 0.25 mA cm−2 and 0.25 mAh cm−2[160].
MATERIAL DESIGNS OF THE STRUCTURE AND CONSTITUTION OF ZN ANODES
The structure and constitution of anodes play an extremely important role in the localized ion concentration distribution and localized current density[161-162], thus significantly affecting the local Zn plating/stripping process. Different structures and constitutions of anodes can be obtained by etching, rolling, 3D printing, template method, CVD, freeze-drying, melting method, electroplate, etc. In this section, Zn surface structure designs, conductive host designs, and alloy anodes for Zn anode protection are discussed.
Zn surface structure designs
At present, AZIBs primarily use commercial Zn metal as anodes, which has crystal heterogeneity and usually suffers from Zn dendrites with an uneven surface electric field. To regulate Zn deposition, the surface structure designs of Zn anodes have aroused researchers’ interest. Special surface structures can be prepared via treating the Zn metal, which can effectively redistribute the electric field evenly for uniform Zn deposition. For instance, 3D ridge-like structure[163], pitted texture[164], hexagonal-hole patterns[165], porous surface architectures[166], etc. can be formed on the surface of the Zn foil just by a simple chemical polishing. It can be attributed to the selective etching of weak crystallographic planes and grain boundaries of Zn. In addition, the surface texture and surficial atomic structure of Zn anodes play significant roles in solving the dendrite problem and improving electrochemical performance. Usually, Zn (002) crystal planes exhibit non-dendrites, non-byproducts, and weak HER in sharp contrast to the other crystal plane[165]. Therefore, many strategies have been designed to form a preferred (002) crystal plane on the Zn surface, such as an organic acid-etching approach[167], a large rolling deformation process[168-169], and a thermal annealing process[170]. The (002)-textured Zn anode exhibits better electrochemical performance than commercial Zn. For instance, the Zn anodes with preferred (002) orientation formed after accumulative roll bonding (ARB) could achieve hundreds of cycles at 40 mA cm−2 and 4 mAh cm−2 in symmetric cells [Figure 6A and B]. By contrast, the pristine Zn anodes failed after only the initial cycles[169]. Nevertheless, the Zn metal anodes after surface designs cannot avoid the dramatic volume deformation, which might not be conducive to the working conditions of the large DOD and large area current.
Figure 6. (A) Schematic diagram for ARB process and the 2D X-ray diffraction patterns of Zn foils before and after different ARB cycles (2θ = 36.2 degrees). (B) The galvanostatic charge/discharge profile of commercial and textured Zn symmetric cells[169]. (C) Schematic diagram of the fabrication process of 3D Ni-Zn anode[178]. (D) Schematic illustrating stratified Zn deposition from bottom to top[179]. (E) Electric field simulation on the Zn/CC anode surface before and after the deposition of CNT on CC[182]. (F) Schematic illustration of graphene and different functional groups of N and corresponding binding energy with Zn atom[188]. (G) Schematic illustration of Zn plating/stripping process on bulk Zn anode and MGA-Zn anode[191]. (H) Schematic of morphology evolution for lamellar Zn88Al12 (at%) alloy anode[31]. (I) Schematic illustration of the 3D Zn-Mn alloy for suppressed dendrite growth[195]. Reproduced from Refs.[169,178-179,182,188,191] and Refs.[31,195] with permission from Wiley-VCH and Springer Nature.
Conductive host designs
To accommodate large volume changes of the Zn anodes, more researchers have focused on the host designs of the anodes. The hosts for Zn anodes mainly include metal-based, carbon-based, and MXene hosts. In addition to providing enough space for Zn deposition, the 3D hosts often process large specific surface areas to cause sufficient contact area. As a result, the 3D hosts can effectively reduce the local current density and simultaneously provide more sites for Zn deposition, which will further inhibit the growth of dendrites.
3D metal-based materials are regarded as popular Zn anode hosts due to their good structure stability and Zn affinity. Zn metal can directly function as the skeleton by designing its 3D structure[171]. 3D-structure Zn metal anodes can induce spatial selective deposition and thus inhibit the dendrite growth[172]. Moreover, they can also achieve an interface-localized concentrated electrolyte due to the space charge effect[173], which is beneficial to suppress the side reactions. Even so, 3D-structure Zn metal anodes still act as both the active material and collector, which could reduce the utilization of Zn. To increase the Zn utilization, non-Zn metal-based hosts have received more attention, such as 3D Cu[174-176], 3D Ti[177], etc. Taking Ni host as an example, a 3D Ni host with multi-channel lattice structures was prepared using 3D printing and electroless plating techniques recently [Figure 6C][178]. Due to the ability to redistribute localized electric field and its super-hydrophilic property, the 3D Ni-Zn anode achieved low Zn nucleation overpotential and induced the uniform Zn deposition without dendrite growth. However, some metal-based metals would result in a “top growth” mode, which can contribute to dendrite growth. To solve this problem, Shen et al.[179] produced a stratified deposition framework (from bottom to top: Cu foam, Ni foam, and NiO) and realized stratified Zn deposition from bottom to top [Figure 6D]. It can be attributed to the different Zn deposition overpotentials and Zn affinity of metals. In addition to the dendrites, HER and other side reactions can also be suppressed via metal-based hosts. For instance, Jian et al.[180] prepared a nanoporous Sn host (NSH) on a Cu mesh by the replacement reaction. With the high HER overpotential of Sn, NSH could suppress side reactions. Moreover, benefitting from the nanoporous structure, NSH also redistributed the Zn2+ flux and electric field to further uniform the Zn deposition. Nonetheless, due to their large weight, metal-based hosts may reduce the energy density to some extent.
Carbon-based 3D hosts have the advantage of light weight, which is conducive to improving the energy density of AZIBs. The excellent mechanical stability of carbon-based 3D hosts also gives them the ability to be used in flexible and wearable devices. Carbon fiber (CF) and carbon cloth (CC) purchased directly have poor hydrophilia and zincophilia[181], which means that these carbon materials alone are insufficient to function as efficient hosts for Zn anodes. Many activated strategies have been developed within the 3D carbon frameworks to introduce Zn deposition sites and enhance the electrochemical performance of Zn anodes. For example, 3D CNT frames grown on CC using the CVD method enhanced the affinity with Zn and decreased the Zn nucleation overpotential[182]. Moreover, the 3D CNT frames exhibited enlarged specific areas and a more uniform electric field distribution [Figure 6E]. Based on the merits, a higher CE of 97.9% and better cycle stability for 200 h at 2 mA cm−2 of anode were endowed. Other methods are also effective for stable Zn plating/stripping, such as depositing 3D CNT on CC[182], printing Ag nanoparticles on CC[183], and coating graphene on CF[184]. Additionally, introducing defects[185], zincophilic atoms[186], or zincophilic functional groups[187] are also efficient ways to activate the carbon matrix and strengthen interfacial interaction between Zn and the carbon matrix. For instance, Cao et al.[188] introduced 3D nitrogen-doped vertical graphene nanosheets into the CC. They found that the binding energies of Zn2+ absorbed on pyrrolic N (prN) (-0.377) and pyridinic N (pnN) (-0.356 eV) were larger than that of the carbon atomic group (-0.263 eV) [Figure 6F]. Therefore, the doped N could serve as nucleation sites for Zn2+ to reduce the Zn nucleation overpotential and lead to the homogeneous Zn deposition.
Apart from metal and carbon hosts, MXene is also a popular candidate for Zn host. It has been proven that Ti3C2Tx MXene paper host can endow the Zn anodes with a suppressive dendrite growth and fast Zn plating/stripping kinetics because of its conductivity and hydrophilicity[189]. Furthermore, an increased Zn deposition stability can be achieved when introducing zincophilic metals into the system[190]. For instance, Tian et al.[190] designed Sb nanoarrays on Ti3C2Tx MXene paper. The Sb could function as a zincophilic nucleation seed to reduce nucleation overpotential and further regulate homogeneous Zn deposition. Nevertheless, 2D MXene paper is insufficient to accommodate large Zn deposition compared to the 3D structure. To address this issue, Zhou et al.[191] constructed a 3D flexible MXene/graphene aerogel (MGA) scaffold, where the Ti3C2Tx sheets were assembled onto the surface of rGO. Benefiting from the novel structure, MGA could effectively pack the deposited Zn. Moreover, the F terminal in the MXene could induce a ZnF2-rich SEI [Figure 6G], which was effective for uniform Zn deposition as well as inhibited byproduct ZHS formation and HER (3.8 × 10−3 mol h−1 cm−2)[191]. In addition to MXene, some other novel hosts have also aroused research interest recently, such as MOF ZIF-8-500[192] and TiOx/Zn/N-doped carbon inverse opal (TZNC IO) host[193]. These novel frameworks are also efficient for a long lifetime of Zn anodes.
Zn alloy anodes
In addition to modulating the physical structure of Zn anodes, alloy design for Zn anodes is also a significant strategy through tuning the chemical constitution of Zn anodes[194]. For instance, Wang et al.[31] introduced an alternating lamellar Zn88Al12 (at%) alloy anode. They found that the Al layers could act as a 2D host to accommodate the deposited Zn, and the insulating Al2O3 shells generated on the Al layers could prevent the electrons transfer from Al to Zn2+ [Figure 6H]. These characters could guide Zn2+ deposition and finally achieve a dendrite-free behavior. Moreover, the alloy strategy can improve the stability of Zn anodes together with a 3D structure. Taking Zn-Mn alloy as an example, a Zn-Mn alloy with a 3D structure was obtained only using the electrodeposition method. The formed alloy anode could not only control the Zn2+ diffusion kinetics through favorable diffusion channels [Figure 6I] but also regulate Zn nucleation by a higher surface binding energy[195]. As a result, the anode with a 3D Zn-Mn alloy could achieve 1900 stable cycles in the harsh conditions of 80 mA cm−2 and 16 mAh cm−2 in sea water-based aqueous electrolytes[195].
To inhibit HER on Zn anodes, Wang et al.[196] introduced Sn to alloy with Zn. The appropriate Sn amount could effectively suppress the HER due to the enlarged ΔGH*. Meanwhile, Sn could offer favorable sites to decrease the energy barrier for Zn nucleation, which would further regulate the Zn deposition and induce a dendrite-free behavior[196]. In addition to Sn, Cu is a promising element to improve the corrosion resistance of alloy anodes. As is known, Cu possesses an intrinsically inert nature. The Cu-Zn alloy could effectively alleviate the HER and Zn corrosion[197]. Moreover, the zincophilic Cu could also provide abundant Zn2+ adsorption sites to promote homogeneous Zn nucleation and deposition[197]. Benefitting from the suppressed dendrites and side reactions, the Cu-Zn alloy anode achieved stable cycles for over 1600 h at 5 mA cm−2 and 2.5 mAh cm−2 in symmetric cells. It is worth noting that this Cu-Zn alloy anode could also enhance the electrochemical performance of cells in the alkaline system[197].
MATERIAL DESIGNS OF ELECTROLYTES
The composition and concentration of electrolytes affect the anode–electrolyte interface, which is directly related to dendrites, HER, and the formation of byproducts. Zn salts used in AZIBs mainly include
Highly concentrated electrolytes
Increasing the electrolyte concentration is a simple and effective strategy to improve the electrochemical performance of AZIBs. The merits of using concentrated electrolytes can be concluded in two ways. (1) Decreasing the amount of water molecules and breaking the hydrogen-bond networks in the bulk electrolyte: These are beneficial for reducing the side reactions, alleviating the active materials’ dissolution, expanding the electrochemical stability window, and lowering the freezing point to some extent. (2) Changing the solvation structure of Zn2+[215] : this can not only further govern the Zn deposition and suppress Zn dendrite growth, but it is also conducive to restrained side reactions. For instance, Zhang et al.[216] found that the concentration of ZnCl2 in aqueous could reach up to 30 m [where m is molality (mol kg−1)], and the electrolyte would transform into a “water-in-salt” (WIS) electrolyte. Usually, with enough water molecules available, Zn2+ could be solvated to form stable [Zn(H2O)6]2+. However, the number of [Zn(H2O)6]2+ was minor in 30 m ZnCl2 electrolyte because few free water molecules existed in this WIS[216]. Moreover, there were fewer and fewer water molecules involved in the solvation structure of the Zn2+ with the increased ZnCl2 concentration from 5 to 30 m. As a result, the concentration of [ZnCl4]2− would increase and that of [Zn(OH2)2Cl4]2− would decrease in the electrolyte. The average CE of the Zn anode could also increase from 73.2% to 95.4%[216]. In addition to improving the reversibility of Zn plating/stripping, the 30 m ZnCl2 WIS electrolyte also has the ability to mitigate the dissolution problem of active material[217-218] and widen the voltage window[218-219] compared to a dilute one.
Common electrolytes, such as ZnSO4 and Zn(CF3SO3)2 electrolytes, can also achieve improved electrochemical performance after increasing their concentration. For instance, 3 M [where M is molarity (mol L−1)] ZnSO4 electrolyte can not only suppress the dissolution of V2O5 cathode but also enhance the stability of the Zn anodes compared to that in 1 and 2 M electrolytes[220]. Furthermore, with the ZnSO4 concentration increasing from 2 to 4.5 M in the presence of 0.1 M MnSO4 electrolyte additives, strongly aggregated ion pairs would replace the water molecules around Zn2+[199]. Therefore, the concentrated electrolyte of 4.2 M ZnSO4 + 0.1 M MnSO4 obtained an improved Zn plating/stripping CE of 99.21%[199]. A similar phenomenon is evidenced in the Zn(CF3SO3)2 electrolyte, where the CE of full batteries in Zn(CF3SO3)2 electrolyte increases with salt concentration boosting from 1 to 4 M[208]. However, the saturation concentration of most Zn salts is limited at ~4 M[221-222] or even smaller[206], where a lot of free water exists and most Zn2+ ions are surrounded by six water molecules. In view of this, researchers attempted to add other salts in Zn electrolytes to obtain highly concentrated electrolytes. These highly concentrated electrolytes can also decrease the water molecules and alter the solvation structure of Zn2+[223-224]. For instance, a saturated WIS electrolyte was investigated by introducing 20 m LiTFSI into 1 m Zn(TFSI)2[225]. In this novel WIS electrolyte, water molecules could be severely confined within the Li+ solvation structures, and the Zn2+-solvation sheath was occupied primarily by TFSI− to form (Zn-TFSI)+ structure[225]. The changed Zn2+-solvation sheath could improve the reversibility of the Zn anodes (CE ~ 100%) and simultaneously restrain the side reactions. However, it is worth noting that Zhang et al.[226] recently found that Zn2+ was still mainly solvated by six waters in the first solvation shell in 1 m Zn(TFSI)2 + 20 m LiTFSI electrolyte. Therefore, more studies need to be developed in this electrolyte system. In addition to the changed Zn2+ solvation structure, researchers found that an enhanced threshold of critical current density for cation depletion could also result in regulated Zn deposition and suppressed dendrite growth in the highly concentrated electrolyte[227].
Electrolyte additives
In addition to the concentration control, the composition change of an electrolyte via a small amount of electrolyte additives can also affect the stability of Zn anodes. Electrolyte additives can be mainly divided into inorganic and organic additives.
Inorganic additives
Some metal ions have more negative reduction potential than Zn2+, such as Mn2+[199], Na+[228], Li+[229], etc. As a result, these metal ions can competitively adsorb on the Zn protrusions and thus inhibit the dendrite growth according to an electrostatic shield mechanism. For instance, Li+ can adsorb on the Zn anode surface and further establish a new interface between the anode and electrolyte. The new interface can guide non-dendrite Zn growth and inhibit the HER[230]. Additionally, the Li2O/Li2CO3 layer could be preferentially formed on the Zn surface to provide a shielding effect when adding 2 M LiCl into 3 M ZnSO4[229]. It is worth noting that, in addition to metal ions, other inorganic cations (such as NH4+[231-233]) can also adsorb on the Zn protrusions and regulate the Zn deposition process. Furthermore, some metal ions can inhibit the Zn dendrite growth by regulating the Zn nucleation process. For instance, Li et al.[234] found that Ce3+ and La3+ could guide the Zn nucleation through a progressive process instead of an instantaneous one, thereby resulting in stable Zn stripping/plating behavior.
In addition to guiding Zn deposition directly, the introduced inorganic salts can also in situ form an SEI protective layer on the Zn surface to stabilize the anode-electrolyte interface[235]. In LIBs, SEI layers can be in situ built at the anode surface through the decomposition of electrolyte components and/or salt anions[236]. However, in AZIBs, SEI with in situ formation remains a huge challenge. It can be attributed to that the relatively high reduction potential of Zn deposition and restricted voltage windows of water. As a result, HER and Zn plating are usually generated prior to the decomposition of salt anions. To in situ form SEI on Zn anodes, several mechanisms or methods have been developed by adding inorganic salts. One of these is to make use of the locally increased OH− caused by HER. For instance, Zeng et al.[237] found that a dense and uniform Zn3(PO4)2·4H2O SEI could form by adding Zn(H2PO4)2 to Zn(CF3SO3)2 electrolyte. As shown in Figure 7A, through the chemical reaction
Figure 7. (A) Schematic diagram of the in situ formation of Zn3(PO4)2·4H2O SEI on Zn anode surface[237]. (B) Schematic diagram of preferential Sn deposition than Zn in the 7.6 m ZnCl2 aqueous electrolyte with 0.05 m SnCl2 additive. The deposited Sn would further facilitate the formation of a uniform zincophobic Zn5(OH)8Cl2·H2O SEI, and the water activity was decreased due to the strong H2O-Cl- interactions[241]. (C) Schematic illustration of the PEG additive in the electrolyte for uniform Zn deposition. (D) The galvanostatic charge/discharge profile of symmetric cells with 0% or 70% PEG additive[284]. (E) Schematic diagram of PVIPS gel electrolyte for changing the solvated structure of Zn2+ and inducing a Zn (002) texture[311]. (F) The MD simulation snapshot of ZCE at 300 K illustrating the enhanced ion dynamics and the diagram illustrating the Zn2+ hopping-type transport mechanism in the ZCE[329]. Reproduced from Refs.[237,241,311,329], and Ref.[284] with permission from Wiley-VCH and Elsevier.
2H2PO4− + 4OH- + 3Zn2+ → Zn3(PO4)2. 4H2O↓ (3)
the Zn3(PO4)2·4H2O film would precipitate on the anode as SEI with the increased OH− leftover from HER. Similarly, ZnF2-Zn5(CO3)2(OH)6-organic bi-layer SEI was produced by introducing Zn(NO3)2 additive into Zn(CF3SO3)2 aqueous electrolyte[238]. This bi-layer SEI could promote Zn2+ diffusion and block water penetration, thus enabling a highly reversible Zn plating/stripping (CE ~ 99.8%)[238]. In addition to utilizing the increased OH−, another method is to add salts that are thermodynamically instable in aqueous environments. Inspired by this, Chu et al.[239] added KPF6 in 2 M ZnSO4 aqueous electrolyte. They found that a composite SEI (ZCS) could be in situ formed on the Zn anode, and the ZCS was mainly composed of Zn3(PO4)2 and ZnF2. It could be attributed to the decomposition of PF6− and the further reaction between the decomposition products and Zn. Benefitting from the in situ formed ZCS, the largest cumulative capacity on the anode could reach up to 2020 mAh cm−2 and the symmetric cells realized stable cycles for 250 h at 10 mA cm−2 and 20 mAh cm−2. In addition to the above two methods, SEI can also be formed in Zn anodes when introducing some metal ions with higher reduction potential than Zn2+, such as Bi3+, Pd2+[240], Sn2+[241-242], In3+[243], etc. These metals could preferentially deposit on the anodes before Zn and thus can act as SEI layers to guide Zn deposition and suppress the side reactions [Figure 7B].
Organic additives
The other popular and effective candidate of electrolyte additives are organic additives, including alcohols[244-247], ether[248-252], aldehydes[253-254], esters[255-256], amino acid[257], polymers[258-259], organic salts[259-266], etc. The organic additives usually function as trace mineral supplements or co-solvent. They can affect Zn deposition in three ways.
Firstly, they prefer to adsorb on the surface or protuberance of the Zn anodes due to their stronger absorptive function than H2O[254] or because of the tip effect[267]. The adsorbed organic additives can not only occupy the active sites for HER and further alleviate side reactions[263] but also serve as zincophilic sites for Zn nucleation and restricting 2D Zn2+ diffusion[268]. As a result, these organic additives can suppress the continuous dendrite growth and even guide different crystallographic orientations and surface textures of deposited Zn[269]. Secondly, organic additives can inhibit the invasion of free water into the Helmholtz plane[270] or change the Zn2+ solvation structure[271-272]. The changed solvation structure would guide Zn deposition and even interrupt the hydrogen bonding network among water molecules[273] to reduce water activity. The reduced water activity can not only broaden the electrochemical stability window[274] but also lower the freezing point of the electrolyte[275]. These are beneficial to the inhibited side reactions and the further expanded usage of AZIBs. More importantly, an SEI could be in situ formed, driven by the decomposition of organic additives[276] or polymerization. For instance, Zeng et al.[277] found that a stable polydopamine SEI could be constructed via an in situ electrochemical polymerization of dopamine additive. In addition to the polymerization, the introduced organic additives can be decomposed on the anodes to form SEI layers due to the inhibited HER after excluding water in the EDL structure[278]. Benefitting from the three effects on the anodes, some organics have been frequently studied as additives in AZIBs, such as DMSO[279-280] and PEG[281-283]. Wu and co-workers[284] found that the PEG additive had several advantages at the same time. These advantages included enabling Zn (002) deposition preferentially, decreasing the water activity, and forming an anion-dominated solvent structure and ZnF2-rich SEI films [Figure 7C]. Benefitting from these merits, the Zn anode in electrolyte with 70% PEG additive achieved a super-high cyclic performance for 9000 or 8000 h at 1 or 2 mA cm−2 [Figure 7D][284].
Hydrogel and solid-state electrolytes
Employing the hydrogel/quasi-solid electrolytes is also considered a promising solution to Zn anode issues because of their limited water content and decent ionic conductivity. They are generally composed of Zn salts and polymer skeleton networks, including non-crosslinked and crosslinked hydrogel electrolytes[285]. Unlike the crosslinked type, non-crosslinked hydrogel electrolytes tend to exhibit amorphous morphology and poor mechanical properties; thus, they require additional separators in most cases. Based on different polymer skeletons, the hydrogel electrolytes used in AZIBs can mainly be divided into PEO-based[286], PVDF-based[287], PAN-based[288], PVA-based[289-293], PAM-based[294-299], natural biomass-based[300-304], etc. Appropriate Zn salts should be selected to match the polymers because some polymers are incompatible with salts. For example, commonly used polymers (PEO, PVA, agar, gelatin, and sodium polyacrylate) in AZIBs would precipitate in electrolytes containing SO42− according to the Hofmeister series[305]. Similar to polymer electrolyte additives, polymer chains of hydrogel electrolytes can closely adsorb onto the Zn anodes, especially on the bulge. These polymer chains can serve as electrostatic shielding layers to inhibit dendrite growth and prevent water from contacting the anodes[306]. Moreover, polymers can guide Zn2+ transport and deposition through local segmental motion because the Zn2+ would coordinate with oxygen-containing functional groups on the polymer chains[302,307-310]. For instance, Hao et al.[311] found that, in polyzwitterionic PVIPS gel electrolyte, the SO3− groups could bond to Zn2+ and change the Zn2+ solvation structure from [Zn(H2O)6]2+ to R-SO3− [Zn(H2O)4]2+ −SO3-R [Figure 7E]. The changed solvated Zn2+ would further guide the Zn2+ transport and inhibit the side reactions. Moreover, the imidazole groups in PVIPS gel electrolyte were able to synergize with SO3− groups to guide Zn2+ nucleation and deposition along the (002) plane. Most crosslinked hydrogel electrolytes exhibit high mechanical integrity, which is beneficial to suppress dendrite formation[312]. However, the hydrogel electrolytes with high modulus may cause poor interfacial contact with anodes. To solve this, Cao et al.[313] developed a mechanoadaptive morphing gel electrolyte (MorphGE). MorphGE with high modulus effectively inhibited Zn dendrite growth under the well interfacial contact with Zn anodes. Moreover, MorphGE also relieved the side reactions due to its anchoring for desolvated Zn2+. As a result, MorphGE induced long cycles for 2400 h at 1 mA cm−2 and 1 mAh cm−2, and even 100 h at 10 mA cm−2 and 10 mAh cm−2 in symmetrical Zn cells. It is worth noting that the hydrogel electrolytes can expand the application of AZIBs in flexible and wearable energy storage devices[314].
All-solid-state electrolytes can fundamentally eliminate the side reactions driven by water, thus improving the utilization of Zn anodes. However, few studies on all-solid electrolytes have been reported[315]. That is because the divalent Zn2+ has a higher charge density, which induces quite sluggish diffusion kinetics in solid materials at room temperature[316]. Water can activate inorganic electrolytes to some extent. For example, MOF-based Zn2+ solid electrolytes[317] and inorganic colloidal electrolytes[318-319] have been explored to obtain good Zn2+ migration and suppression ability for both dendrites and side reactions. However, their Zn2+ conduction mechanism is actually Zn2+ being transported through water. Recently, an inorganic Zn2+ conductor electrolyte (ZHAP-Zn) with a solid-liquid hybrid Zn2+ transport channel has been developed[320]. The ZHAP-Zn enabled a high Zn2+ transference number of 0.75 and achieved dendrite-free Zn plating/stripping over 2000 h at 0.5 mA cm−2. Nevertheless, the use of all-solid-state electrolytes without water can still be a challenge. In general, polymers with lower glass transition temperatures[321] and all-amorphous regime[322] present higher ionic conductivity and can be used in AZIBs. Additionally, the use of inorganic fillers (such as TiO2[323-324], ZrO2[321], Al2O3[325], MXene[326], etc.) and the bulky anionic salts[327-328] can effectively reduce the crystallinity of the polymer to improve Zn2+ conductivity, but their influence on Zn dendrites is rarely researched. Very recently, a Zn2+ conductive solid electrolyte (ZCE) was prepared and studied for the Zn anode by crystallization of Zn(TFSI)2-based deep eutectic solvent[329]. In ZCEs, TFSI− was preferentially adsorbed on the surface of Lewis acid TiO2, thus weakening ion association and resulting in a high Zn2+ transference number (0.64). Moreover, an interface Zn2+ conduction pathway was established via the adsorbed TFSI− on the surface [Figure 7F], which endowed ZCEs with a high ionic conductivity of 5.91 × 10−5 S cm−1 at 30 °C. Benefitting from the good Zn2+ conductivity, sufficient mechanical strength, and water-free characteristics, the ZCEs induced Zn plating/stripping for 4000 h at 0.01 mA cm−2 without dendrites or side reactions. However, the ionic conductivity of all-solid-state electrolytes is still far below the practical application requirements, and the research for all-solid-state Zn-ion batteries is still in the initial stages.
CONCLUSIONS AND OUTLOOK
AZIBs have attracted extensive attention because of their advantages of low price, high safety, and high power density. However, the anode-electrolyte interface is often accompanied by some harmful problems that hinder the performance of AZIBs. Due to the uneven distribution of electric field and Zn2+ concentration field, dendrite growth often exists on the Zn anodes, causing the battery to short circuit. In addition to Zn dendrites, another big issue on Zn anodes is the side reactions (HER, corrosion, and passivation) driven by the thermodynamic activity of Zn metal in an aqueous solution. The side reactions could reduce Zn utilization and the CE of the Zn plating/stripping on Zn anodes. For this, material designs for the anodes and electrolytes have been developed. For instance, on the Zn anodes, protective layers can physically isolate the anodes and bulky electrolytes, thus alleviating the side reactions. Moreover, layers of modified Zn anodes or separators can also regulate Zn2+ transfer kinetics deposition and induce a dendrite-free deposition behavior. In the electrolyte, trace amounts of electrolyte additives can regulate the solvated structure of Zn2+ to guide uniform Zn deposition and mitigated side reactions. More detailed information about material designs for stable Zn anodes can be seen in Table 1. Although the material design for Zn protection has made many advances, there are still some factors limiting their actual application from the lab to commercialization, and further research efforts are needed.
Summary of different material design strategies for stable Zn anodes
Designs | Materials | Synthesis | Important parameter (electrolyte or salt) | Representative indicators | Reference | Remarksb | |||
| Symmetric cells | Asymmetrical cells | ||||||||
| Lifespan (h) (C1, C2)a | CE | Cycles (C1, C2)a | |||||||
| Surface modification on Zn metal | Carbon-based materials | CG separator | Casting | 2 M ZnSO4 | 1750 (2, 1) 400 (20, 10) | 98.68% | 100 (1, 1) | [50] | ·Simple preparation ·Redistribution of surface electric ·Less effect on large DOD |
| HsGDY | In situ growth | 2 M ZnSO4 | 2400 (0.5/1/2, 0.1) | / | / | [55] | |||
| N-C | Casting | 2 M ZnSO4 | 1000 (1, 1) | 98.76% | 120 (2, 2) | [42] | |||
| CNG | Drying | 3 M ZnSO4 | 2956 (1, 0.5) | 99.4% | 300 (/, 0.5) | [46] | |||
| Inorganic compounds | ZrO2 | Casting | 2 M ZnSO4 | 3800 (0.25, 0.125) 2100 (5, 1) | 99.36% | 230 (20, 5) | [57] | ·Simple preparation ·Physical barrier for free water ·Crack on the large DOD | |
| ZnO | Liquid-phase synthesis | 2 M ZnSO4 + 0.1 M MnSO4 | 500 (5, 1.25) | 99.55% | 300 (2, 0.5) | [63] | |||
| ZnS | CVD | 1 M ZnSO4 | 1100 (2, 2) | 99.2% | 200 (2, 1) | [68] | |||
| ZnF2 | In situ ion metathesis | 2 M ZnSO4 | 2500 (1/2/5, 1) | ~99.5% | 1000 (1, 1) | [73] | |||
| MXene | In situ reducing/assembling | 2 M ZnSO4 | 800 (0.2, 0.2) | / | / | [90] | |||
| ZnP | Electrodeposition | 2 M ZnSO4 | 3200 (5, 1.25) 300 (20, 30) | 99.5% | 200 (2, 0.5) | [77] | |||
| CaCO3 | Casting | 3 M ZnSO4 + 0.1 M MnSO4 | 836 (0.25, 0.05) | / | / | [83] | |||
| BaTiO3 | Coating | 4000 (1, 1) 1300 (10, 2) | / | 120 (1, 1) | [85] | ||||
| Organic compounds | PA | Casting | 2 M ZnSO4 | 8000 (0.5, 0.25) | 95.12% | 300 (0.4, 0.4) | [102] | ·Simple preparation ·Physical barrier for free water ·Certain shape adaptability ·Lower ionic conductivity | |
| FCOF | Pulling | 2 M ZnSO4 | 1700 (5, 1) | 97.2% | 320 (80, 1) | [110] | |||
| Gelatin | Casting and crosslinking | 1 M Zn(OTF)2 | 4000 (1, 1) | / | / | [114] | |||
| MOF | Casting | 2 M ZnSO4 | 3000 (0.5, 0.5) | / | / | [116] | |||
| Metal-based materials | ZnSe | CVD | 2 M ZnSO4 | 1500 (1/10, 1) | / | / | [128] | ·Simple preparation ·Redistribution of surface electric ·Certain solutions for side reactions ·Less effect on large DOD | |
| In | Ion exchange | 2 M ZnSO4 | 1400 (0.25, 0.05) | / | / | [136] | |||
| Cu/Zn | Ion exchange and annealing | 3 M ZnSO4 | 1500 (1, 0.5) | 91.8% | 100 (5, 0.5) | [33] | |||
| Ga-In | Coating | 3 M ZnSO4 | 2100 (0.25, 0.05) 1200 (1, 0.1) | / | / | [141] | |||
| Multimaterial composites | AEC (TiO2-PVDF) | Coating | 2 M ZnSO4 | 2000 (0.885, 0.885) | 99.4% | 1000 (1.77, 0.885) | [153] | ·Simple preparation ·Physical barrier for free water ·More complicated mechanism ·At initial stage | |
| Nafion Zn-X | Casting | 2 M ZnSO4 | 2000 (5, 0.5) | 97% | 130 (0.5, 0.5) | [150] | |||
| Ti3C2Tx MXene/ZnS | Coating | 2 M znSO4 | 1100 (1, 0.5/1) 400 (5, 5) | / | / | [159] | |||
| The structure and constitution design of Zn anodes | Zn surface structure designs | Pitted surface texture | Etching | 2 M ZnSO4 | 1000 (1, 1) | / | / | [164] | ·Redistribution of surface electric ·Poor solution for side reactions |
| hexagonal-hole patterns | Etching | 2 M ZnSO4 | 1800 (0.5, 0.5) | 99.57% | 700 (2, 1) | [165] | |||
| 3D porous surface | Etching | 1 M ZnSO4 | 930 (4, 2) | / | / | [166] | |||
| PPZ@Zn | Etching | 2 M ZnSO4 | 3000 (1, 0.5) | / | / | [167] | |||
| Conductive host designs | SDF | Template, deposition and sputtering | 2 M ZnSO4 | 1000 (2, 1) | / | / | [179] | ·Redistribution of surface electric and ion flux ·Beneficial to more Zn and large DOD ·Lower volume energy density ·Poor solution for side reactions | |
| MGA | Freeze-drying | 2 M ZnSO4 | 1050 (10, 1) | 99.67% | 600 (10, 1) | [191] | |||
| 3D Ti-TiO2 | Electrodeposition and dealloying | 2 M ZnSO4 | 2000 (1, 1) | 95.20% | 200 (10, 5) | [177] | |||
| Zn alloy anodes | Zn88Al12 (at%) | Metallurgy | 2 M ZnSO4 without O2 | 2000 (0.5, /) | ∼100%c | / | [31] | ·Dendrite-free ·Certain solutions for side reactions ·Less effect on large DOD | |
| Zn3Mn | Electrodeposition | 2 M ZnSO4 in seawater | 760 (80, 16) | ∼100%c 99.62% | / 2500 (10, /) | [195] | |||
| Design of electrolytes | Highly concentrated electrolytes | 30 m ZnCl2 | / | / | 600 (0.2, ~0.033) | 95.4%d | / | [216] | ·Simple preparation ·Potential for large DOD ·Higher cost ·Higher viscosity ·Lower ionic conductivity |
| 3 m Zn(Otf)2 +17 m NaClO4 | / | / | 1600 (1, 0.25) | ~99.96% | 700 (0.5, 0.25) | [224] | |||
| 1 m Zn(TFSI)2+ 20 m LiTFSI | / | / | 170 (0.2, ~0.033) | ~100%c | / | [225] | |||
| Electrolyte additives | 50 mM NH4Oac | / | 2 M ZnSO4 | 3500 (1, 1) | 99.7% | 1800 (1, 0.5) | [233] | ·Simple preparation ·Low cost ·Potential for large DOD ·More complicated mechanism | |
| 25 mM Zn(H2PO4)2 | / | 1 M Zn(OTF)2 | 1200 (1, 1) | 99.4% | 400 (/, 0.5) | [237] | |||
| 0.05 M KPF6 | / | 2 M ZnSO4 | 1200 (2, 4) | 99.37% | 90 (4, 2) | [239] | |||
| 300 mM In(Otf)3 | / | 3 M Zn(OTF)2 | 5700 (2, 2) | 99.94% | 1931 (0.5, 0.5) | [243] | |||
| 70 wt% PEG | / | 1M Zn(OTF)2 | 9000 (1, 1) 8000 (0.5/2, 1) | / | / | [284] | |||
| 0.5 m Me3EtNOTF | / | 4 m Zn(OTF)2 | 6000 (0.5, 0.25) | 99.8% | 1000 (0.5, 0.5) | [276] | |||
| DMSO | H2O/DMSO = 4.3:1 (vol.%) | 1.3 m ZnCl2 | 1000 (0.5, 0.5) | 99.5% | 400 (1, 0.5) | [280] | |||
| Hydrogel and solid-state electrolytes | PVIPS | Crosslinking | ZnSO4 | 500 (5, 5) | 99.6% | 400 (1, 1) | [311] | ·Certain mechanical strength ·Multifunctional, such as flexible, self-healing, freeze-tolerant ·Reduced amount of free water ·Intolerance for some Zn salt ·Low ionic conductivity ·Low power density ·Aging problem | |
| PVA-B-G | Crosslinking | ZnSO4 + MnSO4 | 1400 (2, 2) | / | / | [292] | |||
| polyzwitterionic PSBMA | Crosslinking | ZnSO4 | 3500 h (0.5, 0.5) | / | / | [303] | |||
| ZS/GL/AN | Thermal initiation method | ZnSO4 | 3000 (0.2, 0.2) 3000 (0.5, 5) | / | / | [307] | |||
| PAMPSZn | Ion exchange and crosslinking | / | 4500 (1, 1) | 99.3% | 400 (/, /) | [309] | |||
| ZHAP-Zn | Ion exchange and pressing | / | 2000 (0.5, 0.125) | / | / | [320] | |||
| ZCE | Crystallizing solvent | Zn(TFSI)2 | 4000 (0.01, 0.005) | / | / | [329] | |||
(1) Surface modification of Zn is recognized as an effective strategy to stabilize Zn metal anodes. At present, numerous inorganic and organic materials have been explored for modifying the Zn anode surface. Zn anodes modified by these materials achieve enhanced electrochemical performance. However, most buffer coatings are made using the doctor-blading method and thus suffer from poor bonding strength. They are prone to peel off due to the repeated volume changes during cycles. Therefore, more methods need to be developed to enhance the contact between the layers and anodes during cycling. For instance, the protective layers realized via in situ formation have a tight bond with Zn. However, the rigid and fragile nature of in situ Zn2+ -conducting materials or organic layers with low ion conductivity may induce limited area capacity and DOD of the Zn anodes. Composite coatings inherit the advantages of several materials and thus can achieve synergistic effects to greatly improve the stability of Zn anodes. Therefore, in situ constructing composite layers with high flexibility and Zn2+ conductivity on the Zn anodes is a strategy worth considering in the future.
(2) The structure and constitution designs of Zn anodes are significant for stable Zn anodes. The change in surface morphology of Zn metal can effectively regulate Zn deposition and alleviate dendrite growth. However, as the deposited Zn increases, the effect of the original surface morphology becomes smaller, and Zn dendrites may still grow. Compared with typical 2D Zn anodes, 3D anodes can not only accommodate more deposited Zn and larger volume variation, but also effectively reduce the local current density and redistribute the electric field due to its high specific surface area and good electrical conductivity. Nevertheless, the 3D skeleton cannot prevent side reactions, and, conversely, the nanoscale host may accelerate the reaction kinetics of side reactions due to its more active sites. The alloy strategy can inhibit HER by introducing elements with inert nature or higher HER overpotential. The formed alloy can guide the Zn deposition to some extent but may also fail when the alloy surface is completely covered by the deposited Zn. Cooperative strategies combining structure design with alloy strategy, surface protection, or hydrogel electrolyte may be workable plans for a higher electrochemical performance of Zn anodes.
(3) Modulating the composition and concentration of electrolytes is a promising way to optimize the anode-electrolyte interface and promote the utilization of Zn anodes. Introducing electrolyte additives is considered an effective method to reduce Zn dendrites. In addition to protecting the anodes, they can also act on the cathode and thus affect the whole battery. For example, organic additives usually lead to increased polarization that further deteriorates the rate performance of AZIBs, and the inorganic ones may complicate the battery system. Therefore, an in-depth study of additives for anodes and cathodes should be considered. Moreover, exploring composite additives is a possible way to obtain a better performance of AZIBs. High concentration electrolytes, especially “water-in-salt” electrolytes, can change the Zn2+ solvation structure, thus restraining the dendrite growth and side reactions. However, the concentrated electrolytes usually cause high cost, high viscosity, and decreased ionic conductivity. Balancing the trade-offs of various aspects, such as enhancing the Zn utilization, keeping high Zn2+ conductivity, and maintaining a low cost, is highly demanded. Hydrogel or solid-state electrolytes can also solve the Zn anode issues due to their less water amount and suitable mechanical strength. Their extra mechanical properties also expand the application of AZIBs to the flexible and wearable fields. However, their poor contact with anodes and low intrinsic ion conductivity are major headaches for their further development. Although some inorganic fillers are conducive to enhancing ionic conductivity of hydrogel or solid-state electrolytes, corresponding studies are rare and their effects on Zn anodes are still not clear. To improve the power density of AZIBs, the development of hydrogel or solid-state electrolytes with high ionic conductivity and high dynamic adaptability is still urgently needed.
(4) Although the material design strategies of Zn anodes have been investigated and developed, the in-depth understanding of these issues and strategies to improve the performance of the Zn anodes are limited and still in the lab. There are still many problems to be solved for achieving the practical application of AZIBs. For instance, the test protocols of Zn anodes are not unified. It means that the different studies and strategies lack comparability to a certain extent. In addition, current material design strategies of Zn anodes are mostly based on excess Zn and small DOD, which may fall short of commercial requirements and cover up the shortcomings of strategies. Moreover, the compatibility of material designs for stabilizing Zn anodes with cathode materials needs more exploration, especially the designs on electrolytes. Besides, the basic advantages of AZIBs, such as low cost and high safety, cannot be thrown away during the development. Therefore, it is recommended that a test standard that meets commercial requirements, including the current density and DOD, should be urgently formulated to accelerate the practical application of AZIBs. The cost and safety should also be evaluated simultaneously for the further large-scale development of AZIBs.
DECLARATIONS
Authors’ contributionsSelecting the topic and conceiving the structure of this paper: Zhou WY, Niu ZQ
Investigation, formal analysis, writing-original draft: Zhang YJ
Writing-review & editing: Bi SS, Niu ZQ, Zhou WY, Zhang YJ
Funding acquisition, supervision: Zhou WY, Xie SS
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by the National Key Research and Development Program of China (Grant No. 2018YFA0208402; No. 2019YFA0705600; No. 2020YFA0714700), the National Natural Science Foundation of China (Grant No. 11634014; No. 52172060; No. 51372269), and the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDA09040202).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2021.
REFERENCES
1. Li Y, Fu J, Zhong C, et al. Recent advances in flexible zinc-based rechargeable batteries. Adv Energy Mater 2019;9:1802605.
2. Yu P, Zeng Y, Zhang H, Yu M, Tong Y, Lu X. Flexible zn-ion batteries: recent progresses and challenges. Small 2019;15:e1804760.
3. Gao X, Zhang H, Liu X, Lu X. Flexible Zn-ion batteries based on manganese oxides: Progress and prospect. Carbon Energy 2020;2:387-407.
4. Zhao S, Zuo Y, Liu T, et al. Multi-functional hydrogels for flexible zinc-based batteries working under extreme conditions. Adv Energy Mater 2021;11:2101749.
5. Kong L, Tang C, Peng H, Huang J, Zhang Q. Advanced energy materials for flexible batteries in energy storage: A review. SmartMat 2020;1:smm2.1007.
6. Wu M, Zhang G, Yang H, et al. Aqueous Zn-based rechargeable batteries: recent progress and future perspectives. InfoMat ; doi: 10.1002/inf2.12265.
7. Ma Y, Ma Y, Diemant T, et al. Unveiling the intricate intercalation mechanism in manganese sesquioxide as positive electrode in aqueous Zn-metal battery. Adv Energy Mater 2021;11:2100962.
8. Zhang N, Cheng F, Liu J, et al. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat Commun 2017;8:405.
9. Huang J, Wang Z, Hou M, et al. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat Commun 2018;9:2906.
10. Chao D, Zhou W, Ye C, et al. An electrolytic Zn-MnO2 battery for high-voltage and scalable energy storage. Angew Chem Int Ed Engl 2019;58:7823-8.
11. Wan F, Niu Z. Design strategies for vanadium-based aqueous Zinc-ion batteries. Angew Chem Int Ed Engl 2019;58:16358-67.
12. Wan F, Huang S, Cao H, Niu Z. Freestanding potassium vanadate/carbon nanotube films for ultralong-life aqueous Zinc-Ion batteries. ACS Nano 2020;14:6752-60.
13. Konarov A, Voronina N, Jo JH, Bakenov Z, Sun Y, Myung S. Present and future perspective on electrode materials for rechargeable Zinc-Ion batteries. ACS Energy Lett 2018;3:2620-40.
14. Ma L, Chen S, Li H, et al. Initiating a mild aqueous electrolyte Co3O4/Zn battery with 2.2 V-high voltage and 5000-cycle lifespan by a Co( iii ) rich-electrode. Energy Environ Sci 2018;11:2521-30.
15. Zhao Y, Wang D, Li X, et al. Initiating a reversible aqueous Zn/Sulfur battery through a “liquid film”. Adv Mater 2020;32:e2003070.
16. Song J, Xu K, Liu N, Reed D, Li X. Crossroads in the renaissance of rechargeable aqueous zinc batteries. Materials Today 2021;45:191-212.
17. Wang X, Wang F, Wang L, et al. An aqueous rechargeable Zn//Co3O4 battery with high energy density and good cycling behavior. Adv Mater 2016;28:4904-11.
18. Yuan X, Wu X, Zeng X, et al. A fully aqueous hybrid electrolyte rechargeable battery with high voltage and high energy density. Adv Energy Mater 2020;10:2001583.
19. Zampardi G, La Mantia F. Open challenges and good experimental practices in the research field of aqueous Zn-ion batteries. Nat Commun 2022;13:687.
20. Yuan X, Ma F, Zuo L, et al. Latest advances in high-voltage and high-energy-density aqueous rechargeable batteries. Electrochem Energ Rev 2021;4:1-34.
21. Wu K, Huang J, Yi J, et al. Recent advances in polymer electrolytes for Zinc ion batteries: mechanisms, properties, and perspectives. Adv Energy Mater 2020;10:1903977.
22. Zhang T, Tang Y, Guo S, et al. Fundamentals and perspectives in developing zinc-ion battery electrolytes: a comprehensive review. Energy Environ Sci 2020;13:4625-65.
23. Yuan L, Hao J, Kao C, et al. Regulation methods for the Zn/electrolyte interphase and the effectiveness evaluation in aqueous Zn-ion batteries. Energy Environ Sci 2021;14:5669-89.
24. Li C, Zhang X, Zhu Y, et al. Modulating the lithiophilicity at electrode/electrolyte interface for high-energy Li-metal batteries. EM 2021; doi: 10.20517/energymater.2021.21.
25. Chang H, Wu Y, Han X, Yi T. Recent developments in advanced anode materials for lithium-ion batteries. EM 2021; doi: 10.20517/energymater.2021.02.
26. Yi Z, Chen G, Hou F, Wang L, Liang J. Strategies for the stabilization of Zn metal anodes for Zn-ion batteries. Advanced Energy Materials 2021;11:2003065.
27. He H, Qin H, Wu J, et al. Engineering interfacial layers to enable Zn metal anodes for aqueous zinc-ion batteries. Energy Storage Materials 2021;43:317-36.
28. Dong H, Li J, Guo J, et al. Insights on flexible Zinc-ion batteries from lab research to commercialization. Adv Mater 2021;33:e2007548.
29. Li C, Wang L, Zhang J, et al. Roadmap on the protective strategies of zinc anodes in aqueous electrolyte. Energy Storage Materials 2022;44:104-35.
30. Yin Y, Wang S, Zhang Q, et al. Dendrite-free Zinc deposition induced by tin-modified multifunctional 3D host for stable zinc-based flow battery. Adv Mater 2020;32:e1906803.
31. Wang SB, Ran Q, Yao RQ, et al. Lamella-nanostructured eutectic zinc-aluminum alloys as reversible and dendrite-free anodes for aqueous rechargeable batteries. Nat Commun 2020;11:1634.
32. Wippermann K, Schultze J, Kessel R, Penninger J. The inhibition of zinc corrosion by bisaminotriazole and other triazole derivatives. Corros Sci 1991;32:205-30.
33. Cai Z, Ou Y, Wang J, et al. Chemically resistant Cu-Zn/Zn composite anode for long cycling aqueous batteries. Energy Stor Mater 2020;27:205-11.
34. Li W, Wang K, Zhou M, Zhan H, Cheng S, Jiang K. Advanced low-cost, high-voltage, long-life aqueous hybrid sodium/zinc batteries enabled by a dendrite-free zinc anode and concentrated electrolyte. ACS Appl Mater Interfaces 2018;10:22059-66.
35. Li Z, Wu L, Dong S, et al. Pencil drawing stable interface for reversible and durable aqueous zinc-ion batteries. Adv Funct Mater 2021;31:2006495.
36. Du Y, Liu C, Liu Y, Han Q, Chi X, Liu Y. Carbon fiber micron film guided uniform plating/stripping of metals: a universal approach for highly stable metal batteries. Electrochimica Acta 2020;339:135867.
37. Li M, He Q, Li Z, et al. A novel dendrite-free Mn 2+ /Zn 2+ hybrid battery with 2.3 V voltage window and 11000-cycle lifespan. Adv Energy Mater 2019;9:1901469.
38. Zheng J, Zhao Q, Tang T, et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 2019;366:645-8.
39. Shen C, Li X, Li N, et al. Graphene-boosted, high-performance aqueous Zn-Ion battery. ACS Appl Mater Interfaces 2018;10:25446-53.
40. Xia A, Pu X, Tao Y, Liu H, Wang Y. Graphene oxide spontaneous reduction and self-assembly on the zinc metal surface enabling a dendrite-free anode for long-life zinc rechargeable aqueous batteries. Appl Surf Sci 2019;481:852-9.
41. Liu P, Liu W, Huang Y, Li P, Yan J, Liu K. Mesoporous hollow carbon spheres boosted, integrated high performance aqueous Zn-Ion energy storage. Energy Storage Materials 2020;25:858-65.
42. Wu C, Xie K, Ren K, Yang S, Wang Q. Dendrite-free Zn anodes enabled by functional nitrogen-doped carbon protective layers for aqueous zinc-ion batteries. Dalton Trans 2020;49:17629-34.
43. Yuksel R, Buyukcakir O, Seong WK, Ruoff RS. Metal-organic framework integrated anodes for aqueous zinc-ion batteries. Adv Energy Mater 2020;10:1904215.
44. Zhai S, Wang N, Tan X, et al. Interface-engineered dendrite-free anode and ultraconductive cathode for durable and high-rate fiber Zn dual-ion microbattery. Adv Funct Mater 2021;31:2008894.
45. Zhou J, Xie M, Wu F, et al. Ultrathin surface coating of nitrogen-doped graphene enables stable zinc anodes for aqueous zinc-ion batteries. Adv Mater 2021;33:e2101649.
46. Zhang X, Li J, Liu D, et al. Ultra-long-life and highly reversible Zn metal anodes enabled by a desolvation and deanionization interface layer. Energy Environ Sci 2021;14:3120-9.
47. Cao J, Zhang D, Zhang X, Sawangphruk M, Qin J, Liu R. A universal and facile approach to suppress dendrite formation for a Zn and Li metal anode. J Mater Chem A 2020;8:9331-44.
48. Yang X, Li W, Lv J, et al. In situ separator modification via CVD-derived N-doped carbon for highly reversible Zn metal anodes. Nano Res ; doi: 10.1007/s12274-021-3957-z.
49. Li C, Sun Z, Yang T, et al. Directly grown vertical graphene carpets as janus separators toward stabilized Zn metal anodes. Adv Mater 2020;32:e2003425.
50. Cao J, Zhang D, Gu C, et al. Manipulating crystallographic orientation of zinc deposition for dendrite-free zinc ion batteries. Adv Energy Mater 2021;11:2101299.
51. Liang Y, Wang Y, Mi H, et al. Functionalized carbon nanofiber interlayer towards dendrite-free, Zn-ion batteries. Chemical Engineering Journal 2021;425:131862.
52. Li G, Li Y, Liu H, Guo Y, Li Y, Zhu D. Architecture of graphdiyne nanoscale films. Chem Commun (Camb) 2010;46:3256-8.
53. Li J, Chen Y, Guo J, Wang F, Liu H, Li Y. Graphdiyne oxide-based high-performance rechargeable aqueous Zn-MnO 2 battery. Adv Funct Mater 2020;30:2004115.
54. Wang F, Xiong Z, Jin W, Liu H, Liu H. Graphdiyne oxide for aqueous zinc ion full battery with ultra-long cycling stability. Nano Today 2022;44:101463.
55. Yang Q, Guo Y, Yan B, et al. Hydrogen-substituted graphdiyne ion tunnels directing concentration redistribution for commercial-grade dendrite-free zinc anodes. Adv Mater 2020;32:e2001755.
56. Yang Q, Li L, Hussain T, et al. Stabilizing interface pH by N-modified graphdiyne for dendrite-free and high-rate aqueous Zn-ion batteries. Angew Chem Int Ed Engl 2022;61:e202112304.
57. Liang P, Yi J, Liu X, et al. Highly reversible Zn anode enabled by controllable formation of nucleation sites for Zn-based batteries. Adv Funct Mater 2020;30:1908528.
58. Cao J, Zhang D, Gu C, et al. Modulating Zn deposition via ceramic-cellulose separator with interfacial polarization effect for durable zinc anode. Nano Energy 2021;89:106322.
59. Zhou M, Guo S, Fang G, et al. Suppressing by-product via stratified adsorption effect to assist highly reversible zinc anode in aqueous electrolyte. Journal of Energy Chemistry 2021;55:549-56.
60. Jin H, Dai S, Xie K, et al. Regulating interfacial desolvation and deposition kinetics enables durable Zn anodes with ultrahigh utilization of 80. Small 2022;18:e2106441.
61. Zhang Q, Luan J, Huang X, et al. Revealing the role of crystal orientation of protective layers for stable zinc anode. Nat Commun 2020;11:3961.
62. Liu H, Wang JG, Hua W, et al. Building ohmic contact interfaces toward ultrastable Zn metal anodes. Adv Sci (Weinh) 2021;8:e2102612.
63. Xie X, Liang S, Gao J, et al. Manipulating the ion-transfer kinetics and interface stability for high-performance zinc metal anodes. Energy Environ Sci 2020;13:503-10.
64. Han X, Leng H, Qi Y, et al. Hydrophilic silica spheres layer as ions shunt for enhanced Zn metal anode. Chemical Engineering Journal 2022;431:133931.
65. Zhou X, Cao P, Wei A, et al. Driving the interfacial ion-transfer kinetics by mesoporous TiO2 spheres for high-performance aqueous Zn-ion batteries. ACS Appl Mater Interfaces 2021;13:8181-90.
66. Zhao K, Wang C, Yu Y, et al. Ultrathin surface coating enables stabilized zinc metal Anode. Adv Mater Interfaces 2018;5:1800848.
67. He H, Tong H, Song X, Song X, Liu J. Highly stable Zn metal anodes enabled by atomic layer deposited Al 2 O 3 coating for aqueous zinc-ion batteries. J Mater Chem A 2020;8:7836-46.
68. Hao J, Li B, Li X, et al. An in-depth study of zn metal surface chemistry for advanced aqueous zn-ion batteries. Adv Mater 2020;32:e2003021.
69. Bhoyate S, Mhin S, Jeon JE, Park K, Kim J, Choi W. Stable and high-energy-density Zn-ion rechargeable batteries based on a MoS2-coated Zn anode. ACS Appl Mater Interfaces 2020;12:27249-57.
70. Jia H, Qiu M, Lan C, et al. Advanced Zinc anode with nitrogen-doping interface induced by plasma surface treatment. Adv Sci (Weinh) 2022;9:e2103952.
71. Zheng J, Cao Z, Ming F, et al. Preferred orientation of TiN coatings enables stable zinc anodes. ACS Energy Lett 2022;7:197-203.
72. Yang Z, Lv C, Li W, et al. Revealing the two-dimensional surface diffusion mechanism for zinc dendrite formation on zinc anode. Small 2021:e2104148.
73. Ma L, Li Q, Ying Y, et al. Toward practical high-areal-capacity aqueous zinc-metal batteries: quantifying hydrogen evolution and a solid-ion conductor for stable zinc anodes. Adv Mater 2021;33:e2007406.
74. Yang Y, Liu C, Lv Z, et al. Synergistic manipulation of Zn2+ ion flux and desolvation effect enabled by anodic growth of a 3D ZnF2 matrix for long-lifespan and dendrite-free Zn metal anodes. Adv Mater 2021;33:e2007388.
75. Han J, Euchner H, Kuenzel M, et al. A thin and uniform fluoride-based artificial interphase for the zinc metal anode enabling reversible Zn/MnO 2 batteries. ACS Energy Lett 2021;6:3063-71.
76. Liang G, Zhu J, Yan B, et al. Gradient fluorinated alloy to enable highly reversible Zn-metal anode chemistry. Energy Environ Sci 2022;15:1086-96.
77. Cao P, Zhou X, Wei A, et al. Fast-charging and ultrahigh-capacity zinc metal anode for high-performance aqueous zinc-ion batteries. Adv Funct Mater 2021;31:2100398.
78. Peng H, Liu C, Wang N, et al. Intercalation of organics into layered structures enables superior interface compatibility and fast charge diffusion for dendrite-free Zn anodes. Energy Environ Sci 2022;15:1682-93.
79. Hong L, Wu X, Ma C, et al. Boosting the Zn-ion transfer kinetics to stabilize the Zn metal interface for high-performance rechargeable Zn-ion batteries. J Mater Chem A 2021;9:16814-23.
80. Yang Y, Liu C, Lv Z, et al. Redistributing Zn-ion flux by interlayer ion channels in Mg-Al layered double hydroxide-based artificial solid electrolyte interface for ultra-stable and dendrite-free Zn metal anodes. Energy Storage Materials 2021;41:230-9.
81. Liu M, Cai J, Ao H, Hou Z, Zhu Y, Qian Y. NaTi 2 (PO 4 ) 3 Solid-state electrolyte protection layer on Zn metal anode for superior long-life aqueous zinc-ion batteries. Adv Funct Mater 2020;30:2004885.
82. Xiao P, Xue L, Guo Y, et al. On-site building of a Zn2+-conductive interfacial layer via short-circuit energization for stable Zn anode. Science Bulletin 2021;66:545-52.
83. Kang L, Cui M, Jiang F, et al. Nanoporous CaCO 3 coatings enabled uniform Zn stripping/plating for long-life zinc rechargeable aqueous batteries. Adv Energy Mater 2018;8:1801090.
84. Wu K, Yi J, Liu X, et al. Regulating Zn deposition via an artificial solid-electrolyte interface with aligned dipoles for long life Zn anode. Nanomicro Lett 2021;13:79.
85. Zou P, Zhang R, Yao L, et al. Ultrahigh-rate and long-life zinc-metal anodes enabled by self-accelerated cation migration. Adv Energy Mater 2021;11:2100982.
86. Chen A, Zhao C, Guo Z, et al. Fast-growing multifunctional ZnMoO4 protection layer enable dendrite-free and hydrogen-suppressed Zn anode. Energy Storage Materials 2022;44:353-9.
87. Ma C, Wang X, Lu W, et al. Achieving stable Zn metal anode via a simple NiCo layered double hydroxides artificial coating for high performance aqueous Zn-ion batteries. Chemical Engineering Journal 2022;429:132576.
88. Su XH, Han CX, Zhang J, Wang ZJ. Preparation and electrochemical performance of CoNiO2/Ti3C2Tx composites. J Chin Ceram Soc 2021;49:1033-1040. DOI: 10.14062/j.issn.0454.
89. Chang C, Chen W, Chen Y, et al. Recent progress on two-dimensional materials. Acta Physico Chimica Sinica 2021;0:2108017-0.
90. Zhang N, Huang S, Yuan Z, Zhu J, Zhao Z, Niu Z. Direct self-assembly of mxene on Zn anodes for dendrite-free aqueous zinc-ion batteries. Angew Chem Int Ed Engl 2021;60:2861-5.
91. Sun C, Wu C, Gu X, Wang C, Wang Q. Interface engineering via Ti3C2Tx mxene electrolyte additive toward dendrite-free zinc deposition. Nanomicro Lett 2021;13:89.
92. Li X, Li M, Luo K, et al. Lattice matching and halogen regulation for synergistically induced uniform zinc electrodeposition by halogenated Ti3C2 mxenes. ACS Nano ;2021:813-22.
93. Zou K, Cai P, Deng X, et al. Highly stable zinc metal anode enabled by oxygen functional groups for advanced Zn-ion supercapacitors. Chem Commun 2021;57:528-31.
94. Li Z, Deng W, Li C, et al. Uniformizing the electric field distribution and ion migration during zinc plating/stripping. via ;8:17725-31.
95. Jiao S, Fu J, Wu M, Hua T, Hu H. Ion sieve: tailoring Zn2+ desolvation kinetics and flux toward dendrite-free metallic zinc anodes. ACS Nano ;2021:1013-24.
96. Du H, Zhao R, Yang Y, Liu Z, Qie L, Huang Y. High-capacity and long-life zinc electrodeposition enabled by a self-healable and desolvation shield for aqueous zinc-ion batteries. Angew Chem Int Ed Engl 2022;61:e202114789.
97. Jiao Y, Li F, Jin X, et al. Engineering polymer glue towards 90% zinc utilization for 1000 hours to make high-performance zn-ion batteries. Adv Funct Mater 2021;31:2107652.
98. Lee D, Kim H, Kim W, et al. Water-repellent ionic liquid skinny gels customized for aqueous Zn-Ion battery anodes. Adv Funct Mater 2021;31:2103850.
99. Zou P, Nykypanchuk D, Doerk G, Xin HL. Hydrophobic molecule monolayer brush-tethered zinc anodes for aqueous zinc batteries. ACS Appl Mater Interfaces 2021;13:60092-8.
100. Hieu LT, So S, Kim IT, Hur J. Zn anode with flexible β-PVDF coating for aqueous Zn-ion batteries with long cycle life. Chemical Engineering Journal 2021;411:128584.
101. Wang Y, Guo T, Yin J, et al. Controlled deposition of zinc-metal anodes via selectively polarized ferroelectric polymers. Adv Mater 2022;34:e2106937.
102. Zhao Z, Zhao J, Hu Z, et al. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ Sci 2019;12:1938-49.
103. Chen P, Zhou W, Xiao Z, et al. An integrated configuration with robust interfacial contact for durable and flexible zinc ion batteries. Nano Energy 2020;74:104905.
104. Cao Z, Zhu X, Xu D, et al. Eliminating Zn dendrites by commercial cyanoacrylate adhesive for zinc ion battery. Energy Storage Materials 2021;36:132-8.
105. Park SH, Byeon SY, Park J, Kim C. Insight into the critical role of surface hydrophilicity for dendrite-free zinc metal anodes. ACS Energy Lett 2021;6:3078-85.
106. Kim S, Yang X, Cho M, Lee Y. Nanostructured conductive polymer shield for highly reversible dendrite-free zinc metal anode. Chem Eng J 2022;427:131954.
107. Chen P, Yuan X, Xia Y, et al. An artificial polyacrylonitrile coating layer confining zinc dendrite growth for highly reversible aqueous zinc-based batteries. Adv Sci (Weinh) 2021;8:e2100309.
108. Liu P, Zhang Z, Hao R, et al. Ultra-highly stable zinc metal anode via 3D-printed g-C3N4 modulating interface for long life energy storage systems. Chem Eng J 2021;403:126425.
109. Park JH, Kwak MJ, Hwang C, et al. Self-assembling films of covalent organic frameworks enable long-term, efficient cycling of zinc-ion batteries. Adv Mater 2021;33:e2101726.
110. Zhao Z, Wang R, Peng C, et al. Horizontally arranged zinc platelet electrodeposits modulated by fluorinated covalent organic framework film for high-rate and durable aqueous zinc ion batteries. Nat Commun 2021;12:6606.
111. Zhao J, Ying Y, Wang G, et al. Covalent organic framework film protected zinc anode for highly stable rechargeable aqueous zinc-ion batteries. Energy Storage Materials 2022;48:82-9.
112. Ding J, Liu Y, Huang S, et al. In Situ construction of a multifunctional quasi-gel layer for long-life aqueous zinc metal anodes. ACS Appl Mater Interfaces 2021;13:29746-54.
113. Zhu M, Hu J, Lu Q, et al. A patternable and in situ formed polymeric zinc blanket for a reversible zinc anode in a skin-mountable microbattery. Adv Mater 2021;33:e2007497.
114. Shin J, Lee J, Kim Y, Park Y, Kim M, Choi JW. Highly reversible, grain-directed zinc deposition in aqueous zinc-ion batteries. Adv Energy Mater 2021;11:2100676.
115. Liu M, Yang L, Liu H, et al. Artificial solid-electrolyte interface facilitating dendrite-free zinc metal anodes via nanowetting effect. ACS Appl Mater Interfaces 2019;11:32046-51.
116. Yang H, Chang Z, Qiao Y, et al. Constructing a super-saturated electrolyte front surface for stable rechargeable aqueous zinc batteries. Angew Chem Int Ed Engl 2020;59:9377-81.
117. He M, Shu C, Hu A, et al. Suppressing dendrite growth and side reactions on Zn metal anode via guiding interfacial anion/cation/H2O distribution by artificial multi-functional interface layer. Energy Storage Materials 2022;44:452-60.
118. Li G. Regulating mass transport behavior for high-performance lithium metal batteries and fast-charging lithium-ion batteries. Adv Energy Mater 2021;11:2002891.
119. Yuan D, Manalastas W Jr, Zhang L, et al. Lignin@Nafion membranes forming Zn solid-electrolyte interfaces enhance the cycle life for rechargeable zinc-ion batteries. ChemSusChem 2019;12:4889-900.
120. Wu B, Wu Y, Lu Z, et al. A cation selective separator induced cathode protective layer and regulated zinc deposition for zinc ion batteries. J Mater Chem A 2021;9:4734-43.
121. Fang Y, Xie X, Zhang B, et al. Regulating zinc deposition behaviors by the conditioner of PAN separator for zinc-ion batteries. Adv Funct Materials 2022;32:2109671.
122. Lee BS, Cui S, Xing X, et al. Dendrite suppression membranes for rechargeable zinc batteries. ACS Appl Mater Interfaces 2018;10:38928-35.
123. Zhi J, Li S, Han M, Chen P. Biomolecule-guided cation regulation for dendrite-free metal anodes. Sci Adv 2020;6:eabb1342.
124. Wu L, Zhang Y, Shang P, Dong Y, Wu Z. Redistributing Zn ion flux by bifunctional graphitic carbon nitride nanosheets for dendrite-free zinc metal anodes. J Mater Chem A 2021;9:27408-14.
125. Liu T, Hong J, Wang J, Xu Y, Wang Y. Uniform distribution of zinc ions achieved by functional supramolecules for stable zinc metal anode with long cycling lifespan. Energy Storage Materials 2022;45:1074-83.
126. Wang Z, Dong L, Huang W, et al. Simultaneously regulating uniform Zn2+ flux and electron conduction by MOF/rGO interlayers for high-performance Zn anodes. Nanomicro Lett 2021;13:73.
127. Yang H, Qiao Y, Chang Z, Deng H, He P, Zhou H. A metal-organic framework as a multifunctional ionic sieve membrane for long-life aqueous zinc-iodide batteries. Adv Mater 2020;32:e2004240.
128. Zhang L, Zhang B, Zhang T, et al. Eliminating dendrites and side reactions via a multifunctional ZnSe protective layer toward advanced aqueous Zn metal batteries. Adv Funct Materials 2021;31:2100186.
129. Li TC, Lim YV, Xie X, et al. ZnSe Modified zinc metal anodes: toward enhanced zincophilicity and ionic diffusion. Small 2021;17:e2101728.
130. Yang X, Li C, Sun Z, et al. Interfacial manipulation via in situ grown ZnSe cultivator toward highly reversible Zn metal anodes. Adv Mater 2021;33:e2105951.
131. Cao P, Tang J, Wei A, et al. Manipulating uniform nucleation to achieve dendrite-free Zn anodes for aqueous Zn-ion batteries. ACS Appl Mater Interfaces 2021;13:48855-64.
132. Cui M, Xiao Y, Kang L, et al. Quasi-isolated Au particles as heterogeneous seeds to guide uniform Zn deposition for aqueous zinc-ion batteries. ACS Appl Energy Mater 2019;2:6490-6.
133. Li Z, Gong Z, Wu X, et al. Dendrite-free and anti-corrosion Zn metal anode enabled by an artificial layer for high-performance Zn ion capacitor. Chin Chem Lett 2021; doi: 10.1016/j.cclet.2021.11.015.
134. Han D, Wu S, Zhang S, et al. A corrosion-resistant and dendrite-free zinc metal anode in aqueous systems. Small 2020;16:e2001736.
135. Cai Z, Ou Y, Zhang B, et al. A replacement reaction enabled interdigitated metal/solid electrolyte architecture for battery cycling at 20 mA cm-2 and 20 mAh cm-2. J Am Chem Soc 2021;143:3143-52.
136. Hu K, Guan X, Lv R, et al. Stabilizing zinc metal anodes by artificial solid electrolyte interphase through a surface ion-exchanging strategy. Chem Eng J 2020;396:125363.
137. Hong L, Wang LY, Wang Y, et al. Toward hydrogen-free and dendrite-free aqueous zinc batteries: formation of zincophilic protective layer on Zn anodes. Adv Sci (Weinh) 2022;9:e2104866.
138. Zhang Y, Wang G, Yu F, et al. Highly reversible and dendrite-free Zn electrodeposition enabled by a thin metallic interfacial layer in aqueous batteries. Chem Eng J 2021;416:128062.
139. Wang Y, Chen Y, Liu W, et al. Uniform and dendrite-free zinc deposition enabled by. in situ ;9:8452-61.
140. Lu Q, Liu C, Du Y, et al. Uniform Zn deposition achieved by Ag coating for improved aqueous zinc-ion batteries. ACS Appl Mater Interfaces 2021;13:16869-75.
141. Liu C, Luo Z, Deng W, et al. Liquid alloy interlayer for aqueous zinc-ion battery. ACS Energy Lett 2021;6:675-83.
142. Jia H, Wang Z, Dirican M, et al. A liquid metal assisted dendrite-free anode for high-performance Zn-ion batteries. J Mater Chem A 2021;9:5597-605.
143. Gu J, Tao Y, Chen H, et al. Stress-release functional liquid metal-mxene layers toward dendrite-free zinc metal anodes. Advanced Energy Materials ; doi: 10.1002/aenm.202200115.
144. Liu C, Lu Q, Omar A, Mikhailova D. A facile chemical method enabling uniform Zn deposition for improved aqueous Zn-ion batteries. Nanomaterials (Basel) 2021;11:764.
145. Xie S, Li Y, Li X, et al. Stable zinc anodes enabled by zincophilic Cu nanowire networks. Nanomicro Lett 2021;14:39.
146. Li S, Fu J, Miao G, et al. Toward planar and dendrite-free Zn electrodepositions by regulating Sn-crystal textured surface. Adv Mater 2021;33:e2008424.
147. Guo W, Zhang Y, Tong X, et al. Multifunctional tin layer enabled long-life and stable anode for aqueous zinc-ion batteries. Materials Today Energy 2021;20:100675.
148. Huang Y, Chang Z, Liu W, et al. Layer-by-layer zinc metal anodes to achieve long-life zinc-ion batteries. Chem Eng J 2022;431:133902.
149. Zhao R, Yang Y, Liu G, et al. Redirected Zn Electrodeposition by an Anti-Corrosion Elastic Constraint for Highly Reversible Zn Anodes. Adv Funct Mater 2021;31:2001867.
150. Cui Y, Zhao Q, Wu X, et al. An interface-bridged organic-inorganic layer that suppresses dendrite formation and side reactions for ultra-long-life aqueous zinc metal anodes. Angew Chem Int Ed Engl 2020;59:16594-601.
151. He H, Liu J. Suppressing Zn dendrite growth by molecular layer deposition to enable long-life and deeply rechargeable aqueous Zn anodes. J Mater Chem A 2020;8:22100-10.
152. Zhou S, Wang Y, Lu H, et al. Anti-corrosive and Zn-ion-regulating composite interlayer enabling long-life Zn metal anodes. Adv Funct Materials 2021;31:2104361.
153. Guo Z, Fan L, Zhao C, et al. A dynamic and self-adapting interface coating for stable Zn-metal anodes. Adv Mater 2022;34:e2105133.
154. Di S, Nie X, Ma G, et al. Zinc anode stabilized by an organic-inorganic hybrid solid electrolyte interphase. Energy Storage Materials 2021;43:375-82.
155. Wu S, Zhang S, Chu Y, Hu Z, Luo J. Stacked lamellar matrix enabling regulated deposition and superior thermo-kinetics for advanced aqueous Zn-ion system under practical conditions. Adv Funct Mater 2021;31:2107397.
156. Zhang Y, Zhu M, Wang G, et al. Dendrites-free Zn metal anodes enabled by an artificial protective layer filled with 2D anionic nanosheets. Small Methods 2021;5:e2100650.
157. Xu X, Chen Y, Zheng D, et al. Ultra-fast and scalable saline immersion strategy enabling uniform Zn nucleation and deposition for high-performance Zn-ion batteries. Small 2021;17:e2101901.
158. Mu Y, Zhou T, Li D, et al. Highly stable and durable Zn-metal anode coated by bi-functional protective layer suppressing uncontrollable dendrites growth and corrosion. Chem Eng J 2022;430:132839.
159. An Y, Tian Y, Liu C, Xiong S, Feng J, Qian Y. Rational design of sulfur-doped three-dimensional Ti3C2T. x ;15:15259-73.
160. Deng W, Zhang N, Wang X. Hybrid interlayer enables dendrite-free and deposition-modulated zinc anodes. Chem Eng J 2022;432:134378.
161. Feng G, Guo J, Tian H, et al. Probe the localized electrochemical environment effects and electrode reaction dynamics for metal batteries using in situ 3D microscopy. Adv Energy Mater 2022;12:2103484.
162. Cui B, Han X, Hu W. Micronanostructured design of dendrite-free zinc anodes and their applications in aqueous zinc-based rechargeable batteries. Small Structures 2021;2:2000128.
163. Wang J, Cai Z, Xiao R, et al. A chemically polished zinc metal electrode with a ridge-like structure for cycle-stable aqueous batteries. ACS Appl Mater Interfaces 2020;12:23028-34.
164. Xu Y, Wang C, Shi Y, Miao G, Fu J, Huang Y. A self-preserving pitted texture enables reversible topographic evolution and cycling on Zn metal anodes. J Mater Chem A 2021;9:25495-501.
165. Zhang Y, Han X, Liu R, et al. Manipulating the zinc deposition behavior in hexagonal patterns at the preferential Zn (100) crystal plane to construct surficial dendrite-free zinc metal anode. Small 2022;18:e2105978.
166. Wang W, Huang G, Wang Y, et al. Organic acid etching strategy for dendrite suppression in aqueous zinc-ion batteries. Advanced Energy Materials 2022;12:2102797.
167. Wang X, Meng J, Lin X, et al. Stable zinc metal anodes with textured crystal faces and functional zinc compound coatings. Adv Funct Materials 2021;31:2106114.
168. Zhou M, Guo S, Li J, et al. Surface-preferred crystal plane for a stable and reversible zinc anode. Adv Mater 2021;33:e2100187.
169. Zheng J, Deng Y, Yin J, et al. Textured electrodes: manipulating built-in crystallographic heterogeneity of metal electrodes via severe plastic deformation. Adv Mater 2022;34:e2106867.
170. Wang J, Zhang B, Cai Z, et al. Stable interphase chemistry of textured Zn anode for rechargeable aqueous batteries. Science Bulletin 2022;67:716-24.
171. Chen K, Guo H, Li W, Wang Y. Dual porous 3D zinc anodes toward dendrite-free and long cycle life zinc-ion batteries. ACS Appl Mater Interfaces 2021;13:54990-6.
172. Liu H, Li J, Zhang X, et al. Ultrathin and ultralight Zn micromesh-induced spatial-selection deposition for flexible high-specific-energy Zn-ion batteries. Adv Funct Materials 2021;31:2106550.
173. Xiao R, Cai Z, Zhan R, et al. Localizing concentrated electrolyte in pore geometry for highly reversible aqueous Zn metal batteries. Chem Eng J 2021;420:129642.
174. Li C, Shi X, Liang S, et al. Spatially homogeneous copper foam as surface dendrite-free host for zinc metal anode. Chem Eng J 2020;379:122248.
175. Zhang Q, Luan J, Huang X, et al. Simultaneously regulating the ion distribution and electric field to achieve dendrite-free Zn anode. Small 2020;16:e2000929.
176. Zeng Y, Sun PX, Pei Z, et al. Nitrogen-doped carbon fibers embedded with zincophilic Cu nanoboxes for stable Zn-metal anodes. Adv Mater 2022:e2200342.
177. An Y, Tian Y, Xiong S, Feng J, Qian Y. Scalable and controllable synthesis of interface-engineered nanoporous host for dendrite-free and high rate zinc metal batteries. ACS Nano ;2021:11828-42.
178. Zhang G, Zhang X, Liu H, Li J, Chen Y, Duan H. 3D-printed multi-channel metal lattices enabling localized electric-field redistribution for dendrite-free aqueous Zn-ion batteries. Adv Energy Mater 2021;11:2003927.
179. Shen Z, Luo L, Li C, et al. Stratified zinc-binding strategy toward prolonged cycling and flexibility of aqueous fibrous zinc metal batteries. Adv Energy Mater 2021;11:2100214.
180. Jian Q, Guo Z, Zhang L, Wu M, Zhao T. A hierarchical porous tin host for dendrite-free, highly reversible zinc anodes. Chem Eng J 2021;425:130643.
181. Qian Y, Meng C, He J, Dong X. A lightweight 3D Zn@Cu nanosheets@activated carbon cloth as long-life anode with large capacity for flexible zinc ion batteries. Journal of Power Sources 2020;480:228871.
182. Zeng Y, Zhang X, Qin R, et al. Dendrite-free zinc deposition induced by multifunctional CNT frameworks for stable flexible Zn-ion batteries. Adv Mater 2019;31:e1903675.
183. Chen T, Wang Y, Yang Y, et al. Heterometallic seed-mediated zinc deposition on inkjet printed silver nanoparticles toward foldable and heat-resistant zinc batteries. Adv Funct Mater 2021;31:2101607.
184. Zheng J, Bock DC, Tang T, et al. Regulating electrodeposition morphology in high-capacity aluminium and zinc battery anodes using interfacial metal-substrate bonding. Nat Energy 2021;6:398-406.
185. Hong C, Yang G, Wang C. Highly reversible Zn electrodeposition enabled by an artificial 3D defect-rich conductive scaffold. ACS Appl Mater Interfaces 2021;13:54088-95.
186. Xie F, Li H, Wang X, et al. Mechanism for zincophilic sites on zinc-metal anode hosts in aqueous batteries. Adv Energy Mater 2021;11:2003419.
187. Wan F, Hao Z, Wang S, et al. A Universal compensation strategy to anchor polar organic molecules in bilayered hydrated vanadates for promoting aqueous zinc-ion storage. Adv Mater 2021;33:e2102701.
188. Cao Q, Gao H, Gao Y, et al. Regulating dendrite-free zinc deposition by 3D zincopilic nitrogen-doped vertical graphene for high-performance flexible Zn-ion batteries. Adv Funct Mater 2021;31:2103922.
190. Tian Y, An Y, Liu C, Xiong S, Feng J, Qian Y. Reversible zinc-based anodes enabled by zincophilic antimony engineered MXene for stable and dendrite-free aqueous zinc batteries. Energy Storage Materials 2021;41:343-53.
191. Zhou J, Xie M, Wu F, et al. Encapsulation of metallic Zn in a hybrid mxene/graphene aerogel as a stable Zn anode for foldable Zn-ion batteries. Adv Mater 2022;34:e2106897.
192. Wang Z, Huang J, Guo Z, et al. A metal-organic framework host for highly reversible dendrite-free zinc metal anodes. Joule 2019;3:1289-300.
193. Sun PX, Cao Z, Zeng YX, et al. Formation of super-assembled TiOx /Zn/N-doped carbon inverse opal towards dendrite-free Zn anodes. Angew Chem Int Ed Engl 2022;61:e202115649.
194. Zhang Y, Howe JD, Ben-yoseph S, Wu Y, Liu N. Unveiling the origin of alloy-seeded and nondendritic growth of Zn for rechargeable aqueous Zn batteries. ACS Energy Lett 2021;6:404-12.
195. Tian H, Li Z, Feng G, et al. Stable, high-performance, dendrite-free, seawater-based aqueous batteries. Nat Commun 2021;12:237.
196. Wang L, Huang W, Guo W, et al. Sn alloying to inhibit hydrogen evolution of Zn metal anode in rechargeable aqueous batteries. Adv Funct Materials 2022;32:2108533.
197. Zhou L, Yang F, Zeng S, et al. Zincophilic Cu sites induce dendrite-free Zn anodes for robust alkaline/neutral aqueous batteries. Adv Funct Materials 2022;32:2110829.
198. Wan F, Wang X, Bi S, Niu Z, Chen J. Freestanding reduced graphene oxide/sodium vanadate composite films for flexible aqueous zinc-ion batteries. Sci China Chem 2019;62:609-15.
199. Olbasa BW, Fenta FW, Chiu S, et al. High-rate and long-cycle stability with a dendrite-free zinc anode in an aqueous Zn-ion battery using concentrated electrolytes. ACS Appl Energy Mater 2020;3:4499-508.
200. Liu Z, Yang Y, Liang S, Lu B, Zhou J. pH-buffer contained electrolyte for self-adjusted cathode-free Zn-MnO 2 batteries with coexistence of dual mechanisms. Small Structures 2021;2:2100119.
201. Chuai M, Yang J, Wang M, et al. High-performance Zn battery with transition metal ions co-regulated electrolytic MnO2. eScience 2021;1:178-85.
202. Du M, Zhang F, Zhang X, et al. Calcium ion pinned vanadium oxide cathode for high-capacity and long-life aqueous rechargeable zinc-ion batteries. Sci China Chem 2020;63:1767-76.
203. Song X, He H, Aboonasr Shiraz MH, Zhu H, Khosrozadeh A, Liu J. Enhanced reversibility and electrochemical window of Zn-ion batteries with an acetonitrile/water-in-salt electrolyte. Chem Commun (Camb) 2021;57:1246-9.
204. Luo LW, Zhang C, Wu X, et al. A Zn-S aqueous primary battery with high energy and flat discharge plateau. Chem Commun (Camb) 2021;57:9918-21.
205. Sun T, Yuan X, Wang K, et al. An ultralow-temperature aqueous zinc-ion battery. J Mater Chem A 2021;9:7042-7.
206. Wang L, Zhang Y, Hu H, et al. A Zn(ClO4)2 electrolyte enabling long-life zinc metal electrodes for rechargeable aqueous zinc Batteries. ACS Appl Mater Interfaces 2019;11:42000-5.
207. Kasiri G, Trócoli R, Bani Hashemi A, La Mantia F. An electrochemical investigation of the aging of copper hexacyanoferrate during the operation in zinc-ion batteries. Electrochimica Acta 2016;222:74-83.
208. Zhang N, Cheng F, Liu Y, et al. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J Am Chem Soc 2016;138:12894-901.
209. Zhang Q, Xia K, Ma Y, et al. Chaotropic anion and fast-kinetics cathode enabling low-temperature aqueous Zn batteries. ACS Energy Lett 2021;6:2704-12.
210. Zhang Y, Zhao L, Liang Y, Wang X, Yao Y. Effect of electrolyte anions on the cycle life of a polymer electrode in aqueous batteries. eScience 2022; doi: 10.1016/j.esci.2022.01.002.
211. Huang Y, Gu Q, Guo Z, et al. Unraveling dynamical behaviors of zinc metal electrodes in aqueous electrolytes through an operando study. Energy Storage Materials 2022;46:243-51.
212. Yuan D, Zhao J, Ren H, et al. Anion texturing towards dendrite-free Zn anode for aqueous rechargeable batteries. Angew Chem Int Ed Engl 2021;60:7213-9.
213. Polu AR, Kumar R, Joshi GM. Effect of zinc salt on transport, structural, and thermal properties of PEG-based polymer electrolytes for battery application. Ionics 2014;20:675-9.
214. Karan S, Sahu TB, Sahu M, Agrawal R. .
215. Wang W, Zhao L, Yan B, Tan X, Qi Y, He B. Effects of concentration and freeze-thaw on the first hydration shell structure of Zn2+ ions. Trans Tianjin Univ 2011;17:381-5.
216. Zhang C, Holoubek J, Wu X, et al. A ZnCl2 water-in-salt electrolyte for a reversible Zn metal anode. Chem Commun (Camb) 2018;54:14097-9.
217. Zhang L, Rodríguez-pérez IA, Jiang H, et al. ZnCl 2 “water-in-salt” electrolyte transforms the performance of vanadium oxide as a Zn battery cathode. Adv Funct Mater 2019;29:1902653.
218. Tang X, Wang P, Bai M, et al. Unveiling the reversibility and stability origin of the aqueous V2 O5 -Zn batteries with a ZnCl2 “water-in-salt” electrolyte. Adv Sci (Weinh) 2021;8:e2102053.
219. Wu X, Xu Y, Zhang C, et al. Reverse dual-ion battery via a ZnCl2 water-in-salt electrolyte. J Am Chem Soc 2019;141:6338-44.
220. Dong Y, Jia M, Wang Y, et al. Long-life zinc/vanadium pentoxide battery enabled by a concentrated aqueous ZnSO 4 electrolyte with proton and zinc ion Co-intercalation. ACS Appl Energy Mater 2020;3:11183-92.
221. Patil N, Cruz C, Ciurduc D, Mavrandonakis A, Palma J, Marcilla R. An ultrahigh performance zinc-organic battery using poly(catechol) cathode in Zn(TFSI) 2 -based concentrated aqueous electrolytes. Adv Energy Mater 2021;11:2100939.
222. Olbasa BW, Huang C, Fenta FW, et al. Highly reversible Zn metal anode stabilized by dense and anion-derived passivation layer obtained from concentrated hybrid aqueous electrolyte. Adv Funct Materials 2022;32:2103959.
223. Huang J, Chi X, Wu J, Liu J, Liu Y. High-concentration dual-complex electrolyte enabled a neutral aqueous zinc-manganese electrolytic battery with superior stability. Chem Eng J 2022;430:133058.
224. Ao H, Zhu W, Liu M, et al. High-voltage and super-stable aqueous sodium-zinc hybrid ion batteries enabled by double solvation structures in concentrated electrolyte. Small Methods 2021;5:e2100418.
225. Wang F, Borodin O, Gao T, et al. Highly reversible zinc metal anode for aqueous batteries. Nat Mater 2018;17:543-9.
227. Chen S, Lan R, Humphreys J, Tao S. Salt-concentrated acetate electrolytes for a high voltage aqueous Zn/MnO2 battery. Energy Storage Materials 2020;28:205-15.
228. Wan F, Zhang L, Dai X, Wang X, Niu Z, Chen J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat Commun 2018;9:1656.
229. Guo X, Zhang Z, Li J, et al. Alleviation of dendrite formation on zinc anodes via electrolyte additives. ACS Energy Lett 2021;6:395-403.
230. Zhu M, Wang X, Tang H, et al. Antifreezing hydrogel with high zinc reversibility for flexible and durable aqueous batteries by cooperative hydrated cations. Adv Funct Mater 2019;30:1907218.
231. Wu H, Gu X, Huang P, et al. Polyoxometalate driven dendrite-free zinc electrodes with synergistic effects of cation and anion cluster regulation. J Mater Chem A 2021;9:7025-33.
232. Jian Q, Wu M, Jiang H, Lin Y, Zhao T. A trifunctional electrolyte for high-performance zinc-iodine flow batteries. J Power Sources 2021;484:229238.
233. Han D, Wang Z, Lu H, et al. A self-regulated interface toward highly reversible aqueous zinc batteries. Adv Energy Mater 2022;12:2102982.
234. Li Y, Wu P, Zhong W, et al. A progressive nucleation mechanism enables stable zinc stripping–plating behavior. Energy Environ Sci 2021;14:5563-71.
235. Yuan W, Ma G, Nie X, et al. In-situ construction of a hydroxide-based solid electrolyte interphase for robust zinc anodes. Chem Eng J 2022;431:134076.
236. Xiao Y, Xu R, Xu L, Ding J, Huang J. Recent advances on anion-derived sei for fast-charging and stable lithium batteries. EM 2021; doi: 10.20517/energymater.2021.17.
237. Zeng X, Mao J, Hao J, et al. Electrolyte design for in situ construction of highly Zn2+ -conductive solid electrolyte interphase to enable high-performance aqueous Zn-ion batteries under practical conditions. Adv Mater 2021;33:e2007416.
238. Li D, Cao L, Deng T, Liu S, Wang C. Design of a solid electrolyte interphase for aqueous Zn batteries. Angew Chem Int Ed Engl 2021;60:13035-41.
239. Chu Y, Zhang S, Wu S, Hu Z, Cui G, Luo J. In situ built interphase with high interface energy and fast kinetics for high performance Zn metal anodes. Energy Environ Sci 2021;14:3609-20.
240. Otani T, Fukunaka Y, Homma T. Effect of lead and tin additives on surface morphology evolution of electrodeposited zinc. Electrochimica Acta 2017;242:364-72.
241. Cao L, Li D, Soto FA, et al. Highly reversible aqueous zinc batteries enabled by zincophilic-zincophobic interfacial layers and interrupted hydrogen-bond electrolytes. Angew Chem Int Ed Engl 2021;60:18845-51.
242. Liu Z, Ren J, Wang F, et al. Tuning surface energy of Zn anodes via Sn heteroatom doping enabled by a codeposition for ultralong life span dendrite-free aqueous Zn-ion batteries. ACS Appl Mater Interfaces 2021;13:27085-95.
243. Ouyang K, Ma D, Zhao N, et al. A new insight into ultrastable Zn metal batteries enabled by in situ built multifunctional metallic interphase. Adv Funct Materials 2022;32:2109749.
244. Hao J, Yuan L, Ye C, et al. Boosting zinc electrode reversibility in aqueous electrolytes by using low-cost antisolvents. Angew Chem Int Ed Engl 2021;60:7366-75.
245. Zhang Y, Zhu M, Wu K, et al. An in-depth insight of a highly reversible and dendrite-free Zn metal anode in an hybrid electrolyte. J Mater Chem A 2021;9:4253-61.
246. Qin R, Wang Y, Zhang M, et al. Tuning Zn2+ coordination environment to suppress dendrite formation for high-performance Zn-ion batteries. Nano Energy 2021;80:105478.
247. Li F, Yu L, Hu Q, et al. Fabricating low-temperature-tolerant and durable Zn-ion capacitors via modulation of co-solvent molecular interaction and cation solvation. Sci China Mater 2021;64:1609-20.
248. Xu W, Zhao K, Huo W, et al. Diethyl ether as self-healing electrolyte additive enabled long-life rechargeable aqueous zinc ion batteries. Nano Energy 2019;62:275-81.
249. Cui J, Liu X, Xie Y, et al. Improved electrochemical reversibility of Zn plating/stripping: a promising approach to suppress water-induced issues through the formation of H-bonding. Mater Today Energy 2020;18:100563.
250. Feng R, Chi X, Qiu Q, et al. Cyclic ether-water hybrid electrolyte-guided dendrite-free lamellar zinc deposition by tuning the solvation structure for high-performance aqueous zinc-ion batteries. ACS Appl Mater Interfaces 2021;13:40638-47.
251. Chen S, Nian Q, Zheng L, et al. Highly reversible aqueous zinc metal batteries enabled by fluorinated interphases in localized high concentration electrolytes. J Mater Chem A 2021;9:22347-52.
252. Du H, Wang K, Sun T, et al. Improving zinc anode reversibility by hydrogen bond in hybrid aqueous electrolyte. Chem Eng J 2022;427:131705.
253. Sun P, Ma L, Zhou W, et al. Simultaneous regulation on solvation shell and electrode interface for dendrite-free Zn ion batteries achieved by a low-cost glucose additive. Angew Chem Int Ed Engl 2021;60:18247-55.
254. Qiu M, Ma L, Sun P, Wang Z, Cui G, Mai W. Manipulating Interfacial stability via absorption-competition mechanism for long-lifespan Zn anode. Nanomicro Lett 2021;14:31.
255. Dong Y, Miao L, Ma G, et al. Non-concentrated aqueous electrolytes with organic solvent additives for stable zinc batteries. Chem Sci 2021;12:5843-52.
256. Liu S, Mao J, Pang WK, et al. Tuning the electrolyte solvation structure to suppress cathode dissolution, water reactivity, and Zn dendrite growth in zinc-ion batteries. Adv Funct Mater 2021;31:2104281.
257. Lu H, Zhang X, Luo M, et al. Amino acid-induced interface charge engineering enables highly reversible Zn anode. Adv Funct Mater 2021;31:2103514.
258. Zhang Q, Luan J, Fu L, et al. The three-dimensional dendrite-free zinc anode on a copper mesh with a zinc-oriented polyacrylamide electrolyte additive. Angew Chem Int Ed Engl 2019;58:15841-7.
259. Yan M, Dong N, Zhao X, Sun Y, Pan H. Tailoring the stability and kinetics of Zn anodes through trace organic polymer additives in dilute aqueous electrolyte. ACS Energy Lett 2021;6:3236-43.
260. Hao J, Long J, Li B, et al. Toward high-performance hybrid Zn-based batteries via deeply understanding their mechanism and using electrolyte additive. Adv Funct Mater 2019;29:1903605.
261. Bayaguud A, Luo X, Fu Y, Zhu C. Cationic surfactant-type electrolyte additive enables three-dimensional dendrite-free zinc anode for stable zinc-ion batteries. ACS Energy Lett 2020;5:3012-20.
262. Zhang Q, Ma Y, Lu Y, et al. Designing anion-type water-free Zn2+ solvation structure for robust Zn metal anode. Angew Chem Int Ed Engl 2021;60:23357-64.
263. Zhang S, Hao J, Luo D, et al. Dual-function electrolyte additive for highly reversible Zn anode. Adv Energy Mater 2021;11:2102010.
264. Xi M, Liu Z, Ding J, Cheng W, Jia D, Lin H. Saccharin anion acts as a “traffic assistant” of Zn2+ to achieve a long-life and dendritic-free zinc plate anode. ACS Appl Mater Interfaces 2021;13:29631-40.
265. Wang N, Zhai S, Ma Y, et al. Tridentate citrate chelation towards stable fiber zinc-polypyrrole battery with hybrid mechanism. Energy Stor Mater 2021;43:585-94.
266. Qian L, Yao W, Yao R, et al. Cations coordination-regulated reversibility enhancement for aqueous Zn-ion battery. Adv Funct Mater 2021;31:2105736.
267. Chen Z, Chen H, Che Y, et al. Arginine cations inhibiting charge accumulation of dendrites and boosting Zn metal reversibility in aqueous rechargeable batteries. ACS Sustainable Chem Eng 2021;9:6855-63.
268. Zhang L, Miao L, Xin W, Peng H, Yan Z, Zhu Z. Engineering zincophilic sites on Zn surface via plant extract additives for dendrite-free Zn anode. Energy Storage Materials 2022;44:408-15.
269. Sun KE, Hoang TK, Doan TN, et al. Suppression of dendrite formation and corrosion on zinc anode of secondary aqueous batteries. ACS Appl Mater Interfaces 2017;9:9681-7.
270. Guan K, Tao L, Yang R, et al. Anti-corrosion for reversible zinc anode via a hydrophobic interface in aqueous zinc batteries. Adv Energy Mater 2022;12:2103557.
271. Yao R, Qian L, Sui Y, et al. A versatile cation additive enabled highly reversible zinc metal anode. Adv Energy Mater 2022;12:2102780.
272. Li R, Li M, Chao Y, et al. Hexaoxacyclooctadecane induced interfacial engineering to achieve dendrite-free Zn ion batteries. Energy Stor Mater 2022;46:605-12.
273. Li C, Kingsbury R, Zhou L, Shyamsunder A, Persson KA, Nazar LF. Tuning the solvation structure in aqueous zinc batteries to maximize Zn-ion intercalation and optimize dendrite-free zinc plating. ACS Energy Lett 2022;7:533-40.
274. Huang Z, Wang T, Li X, et al. Small-dipole-molecule-containing electrolytes for high-voltage aqueous rechargeable batteries. Adv Mater 2022;34:e2106180.
275. Hou Z, Lu Z, Chen Q, Zhang B. Realizing wide-temperature Zn metal anodes through concurrent interface stability regulation and solvation structure modulation. Energy Stor Mater 2021;42:517-25.
276. Cao L, Li D, Pollard T, et al. Fluorinated interphase enables reversible aqueous zinc battery chemistries. Nat Nanotechnol 2021;16:902-10.
278. Ma L, Pollard TP, Zhang Y, et al. Functionalized phosphonium cations enable zinc metal reversibility in aqueous electrolytes. Angew Chem Int Ed Engl 2021;60:12438-45.
279. Feng D, Cao F, Hou L, Li T, Jiao Y, Wu P. Immunizing aqueous Zn batteries against dendrite formation and side reactions at various temperatures via electrolyte additives. Small 2021;17:e2103195.
280. Cao L, Li D, Hu E, et al. Solvation structure design for aqueous Zn metal batteries. J Am Chem Soc 2020;142:21404-9.
281. Ballesteros J, Díaz-arista P, Meas Y, Ortega R, Trejo G. Zinc electrodeposition in the presence of polyethylene glycol 20000. Electrochimica Acta 2007;52:3686-96.
282. Mitha A, Yazdi AZ, Ahmed M, Chen P. Surface adsorption of polyethylene glycol to suppress dendrite formation on zinc anodes in rechargeable aqueous batteries. Chem Electro Chem 2018;5:2409-18.
283. Cao Z, Zhu X, Gao S, et al. Ultrastable zinc anode by simultaneously manipulating solvation sheath and inducing oriented deposition with PEG stability promoter. Small 2022;18:e2103345.
284. Wu Y, Zhu Z, Shen D, et al. Electrolyte engineering enables stable Zn-ion deposition for long-cycling life aqueous Zn-ion batteries. Energy Stor Mater 2022;45:1084-91.
285. Huang S, Zhu J, Tian J, Niu Z. Recent progress in the electrolytes of aqueous zinc-ion batteries. Chemistry 2019;25:14480-94.
286. Zhao J, Sonigara KK, Li J, et al. A smart flexible zinc battery with cooling recovery ability. Angew Chem Int Ed Engl 2017;56:7871-5.
287. Kumar G. Electrochemical characterization of poly(vinylidenefluoride)-zinc triflate gel polymer electrolyte and its application in solid-state zinc batteries. Solid State Ionics 2003;160:289-300.
288. Kumar G, Sampath S. Spectroscopic characterization of a gel polymer electrolyte of zinc triflate and polyacrylonitrile. Polymer 2004;45:2889-95.
289. Zeng Y, Zhang X, Meng Y, et al. Achieving ultrahigh energy density and long durability in a flexible rechargeable quasi-solid-state Zn-MnO2 battery. Adv Mater 2017;29:1700274.
290. Li Q, Cui X, Pan Q. Self-healable hydrogel electrolyte toward high-performance and reliable quasi-solid-state Zn-MnO2 batteries. ACS Appl Mater Interfaces 2019;11:38762-70.
291. Liu J, Long J, Shen Z, et al. A self-healing flexible quasi-solid zinc-ion battery using all-in-one electrodes. Adv Sci (Weinh) 2021;8:2004689.
292. Chen M, Zhou W, Wang A, et al. Anti-freezing flexible aqueous Zn-MnO 2 batteries working at -35 °C enabled by a borax-crosslinked polyvinyl alcohol/glycerol gel electrolyte. J Mater Chem A 2020;8:6828-41.
293. Zhou W, Chen J, Chen M, et al. An environmentally adaptive quasi-solid-state zinc-ion battery based on magnesium vanadate hydrate with commercial-level mass loading and anti-freezing gel electrolyte. J Mater Chem A 2020;8:8397-409.
294. Li H, Liu Z, Liang G, et al. Waterproof and tailorable elastic rechargeable yarn zinc ion batteries by a cross-linked polyacrylamide electrolyte. ACS Nano 2018;12:3140-8.
295. Wang D, Wang L, Liang G, et al. A superior δ-MnO2 cathode and a self-healing Zn-δ-MnO2 battery. ACS Nano 2019;13:10643-52.
296. Li H, Han C, Huang Y, et al. An extremely safe and wearable solid-state zinc ion battery based on a hierarchical structured polymer electrolyte. Energy Environ Sci 2018;11:941-51.
297. Jin X, Song L, Dai C, et al. A self-healing zinc ion battery under -20 °C. Energy Stor Mater 2022;44:517-26.
298. Mo F, Liang G, Meng Q, et al. A flexible rechargeable aqueous zinc manganese-dioxide battery working at -20 °C. Energy Environ Sci 2019;12:706-15.
299. Wang Z, Mo F, Ma L, et al. Highly compressible cross-linked polyacrylamide hydrogel-enabled compressible Zn-MnO2 battery and a flexible battery-sensor system. ACS Appl Mater Interfaces 2018;10:44527-34.
300. Han Q, Chi X, Zhang S, et al. Durable, flexible self-standing hydrogel electrolytes enabling high-safety rechargeable solid-state zinc metal batteries. J Mater Chem A 2018;6:23046-54.
301. Huang Y, Liu J, Zhang J, et al. Flexible quasi-solid-state zinc ion batteries enabled by highly conductive carrageenan bio-polymer electrolyte. RSC Adv 2019;9:16313-9.
302. Mo F, Chen Z, Liang G, et al. Zwitterionic sulfobetaine hydrogel electrolyte building separated positive/negative ion migration channels for aqueous Zn-MnO 2 batteries with superior rate capabilities. Adv Energy Mater 2020;10:2000035.
303. Leng K, Li G, Guo J, et al. A safe polyzwitterionic hydrogel electrolyte for long-life quasi-solid state zinc metal batteries. Adv Funct Mater 2020;30:2001317.
304. Wang J, Huang Y, Liu B, et al. Flexible and anti-freezing zinc-ion batteries using a guar-gum/sodium-alginate/ethylene-glycol hydrogel electrolyte. Energy Storage Materials 2021;41:599-605.
305. Zhang S, Yu N, Zeng S, et al. An adaptive and stable bio-electrolyte for rechargeable Zn-ion batteries. J Mater Chem A 2018;6:12237-43.
306. Chen M, Chen J, Zhou W, Han X, Yao Y, Wong CP. Realizing an all-round hydrogel electrolyte toward environmentally adaptive dendrite-free aqueous Zn-MnO2 batteries. Adv Mater 2021;33:e2007559.
307. Wei T, Ren Y, Li Z, Zhang X, Ji D, Hu L. Bonding interaction regulation in hydrogel electrolyte enable dendrite-free aqueous zinc-ion batteries from -20 to 60 °C. Chem Eng J 2022;434:134646.
308. Tang Y, Liu C, Zhu H, et al. Ion-confinement effect enabled by gel electrolyte for highly reversible dendrite-free zinc metal anode. Energy Stor Mater 2020;27:109-16.
309. Cong J, Shen X, Wen Z, et al. Ultra-stable and highly reversible aqueous zinc metal anodes with high preferred orientation deposition achieved by a polyanionic hydrogel electrolyte. Energy Stor Mater 2021;35:586-94.
310. Zhang B, Qin L, Fang Y, et al. Tuning Zn2+ coordination tunnel by hierarchical gel electrolyte for dendrite-free zinc anode. Science Bulletin 2022; doi: 10.1016/j.scib.2022.01.027.
311. Hao Y, Feng D, Hou L, Li T, Jiao Y, Wu P. Gel electrolyte constructing Zn (002) deposition crystal plane toward highly stable Zn anode. Adv Sci (Weinh) 2022;9:e2104832.
312. Xu P, Wang C, Zhao B, Zhou Y, Cheng H. A high-strength and ultra-stable halloysite nanotubes-crosslinked polyacrylamide hydrogel electrolyte for flexible zinc-ion batteries. J Power Sources 2021;506:230196.
313. Cao F, Wu B, Li T, Sun S, Jiao Y, Wu P. Mechanoadaptive morphing gel electrolyte enables flexible and fast-charging Zn-ion batteries with outstanding dendrite suppression performance. Nano Res 2022;15:2030-9.
314. Nguyen TN, Iranpour B, Cheng E, Madden JDW. Washable and stretchable Zn–MnO 2 rechargeable cell. Adv Energy Mater 2022;12:2103148.
315. Liu D, Tang Z, Luo L, et al. Self-healing solid polymer electrolyte with high ion conductivity and super stretchability for all-solid zinc-ion batteries. ACS Appl Mater Interfaces 2021;13:36320-9.
316. Martinolich AJ, Lee C, Lu I, et al. Solid-state divalent ion conduction in ZnPS 3. Chem Mater 2019;31:3652-61.
317. Wang Z, Hu J, Han L, et al. A MOF-based single-ion Zn2+ solid electrolyte leading to dendrite-free rechargeable Zn batteries. Nano Energy 2019;56:92-9.
318. Gao J, Xie X, Liang S, Lu B, Zhou J. Inorganic colloidal electrolyte for highly robust zinc-ion batteries. Nanomicro Lett 2021;13:69.
319. Cao J, Zhang D, Yue Y, et al. Regulating solvation structure to stabilize zinc anode by fastening the free water molecules with an inorganic colloidal electrolyte. Nano Energy 2022;93:106839.
320. Xing Z, Xu G, Xie X, et al. Highly reversible zinc-ion battery enabled by suppressing vanadium dissolution through inorganic Zn2+ conductor electrolyte. Nano Energy 2021;90:106621.
321. Johnsi M, Suthanthiraraj SA. Compositional effect of ZrO2 nanofillers on a PVDF-co-HFP based polymer electrolyte system for solid state zinc batteries. Chin J Polym Sci 2016;34:332-43.
322. Zhao Z, Wang J, Lv Z, et al. In-situ formed all-amorphous poly (ethylene oxide)-based electrolytes enabling solid-state Zn electrochemistry. Chem Eng J 2021;417:128096.
323. Johnsi M, Suthanthiraraj SA. Preparation, zinc ion transport properties, and battery application based on poly(vinilydene fluoride-. co ;27:877-85.
324. Pucic I, Turkovic A. Radiation modification of (PEO)ZnCl polyelectrolyte and nanocomposite. Solid State Ionics 2005;176:1797-800.
325. Nancy AC, Suthanthiraraj SA. Effect of Al2O3 nanofiller on the electrical, thermal and structural properties of PEO:PPG based nanocomposite polymer electrolyte. Ionics 2017;23:1439-49.
326. Chen Z, Li X, Wang D, et al. Grafted mxene/polymer electrolyte for high performance solid zinc batteries with enhanced shelf life at low/high temperatures. Energy Environ Sci 2021;14:3492-501.
327. Karan S, Sahu TB, Sahu M, Mahipal YK, Agrawal RC. Characterization of ion transport property in hot-press cast solid polymer electrolyte (SPE) films: [PEO: Zn(CF3SO3)2]. Ionics 2017;23:2721-6.
328. Liu J, Khanam Z, Muchakayala R, Song S. Fabrication and characterization of Zn-ion-conducting solid polymer electrolyte films based on PVdF-HFP/Zn(Tf)2 complex system. J Mater Sci: Mater Electron 2020;31:6160-73.
329. Qiu H, Hu R, Du X, et al. Eutectic crystallization activates solid-state zinc-ion conduction. Angew Chem Int Ed Engl 2022;61:e202113086.
330. Adams BD, Zheng J, Ren X, Xu W, Zhang J. Accurate determination of coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv Energy Mater 2018;8:1702097.
331. Xiao J, Li Q, Bi Y, et al. Understanding and applying coulombic efficiency in lithium metal batteries. Nat Energy 2020;5:561-8.
332. Ma L, Schroeder MA, Pollard TP, et al. Critical factors dictating reversibility of the zinc metal anode. Energy Environ Mater 2020;3:516-21.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zhang Y, Bi S, Niu Z, Zhou W, Xie S. Design of Zn anode protection materials for mild aqueous Zn-ion batteries. Energy Mater 2022;2:200012. http://dx.doi.org/10.20517/energymater.2022.08
AMA Style
Zhang Y, Bi S, Niu Z, Zhou W, Xie S. Design of Zn anode protection materials for mild aqueous Zn-ion batteries. Energy Materials. 2022; 2(2): 200012. http://dx.doi.org/10.20517/energymater.2022.08
Chicago/Turabian Style
Zhang, Yuejuan, Songshan Bi, Zhiqiang Niu, Weiya Zhou, Sishen Xie. 2022. "Design of Zn anode protection materials for mild aqueous Zn-ion batteries" Energy Materials. 2, no.2: 200012. http://dx.doi.org/10.20517/energymater.2022.08
ACS Style
Zhang, Y.; Bi S.; Niu Z.; Zhou W.; Xie S. Design of Zn anode protection materials for mild aqueous Zn-ion batteries. Energy Mater. 2022, 2, 200012. http://dx.doi.org/10.20517/energymater.2022.08
About This Article
Copyright
Data & Comments
Data
 Cite This Article 66 clicks
Cite This Article 66 clicks


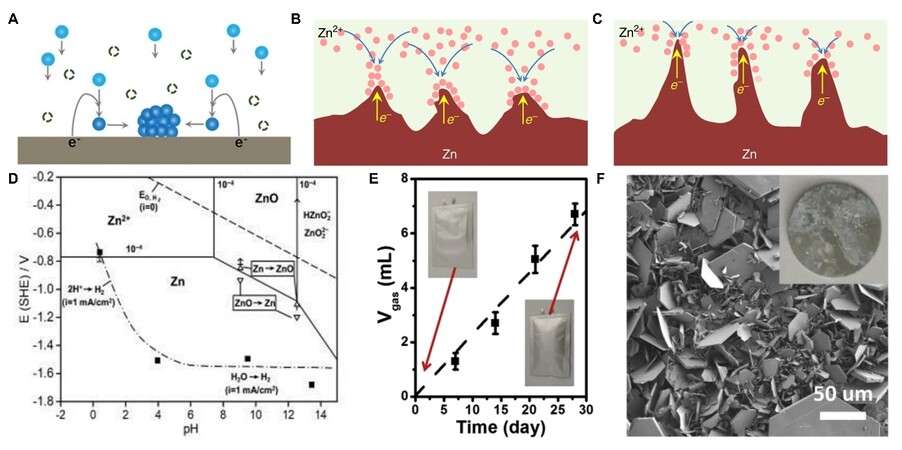
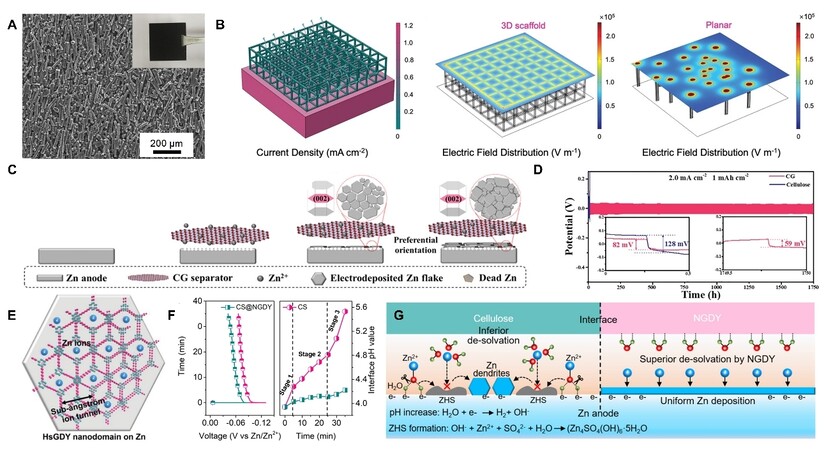
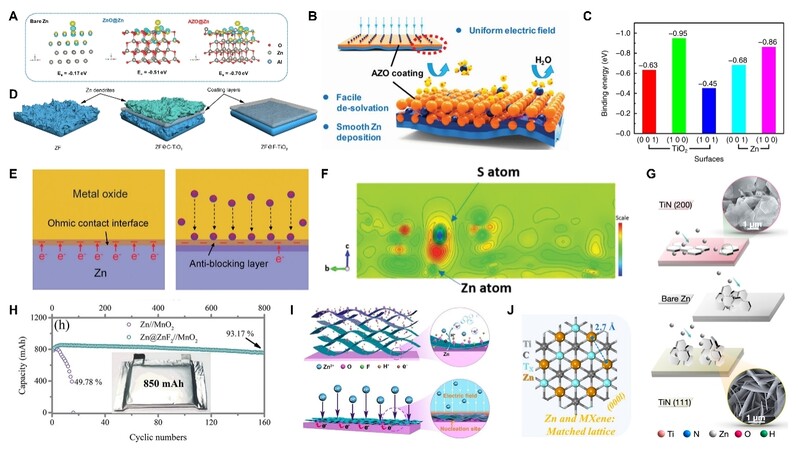
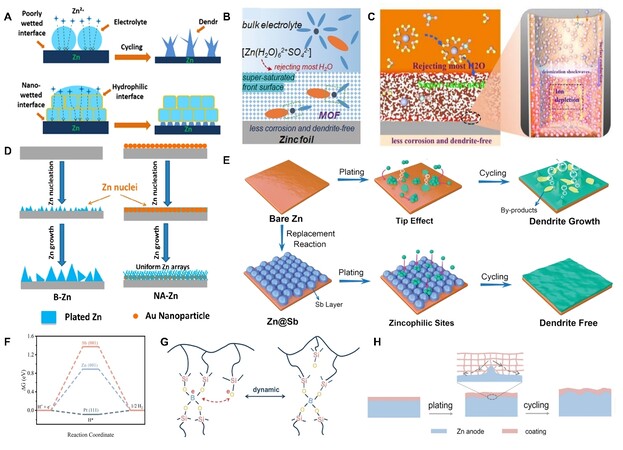
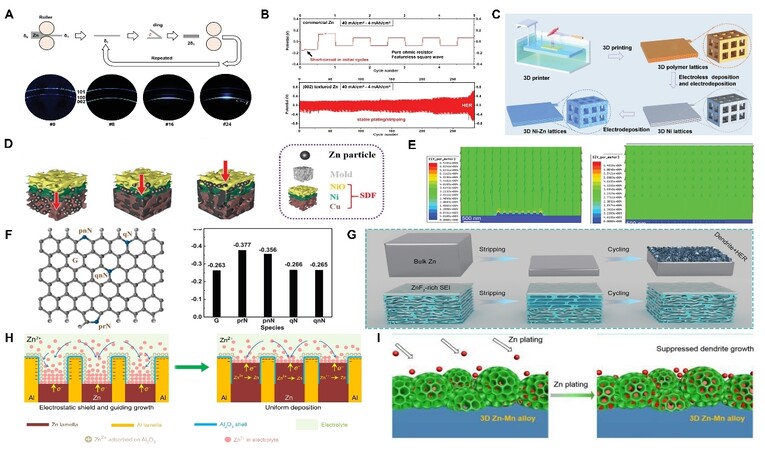
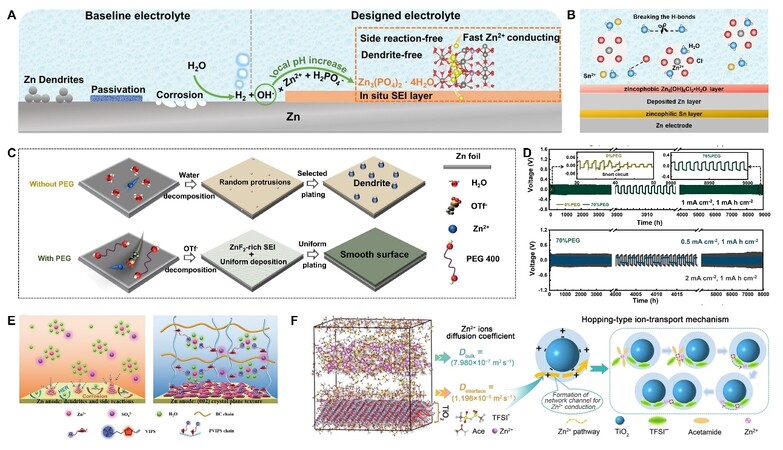











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.