Research progress on the surface/interface modification of high-voltage lithium oxide cathode materials
Abstract
Lithium oxides are the most promising cathode candidates for high-performance lithium-ion batteries (LIBs), owing to their high theoretical capacity and average working voltage, which are conducive to achieving the ultimate goal of upgrading energy density. By raising the upper limit of the cutoff voltage, we may be able to further improve both the practical capacity and average voltage of lithium oxide cathodes. Unfortunately, the high-voltage operation of these cathodes results in significant challenges, namely, reduced surface structural stability and interfacial stability with electrolytes, thus degrading the electrochemical performance. Accordingly, surface/interface modification strategies, including surface coating, electrolyte regulation, binder design, and special surface treatments, are systematically summarized and comprehensively analyzed for high-voltage lithium oxide cathode materials in this review. Furthermore, the corresponding modification mechanisms are discussed in detail to better grasp the internal mechanisms for the enhanced electrochemical performance. Based on recent progress, we further propose predictable development directions for high-performance LIBs in future practical applications. This review provides new insights into various high-voltage lithium oxide cathodes and their universal surface/interface modification strategies towards advanced next-generation LIBs with high energy and power density and long cycle life.
Keywords
INTRODUCTION
Lithium-ion batteries (LIBs) have attracted ever-increasing attention since they were first successfully commercialized by SONY in 1991. After decades of relentless exploration, LIBs are now considered the most promising energy storage systems for portable electronics (e.g., mobile phones) and electric vehicles (e.g., electric buses), owing to their excellent characteristics of high energy and power density, working voltage, and safety and long cycle life[1-3]. Persistent efforts are still needed to further develop next-generation advanced LIBs and fulfill the growing practical application requirements. Energy density is well known as an important parameter to evaluate battery performance and satisfies the relationship of energy density = specific capacity × average working potential. Among the components of LIBs, including the cathode, anode, electrolyte, and separator, cathode materials greatly limit the energy density of LIBs[4,5]. Therefore, the design and optimization of cathode materials with high capacity and working potential are urgently required to achieve advanced LIBs with higher energy density.
Current cathode materials mainly include transition metal oxides and polyanion compounds[6]. Transition metal oxides play a particularly dominant role on account of their advantages of high theoretical specific capacity and working voltage and mature preparation processes[7-9]. Transition metal oxides can generally be divided into four categories in accordance with their crystal structure and composition: (1) layered lithium cobalt oxides, LiCoO2 (LCO); (2) layered lithium ternary oxides, like LiNixCoyMn1-x-yO2 (NCM) and
In order to solve these issues associated with LCO, layered ternary oxide materials have also been widely investigated as prospective candidates by replacing part of the expensive and toxic Co element with more abundant Ni, Mn, or Al elements. Ternary oxide materials are therefore lower in cost compared with LCO[11-13]. Additionally, Ni-rich ternary oxide cathodes usually deliver a higher discharge specific capacity that increases with increasing Ni content[14]. However, in contrast, high Ni contents inevitably result in poor structural and cyclic stability. Different from layered oxides, spinel LNMO can exhibit a high working voltage and stable crystal structure[15] and therefore is a good cathode material for high-voltage LIBs. Nevertheless, LNMO usually simultaneously delivers a low discharge capacity, and novel electrolytes with wide electrochemical windows are urgently required. Compared to these cathode materials, Li-rich cathodes show an extremely high specific capacity of 250-300 mAh g-1 based on the combination of transition metal cation and oxygen anion redox. However, these materials also feature a series of issues, including insufficient initial coulombic efficiency (CE), fast capacity and voltage loss, oxygen release, and insufficient rate capability[16].
Although these transition metal oxide cathode materials have been extensively studied regarding their structural and electrochemical performance, several problems remain, including structural and cycling instability and complicated decay mechanisms, especially under elevated cutoff voltages, which must be solved for next-generation LIBs with distinguished electrochemical performance. The instability of layered crystal structures mainly originates from aggravating irreversible phase transformations, lattice oxygen loss, and interfacial side reactions between oxide cathodes and electrolytes under high-voltage operation, thus hindering their electrochemical performance for further commercial applications.
Specifically, high-voltage operation causes transition metal oxide cathode materials to suffer from the following challenges. The first issue is surface degradation, which can result from transition metal migration and dissolution, phase transformations, and the generation of microcracks[17-19]. For instance, as more Li+ ions are extracted from the crystal structure of LCO under high voltage, the LCO surface may suffer from an irreversible phase transformation from the layered to spinel and even rock salt phase, thereby deteriorating the electrochemical performance[20]. In particular, Ni-rich oxides usually go through severe Li/Ni mixing because of the similar ionic radii of Li+ and Ni2+, which causes slow ion diffusion and increased internal resistance[21].
The second challenge is interfacial side reactions, which become more dramatic when the upper cutoff voltage increases[22,23]. These harmful side reactions will consume the electrolyte and Li+ from the active materials. Furthermore, the formation of an unstable and thick cathode electrolyte interface (CEI) or solid electrolyte interface (SEI) can lead to increased internal resistance. As discussed in the literature, interfacial side reactions, especially the parasitic reactions between electrolytes and charged electrodes, tend to take place under high-voltage operation[24,25]. Pham et al. investigated cathode-electrolyte interfacial processes with increasing charge cutoff voltages of 4.5, 4.8, 5.0, 5.2, and 5.5 V based on a Li-rich layered oxide cathode and a model electrolyte of 0.1 M LiPF6 in di-(2,2,2 trifluoroethyl) carbonate[26]. It was found that the oxidative decomposition of the carbonate begins at 4.7 V, forming a protective film on the cathode surface, thereby inhibiting or reducing the further structural degradation of active materials. Nonetheless, cathode-electrolyte interfacial stabilization can be compromised with persistent surface deterioration and the accumulation of byproducts from electrolyte decomposition when the voltage reaches 5.2-5.5 V. For conventional electrolytes, the tolerable limit of the charge cutoff voltage will be lower.
The third issue is gas evolution[19,27,28], mainly including O2 and CO2. O2 release causes damage to the lattice oxygen and the migration of transition metal ions. CO2 release is attributed to the oxidative decomposition of carbonate solvents, which have adverse effects on the electrochemical performance and safety of LIBs. The final challenge is incompatible electrolytes. High-voltage operation is also a significant challenge to the stability of electrolytes[29-32]. Generally, conventional carbonate-based electrolytes will undergo oxidative decomposition above 4.5 V. The resulting HF can attack cathode materials and thus contribute to the dissolution of transition metal ions. So far, it remains highly challenging to pursue advanced LIBs with improved structural stability and electrochemical performance by alleviating these unfavorable factors at high voltages.
Accordingly, based on the above considerations, this review concentrates on recent progress regarding surface/interface modification strategies for high-voltage lithium oxide cathode materials in advanced LIBs with prominently meliorative electrochemical performance, as shown in Figure 1[20,23,32-36]. Herein, we comprehensively summarize several commonly used surface/interface modification strategies and further elaborate on their underlying working mechanisms. First, surface coating with metal oxides, fluorides, phosphates, fast ionic conductors, and polymers has been regarded as the most simple and effective modification strategy, which generally functions by constructing a protective thin layer on the active material surface to avoid electrolyte corrosion and transition metal ion dissolution. In addition, reasonable electrolyte regulation with novel functional additives can also effectively enhance the cycling stability under high-voltage operation, where the additive can be preferentially decomposed to construct a uniform and stable interface layer in situ. Furthermore, binder design has also proved to be a novel strategy. Some binders with superior binding ability and electrochemical windows can help to create robust CEIs, which contribute to improved electrochemical stability under high voltages. Binders with polar functional groups can also coordinate with transition metal ions to reduce their dissolution. Finally, special surface treatments with chemical substances are considered. These simple treatments can make artificial surface reconstructions to protect active materials and improve their electrochemical performance. Based on the above discussions, several possible development directions can be proposed for high-performance LIBs in the future. This review provides new insights into advanced LIBs for practical applications.
CHALLENGES FACING HIGH-VOLTAGE LITHIUM OXIDE CATHODE MATERIALS
At present, high-voltage oxide cathode materials for LIBs mainly include four types: layered high-voltage LCO; layered lithium ternary oxides (e.g., NCM and NCA); spinel lithium manganese oxides and derivatives (e.g., LNMO); Li-rich layered oxides xLiMnO2∙(1-x)Li2MnO3. In recent years, substantial developments have been made regarding crystal structures, degradation mechanisms, and modification strategies for next-generation advanced LIBs. The detailed structural features, challenges, mechanisms, and modification strategies for these oxide cathode materials are discussed in the following section to further our understanding of their different electrochemical characteristics. In particular, a series of similar challenges regarding electrochemical degradation are presented, from the instability of the active material surface to interfacial instability between the electrode and electrolyte under high-voltage operation. As a result, the corresponding surface/interface modification strategies are extensively discussed.
High-voltage layered lithium cobalt oxide cathodes
LCO is widely regarded as one of the most popular layered oxide cathode materials for high-performance LIBs. LCO is composed of edge-sharing LiO6 and CoO6 octahedra. Its crystal structure is similar to that of layered α-NaFeO2, belonging to a hexagonal crystal system with the R-3m space group, in which O2- ions arrange in cubic close packing (6c site) and Li+ and Co3+ occupy alternate octahedral 3a and 3b sites in the oxygen layer, respectively[37]. Encouragingly, LCO delivers a high theoretical capacity and density (~4.2 g cm-3). However, it only shows an initial discharge specific capacity of ~140 mAh g-1 with an average voltage of 3.9 V from 3.0 to 4.2 V. Many studies have reported a remarkable increase in reversible capacity from 155 to 170 and 185 mAh g-1, along with a slightly increasing average voltage that can be easily implemented by raising the upper cutoff voltage from 4.3 to 4.4 and 4.5 V, thereby leading to a considerable improvement in energy density[38]. In particular, the reversible capacity can reach 220 mAh g-1 when the upper cutoff voltage is increased to 4.6 V[39].
In spite of these substantial improvements in capacity and energy density, there are still some inherent issues caused by the high-voltage operation. For example, the layered crystal structure of LCO becomes unstable with more Li+ (de) intercalation from the lattice when charging to a higher voltage. Simultaneously, traditional organic electrolytes are also subjected to oxidative decomposition, which further results in a series of issues, mainly including deteriorative cyclic stability and safety, increased interfacial impedance, aggravated irreversible phase transformation and transition metal dissolution, and obstructed Li+ diffusion[10]. Moreover, significant research has been carried out to better understand the specific decay mechanisms of high-voltage LCO cathodes. The structural instability of LCO at high working voltages is well known as the major obstacle to achieving a high capacity with prominent capacity retention. Li et al. investigated the origin of high-voltage instability for the LCO crystal structure through an advanced three-dimensional (3D) continuous rotation electron diffraction technique in combination with high-resolution transmission electron microscopy, with an atomistic understanding proposed[40]. Curved cobalt oxide layers, especially at the near surface, are identified as the fundamental reason for poor structural stability and unsatisfactory electrochemical performance. Furthermore, the experimental result was proved to be in good agreement with theoretical calculations.
In order to effectively enhance the surface stability of LCO and interfacial stability between a cathode and electrolyte, modification strategies, like surface coating and electrolyte regulation, have been widely developed. Guo and co-workers[41] designed a surface multilayer, including Zn-rich surface coating, rock-salt buffer, and surface gradient Al doping layers, to modify LCO, which synergistically improved the electrochemical performance when operating at 4.6 V. As a consequence, severe side reactions between the electrode and electrolyte, interfacial impedance and irreversible collapse of the crystal structure all can be vigorously mitigated, so that 65.7% of the initial capacity can be well maintained, even after 500 cycles. Unremitting efforts are still being devoted to the development of advanced LCO cathodes with higher energy density and longer cycle life.
Layered lithium ternary oxide cathodes
Considering the limitations of LCO regarding limited discharge capacity, cost, and toxic Co, layered lithium ternary oxide materials derived from LCO have been extensively developed and researched by means of partially replacing Co with Ni, Mn, or Al. In ternary oxide materials, Ni2+/4+ with a redox reaction of two electrons contributes to the high specific capacity, Co3+ is beneficial to the electronic conductivity and rate capacity, while Mn4+ and Al3+ are electrochemically inactive but stabilize the crystal structure[5]. Generally, these materials have a similar crystal structure and theoretical capacity to LCO, and their chemical compositions, discharge specific capacity, and electrochemical behaviors can be easily adjusted. Ternary oxides, especially Ni-rich ternary oxides, like LiNi0.8Co0.1Mn0.1O2 (NCM811) and LiNi0.6Co0.2Mn0.2O2 (NCM622), have been identified as highly attractive cathode materials, owing to their high reversible capacity and operating potential. Increasing the Ni content while reducing the content of Co has been a tendency that can improve the capacity of oxide cathodes and simultaneously reduce the cost[14]. However, this also results in several problems, including reduced thermal stability and increased sensitivity to air and moisture[42]. In addition, due to the similar ionic radii of Ni2+ (0.69 Å) and Li+ (0.76 Å), cation mixing usually occurs in Ni-rich oxides. Consequently, Ni-rich ternary oxide cathode materials inevitably suffer from fast capacity loss during cycling at a high voltage or temperature[43,44].
The degradation mechanisms of these cathode materials can be divided into four main categories, namely, detrimental phase transformations, irreversible electrolyte breakdown and gas release, transition metal dissolution, and microcrack generation. There have been a number of modification strategies to ameliorate these issues of structural instability and deficiencies in electrochemical performance. In addition to conventional surface coating and bulk doping strategies, the rational design of novel functional electrolytes has also been proved to be a facile and feasible method to effectively promote the electrochemical performance. Recently, Huang and his team[45] successfully introduced dual additives, namely, lithium bis(oxalate)borate and dopamine, into a traditional carbonate electrolyte, where the additives with elevated highest occupied molecular orbital energy levels tend to be preferentially oxidized. They also subsequently generated a uniform and robust N, B, and O-rich inorganic/polymer interphase on the surface of a Ni-rich NCM811 cathode. More significantly, the formed CEI can contribute significantly to strengthening the interfacial stability and reducing the detrimental side reactions. Thus, the electrochemical performance can be remarkably promoted, including an enhanced capacity retention of over 90% after 200 cycles and an outstanding rate capability with 118 mAh g-1 at 20 C. Although reasonable progress has been made regarding layered Ni-rich ternary oxide cathode materials, they still have significant development prospects.
Spinel lithium manganese oxides and substituted cathodes
In comparison to the above layered oxides, the spinel framework with a robust 3D network is capable of providing fast Li+ diffusion channels and thus endowing spinel active materials with superior thermal stability and excellent rate and power capability. Typically, spinel LiMn2O4, one of the most attractive commercial cathode materials for LIBs, belongs to the Fd-3m space group[46]. Its electrochemical behavior is studied with a Mn3+/4+ redox couple operating at ~4.0 V and a discharge capacity of ~140 mAh g-1 (approaching its theoretical capacity of 148 mAh g-1). Moreover, it has the advantages of abundant resources, low cost, and easy synthesis, and is thus widely used in the field of electric vehicles. However, in addition to a low discharge specific capacity, its structural and cycling stability under high temperatures still need to be enhanced for further applications.
The attenuation mechanism of LiMn2O4 has been extensively studied, and the overwhelming majority of studies attribute its fast capacity loss to the malignant dissolution of Mn2+ and the Jahn-Teller distortion of Mn3+[47]. Another representative spinel cathode material is LNMO, in which partial Mn ions are replaced with Ni2+, leading to a more ordered crystal structure with an increased average valence state of Mn ions and a mitigated Jahn-Teller effect. LNMO delivers an extremely high working voltage (4.7 V vs. Li+/Li) and moderate discharge capacity (~148 mAh g-1) based on the Ni2+/4+ redox couple and has been considered as the most prospective 5 V cathode material for high energy density LIBs[48]. Nevertheless, the high-voltage operation will inevitably lead to severe interfacial instability between the electrode and electrolyte, as well as electrolyte decomposition. Therefore, its practical application is still limited by the development of compatible high-voltage electrolytes. At present, commonly used modification strategies, including surface coating, bulk doping, particle morphology control, and electrolyte and binder optimization, are also applied to spinel cathode systems. More detailed developments on modification strategies for spinel lithium oxide cathode materials are discussed later.
Layered Li-rich oxide cathodes
Since Li-rich layered oxides were discovered, the discussions regarding their crystal structure have remained controversial. On the one hand, it is well recognized that Li-rich layered oxides are composed of LiMO2 (
In order to ameliorate the issue of irreversible oxygen evolution, Kim and his colleagues[51] recently reported a layered Li-rich Mn-rich cathode material with a polydopamine (PDA) surface coating that demonstrated an enhanced rate capability and suppressed capacity fading during cycling. It is noteworthy that the PDA layer can function as an oxygen radical scavenger, which can effectively protect cathode materials and thus diminish surface transformation and oxygen release. In addition, numerous modification strategies, including conventional coating, doping, and some novel surface treatments, have been investigated to overcome these difficulties.
SURFACE/INTERFACE MODIFICATION OF HIGH-VOLTAGE LITHIUM OXIDE CATHODE MATERIALS
There are several modification strategies to improve the surface stability of oxide cathode materials and the interfacial stability between the electrode and electrolyte under high voltages[52-58], such as coatings, electrolyte regulation, binder design, and special surface treatments. Bulk doping[59-61] is also a well-known effective approach that focuses on enhancing the intrinsic crystal structural stability of a cathode material and is therefore not discussed in detail in this review.
Surface coating
Surface coating is the most widely used modification strategy, which has a significant effect on surface structural stability, interfacial stability between a cathode and electrolyte, and comprehensive electrochemical performance for lithium oxide cathode materials under high voltages. In contrast to bulk doping, the surface coating does not change the crystal structure of the bulk material but plays a pivotal role in the surface structure. Generally, the role of surface coating can be summarized as follows: (1) an effective projective layer to improve the surface stability, which avoids the detrimental side reaction between the cathode and electrolyte, such as metal fluorides; (2) HF scavengers to remove the byproducts from electrolyte decomposition and reduce attack on the material surface. A series of surface modifications in terms of coating material and method and electrochemical performance enhancement for various oxide cathode materials are systematically summarized in Tables 1-5[34,51,62-138] and discussed in detail in the following sections.
Summary of recent surface modifications using metal oxides and fluorides
| Coating material | Coating method | Voltage range | Capacity retention | Rate capacity (mAh g-1) at C-rate | Ref. | |
| Al2O3 | Wet-chemical | LCO | 3.0-4.5 | 82.6%, 500th at 1C | 130 at 10C | [62] |
| Al2O3 | Solid-state | NCM811 | 2.8-4.3 | 95.42%, 200th at 1C | - | [63] |
| Al2O3 | ALD | LNMO | 3.5-5.0 | - | - | [64] |
| Al2O3 | Solid-state | Li1.2Ni0.13Co0.13Mn0.54O2 | 2.0-4.8 | 86.3%, 200th at 2C | 105.6 at 10C | [65] |
| SiO2 | Solid-state | NCM811 | 3.0-4.3 | 87.3%, 100th at 0.5C | 165 at 2C | [66] |
| SiO2 | Ball milling | LNMO | 3.5-4.9 | 82.4%, 400th at 80C | 106 at 80C | [67] |
| SiO2 | Sol-gel | Li1.2Mn0.54Ni0.13Co0.13O2 | 2.0-4.8 | 89.7%, 200th at 1C | 150.4 at 5C | [68] |
| TiO2 | Magnetron sputtering | LCO | 3.0-4.5 | 86.5%, 100th at 1C | 109 at 10C | [69] |
| TiO2 | Hydrolyzation | NCM811 | - | 104.9%, 100th at 0.1C | - | [70] |
| TiO2 | Modified sol-gel | LiMn2O4 | 3.0-4.5 | 90.1%, 250th at 0.5C | - | [71] |
| Li2TiO3 | Molten salt | Li1.08Mn0.54Co0.13Ni0.13 | 2.0-4.8 | 99.7%, 125th at 0.2C | 225 at 1C | [72] |
| ZnO | ALD | NCM523 | 2.5-4.5 | 91.5%, 60th at 2C | 148.2 at 5C | [73] |
| ZnO | Sol-gel | LiNi0.8Co0.15Al0.05O2 | 2.8-4.3 | 91.3%, 100th at 0.2C | 135 at 10C | [74] |
| AZO | Wet-mixing | LCO | 3.0-4.5 | 80%, 650th at 0.2C | 96 at 8C | [75] |
| SnO2 | CVD | LCO | 3.0-4.5 | > 60%, 500th at 1C | 112 at 10C | [76] |
| SnO2 | Coprecipitation | LiNi0.8Co0.15Al0.05 | 2.8-4.3 | 70.1%, 400th at 1C | 115.2 at 2C | [77] |
| SnO2 | Solid-state | Li1.2Ni0.13Co0.13Mn0.54O2 | 2.0-4.8 | 89.9%, 200th at 1C | 189.7 at 1C | [78] |
| Fe2O3 | Ball milling | LCO | 3.0-4.5 | 92.6%, 50th at 1C | 126 at 5C | [79] |
| Co3O4 | Wet-chemical | LiNi0.8Co0.15Al0.05O2 | 2.8-4.3 | 91.6%, 100th at 1C | 145 at 10C | [80] |
| CeO2 | Wet-chemical | NCM523 | 2.8-4.6 | 57.7%, 100th at 10C | 97.4 at 10C | [81] |
| MoO3 | Precipitation | LNMO | 3.5-5.0 | 80.1%, 500th at 10C | 124 at 10C | [82] |
| MoO3/Li2MoO4 | Drying coating | NCM811 | 2.8-4.3 | 94.8%, 100th at 1C | - | [83] |
| Nd2O3 | Liquid phase coating | NCM811 | 3.0-4.4 | 88%, 100th at 1C | 164 at 8C | [84] |
| RuO2 | Wet-chemical | LNMO | 3.0-5.0 | 101.9%, 150th at 0.5C | 100 at 1C | [85] |
| La2O3 | Wet-chemical | LiNi0.91Co0.06Mn0.03O2 | 3.0-4.3 | 87.2%, 100th at 0.5C | 80.3 at 5C | [86] |
| Y2O3 | Sol-gel | NCM523 | 2.8-4.6 | 76.3%, 100th at 10C | 145.9 at 20C | [87] |
| ZrO2 | Ball-milling | NCM622 | 2.8-4.5 | 82.5%, 100th at 0.5C | 112.7 at 10C | [88] |
| AlF3 | ALD | LNMO | 3.5-4.9 | - | ~68 at 0.8C | [89] |
| AlF3 | Wet-chemical | Li1.2Ni0.13Co0.13Mn0.54O2 | 2.0-4.8 | ~100%, 50th at 0.1C | 208.8 at 1C | [90] |
| CaF2 | Coprecipitation | NCM811 | 2.7-4.3 | 85.36%, 200th at 1C | 181.7 at 5C | [91] |
| LiF | Coprecipitation | LCO | 3.0-4.5 | 71.4%, 300th at 0.1C | 141 at 4C | [92] |
| LiF | ALD | LNMO | 3.5-4.9 | ~64%, 100th at 0.1C | 43 at 1.6C | [93] |
| LiF/FeF3 | Aqueous solution | Li1.2Ni0.2Mn0.6O2 | 2.0-4.8 | 89.14%, 60th at 0.1C | 129.9 at 20C | [94] |
| LaF3 | Coprecipitation | LNMO | 3.5-4.9 | 92%, 150th at 0.1C | ~102 at 1C | [95] |
Summary of recent surface modifications using phosphates
| Coating material | Coating Technology | Voltage range | Capacity retention | Rate capacity | Ref. | |
| Li3PO4 | Magnetron sputtering | LCO | 3.0-4.5 | 79.3%, 100th at 1C | 127 at 12C | [96] |
| Li3PO4 | Sol-gel | NCM622 | 2.8-4.7 | 79.7%, 100th at 1C | 86.6 at 10C | [97] |
| Li3PO4 | Coprecipitation | Li1.18Co0.15Ni0.15Mn0.52O2 | 2.0-4.8 | 94.5%, 70th at 0.2C | 121.1 at 10C | [98] |
| AlPO4 | Sol-gel | LiNi0.7Co0.15Mn0.15O2 | 3.0-4.5 | 81.5%, 100th at 1C | - | [99] |
| AlPO4 | Sol-gel | LiNi0.8Co0.2O2 | 3.0-4.3 | 92%, 60th at 100 mA g-1 | - | [100] |
| AlPO4 | Solid-state | NCM111 | 3.0-4.6 | 95.6%, 100th at 0.5C | - | [101] |
| AlPO4 | Sol-gel | Li1.2Fe0.1Ni0.15Mn0.55O2 | 2.0-5.0 | 74.4%, 50th at 0.1C | 136.1 at 10C | [102] |
| MnPO4 | Sol-gel | LiNi0.4Co0.2Mn0.4O2 | 3.0-4.5 | 92.2%, 100th at 10C | 129.2 at 10C | [103] |
| FePO4 | Hydrothermal method | LCO | 3.0-4.7 | 92%, 50th at 1C | - | [104] |
| FePO4 | Coprecipitation | Li1.2Mn0.54Ni0.13Co0.13 | 2.0-4.8 | 95%, 100th at C/2 | 166 at 2C | [105] |
| NiPO4 | Solid-state | LiNi0.8Co0.15Al0.05O2 | 2.7-4.3 | 75%, 100th at 0.5C | 149 at 10C | [106] |
| YPO4 | Wet-chemical | LNMO | 3.5-4.9 | 77.5%, 240th at 0.1C | ~77 at 5C | [107] |
Summary of recent surface modifications using Li-ion conductors
| Coating material | Coating method | Voltage range | Capacity retention | Rate capacity (mAh g-1) at C-rate | Ref. | |
| Li1.5Al0.5Ti1.5(PO4)3 | Solid-state | LCO | 3.0-4.6 | 88.5%, 100th 0.5C | 118.6 at 5C | [108] |
| Li1.4Al0.4Ti1.6(PO4)3 | Sol-gel | LCO | 3.0-4.5 | 93.1%, 50th at 0.2C | 120 at 5C | [109] |
| Li1.5Al0.5Ge1.5(PO4)3 | Solid-state | LCO | 3.0-4.5 | 88.3%, 400th at 0.1C | 163 at 6C | [110] |
| Li1.3Al0.3Ti1.7(PO4)3 | Solution method | Li1.2Ni0.2Mn0.6O2 | 2.0-4.8 | 94.3%, 80th at 0.2C | 68.9 at 10C | [111] |
| LiZr2(PO4)3 | Sol-gel | LiNi0.82Co0.15Al0.03O2 | 2.7-4.5 | 49%, 500th at 1C | 204 at 1C | [34] |
| Li2O-BPO4 | Wet-chemical | LiNi0.87Co0.1Al0.03O2 | 2.7-4.3 | 86.83%, 100th at 1C | 140 at 10C | [112] |
| LiAlSiO4 | Sol-gel | LNMO | 3.5-4.9 | 97.1%, 150th at 1C | - | [113] |
| Li1.3Ti1.7Al0.3(PO4)3 | Sol-gel | Li1.2Mn0.54Ni0.13Co0.13 | 2.0-4.8 | -, 500th at 1C | 169.7 at 5C | [114] |
| Li2O-Al2O3-TiO2-P2O5 | Coprecipitation | LNMO | 3.5-5.0 | 83%, 100th at 2C (55℃) | 92.1 at 10C (55 ℃) | [115] |
| LiTaO3 | Solid-state | LiNi0.9Co0.06Mn0.04 | 2.8-4.3 | -, 100th at 0.2C | 140 at 10C | [116] |
| LiTi2(PO4)3 | Charge attraction | NCM622 | 2.7-4.3 | 94%, 100th at 1C | 152.1 at 5C | [117] |
Summary of recent surface modifications using conductive polymers
| Coating material | Coating method | Cathode material | Voltage range | Capacity retention | Rate capacity (mAh g-1) at C-rate | Ref. |
| PI | Thermal imidization | LCO | 3.0-4.4 | 85%, 50th at 0.5C | ~119 at 2C | [118] |
| PI/PVP | Thermal imidization | LCO | 3.0-4.4 | 85%, 80th at 0.5C | - | [119] |
| Polysiloxane | In situ hydrolysis-condensation | NCM811 | 3.0-4.3 | 91.5%, 120th at 1C | 171.4 at 5C | [120] |
| PPC | In situ photopolymerization | NCM811 | 3.0-4.5 | 102.66%, 200th at 1C | 123.1 at 10C | [121] |
| half-cyclized polyacrylonitrile | Wet-chemical | NCM811 | 2.8-4.3 | 94.24%, 100th at 1C | 133.9 at 10C | [122] |
| PANI-PEG | Wet-chemical | NCM811 | 2.8-4.3 | 92.4%, 100th at 1C | 156.7 at 10C | [123] |
| PANI-PVP | Wet-chemical | NCM811 | 2.8-4.3 | 88.7%, 100th at 1C | 152 at 5C | [124] |
| PPy | Wet-chemical | LNMO | 3.5-4.9 | 91%, 300th at 1C | 85 at 5C | [125] |
| PI | Thermal imidization | Li1.2Ni0.13Mn0.54Co0.13O2 | 2.0-4.8 | 90.65%, 50th at 0.1C | 191.5 at 2C | [126] |
| PDA | Wet-chemical | LRLO | 2.0-4.7 | ~82%, 200th at 1C | - | [51] |
Summary of recent surface modifications using electrode and other materials
| Coating material | Coating method | Voltage range | Capacity retention | Rate capacity (mAh g-1) at C-rate | Ref. | |
| LiCoPO4 | Solid-state | LCO | 3.0-4.6 | 87%, 300th at 1C | 178 at 10C | [127] |
| Li1.2Mn0.6Ni0.2O2 | Sol-gel | LCO | 3.0-4.5 | 96.4%, 100th at 0.2C | ~167 at 2C | [128] |
| Li3V2(PO4)3 | Chemical deposition | Li1.17Ni0.2Co0.05Mn0.58 | 2.0-4.8 | 90.1%, 50th at 0.2C | 153.4 at 5C | [129] |
| Li4V2Mn(PO4)4 | Solid-state | Li1.2Mn0.54Ni0.13Co0.13O2 | 2.0-4.8 | 78.1%, 200th at 1C | 157.5 at 2C | [130] |
| LiFePO4@C | Physical mixing | NCM622 | 2.7-4.6 | 84.6%, 100th at 0.5C | - | [131] |
| pyrazine-linked 2D sheet | Wet-chemical | NCM811 | 2.8-4.5 | 88.8%, 100th at 1C | 161.2 at 2C | [132] |
| PVDF | Wet-chemical | LNMO | 3.5-4.9 | 97.8%, 300th at 1C | 119.2 at 10C | [133] |
| CNT | Wet-chemical | Li1.15Mn0.55Ni0.2Co0.1O2 | 2.0-4.8 | 92.3%, 200th at 1C | 146 at 10C | [134] |
| Nd0.6Sr0.4CoO3 | Sol-gel | Li1.2Mn0.54Ni0.13Co0.13O2 | 2.0-4.8 | 91%, 300th at 1C | 53 at 50C | [135] |
| LiBH4 | Wet-chemical | LCO | 3.0-4.6 | 95.1%, 100th at 1C | 173 at 5C | [136] |
| LiAlH4 | Wet-chemical | LCO | 3.0-4.6 | 70.4%, 1000th at 1C | 168.9 at 10C | [137] |
| WSe2 | CVD | LNMO | 3.5-4.9 | 99%, ~400th at 1C | - | [138] |
Metal oxides, fluorides and phosphates
Considering the simple preparation and high chemical stability of metal oxides, a series of metal oxides, such as Al2O3, SiO2, TiO2, ZnO, SnO2, and ZrO2, have been widely studied as surface coatings of oxide cathode materials. They can effectively protect oxide cathode materials from liquid electrolytes and hence reduce the side reactions between them, eventually resulting in prominently improved electrochemical performance, including superior cycling stability and rate capability under high-voltage operation. There are several coating methods used, including wet-chemical, ALD, magnetron sputtering, and so on. Although the wet-chemical coating method is easy to operate, it is difficult to control the uniformity and thickness of the coating layer. In contrast, control is simple with ALD, but it has a higher cost and is impractical for large-scale applications. The magnetron sputtering approach combines the advantages and disadvantages of these two methods.
Zhou et al. proposed a low-cost and environmentally-friendly wet-chemical method to coat Al2O3 on LCO using only an Al2(SO4)3 aqueous solution via a hydrolysis reaction and strong adsorption of intermediate Al(OH)3 on the LCO surface[62]. In sharp contrast to bare LCO, the modified LCO electrode exhibited outstanding high-voltage performance under 4.5 V, including excellent cycling stability (82.6% of capacity retention after 500 cycles at 1C) and preeminent rate capability (130 mAh g-1 at 10C). This can be explained by the fact that the Al2O3 coating can not only suppress electrolyte decomposition under high voltages but also decreases the irreversible CEI/SEI formation. Additionally, Nie et al. reported a simple wet-mixing method to obtain a uniform AZO coating for LCO to achieve superior long-term cycling stability and rate capability simultaneously, even under 4.5 V[75]. Furthermore, due to the establishment of a stable surface and SEI layer, the phenomenon of surface degradation and Co dissolution can be significantly inhibited for AZO-coated LCO, as shown in Figure 2A. Excellent ionic and electronic conductivity were found for the AZO-coated LCO.
Figure 2. Metal oxide and fluoride surface modification for lithium oxide cathode materials. (A) Schematic of the working mechanism of AZO coating layer and improved cycling stability. Reproduced from Ref.[75] with permission from Elsevier. (B) Cyclic performance enhancement of in situ ZrO2-coated NCM622 cathode and scanning electron microscopy (SEM) images after 100 cycles for NCM622 with and without the coating. Reproduced from Ref.[88] with permission from Elsevier. (C) Schematic of TiO2 coating process for
Similar to the surface coating of LCO, other oxide cathode materials can also benefit from these coating materials, methods and mechanisms. As shown in Figure 2B, Yao et al. recently reported a thin amorphous ZrO2 layer that was generated in situ on a Ni-rich cathode (NCM622) surface using a Zr-based metal-organic framework (UiO-66) as a coating precursor (u-NCM622)[88]. This could provide a significant amount of active sites for fast Li+ diffusion and effectively inhibit severe side reactions and microcrack generation to maintain the structural integrity upon cycling. As a result, significant improvements in electrochemical performance for u-NCM622 cathode materials can be achieved for large-scale practical applications in high-performance LIBs.
For spinel LiMn2O4, a uniform, continuous and porous nanosized TiO2 coating material was successfully developed by Zhang et al. via a modified sol-gel method based on a kinetically-controlled coating process[71]. The prepared TiO2 layer covering the LiMn2O4 cathode surface is so robust that it is able to greatly inhibit Mn dissolution and capacity deterioration during long-term cycling, even at elevated temperatures and voltage windows. Otherwise, for the traditional chemical coating method, it usually fails to completely coat the surface of active materials, inevitably leading to continuous degradation of the materials during the repeated charging and discharging process [Figure 2C].
Likewise, metal oxides also have been successfully introduced into Li-rich cathode material systems to mitigate their intrinsic issues, e.g., fast capacity and voltage decay. Recently, Zhai et al. reported a Li-rich layered oxide (Li1.2Mn0.54Ni0.13Co0.13O2) decorated with a thin SiO2 layer to enhance its structural and electrochemical stability[68]. In addition, the surface modification mechanism of the SiO2 coating layer was also explained in detail, as shown in Figure 2D. After chemically grafting SiO2 on the active material through the hydrogen bond interaction between -Si-OH from the hydrolysis of tetraethyl orthosilicate and
Considering the general reaction between metal oxides and HF from electrolyte decomposition (e.g.,
Like the metal oxides and fluorides mentioned above, a variety of metal phosphates[96,99,103], including Li3PO4, AlPO4, MnPO4, FePO4, and Ni3(PO4)2, with inferior electronic conductivity but superior ionic conductivity and chemical stability have been extensively exploited as typical surface coating layers for improving the stability of lithium oxide cathode materials under extended operating voltage windows and temperatures. The underlying mechanisms of the electrochemical performance enhancement for various lithium oxide cathode materials with phosphate coatings have been explored in detail. Typically, Li3PO4 is proved to act as an ideal coating layer on the surface of LCO, which not only induces a spinel phase by ALD to accelerate Li+ transport but also protects the surface by effectively hindering the interfacial side reactions and suppressing the irreversible oxygen release. Similarly, AlPO4 coating films have also been introduced to modify LCO, and their features are responsible for the significant enhancement in electrochemical performance.
Inorganic fast Li-ion conductors
Even though advanced progress has been made through extensive surface modification strategies, most surface coating materials exhibit poor Li+ and electron transport and limited structural stability simultaneously. Therefore, significant efforts are still required to develop novel surface coating materials that have both fast ion transport paths and good chemical stability. Inorganic fast Li-ion conductors mainly include solid electrolytes, such as Li1.4Al0.4Ti1.6(PO4)3 and LiTi2(PO4)3, and have also been intensively researched as coating materials to endow oxide cathodes with improved structural stability and electrochemical performance.
For example, as displayed in Figure 3A, a facile solid-state method was developed to modify a commercial bare LCO sample with Li1.5Al0.5Ti1.5(PO4)3 (LATP) (LATP@LCO) nanoparticles[108]. Further studies explored the effects of different annealing temperatures on the electrochemical performance and demonstrated that the LATP@LCO sample with an annealing temperature of 700 ℃ (LATP@LCO-700) exhibited the best cycling stability, which may originate from the surface structure under different annealing temperatures. It was found that a series of continuous reactions between LCO and LATP occur as the temperature increases, and there are only the olivine Li3PO4 phase and spinel species (Co3O4, CoAl2O4, and Co2TiO4) on the LATP@LCO-700 surface, as depicted in Figure 3B. Schematic diagrams of an in situ formed thin and uniform surface layer with the detailed composition and structure are presented in Figure 3C. On the one hand, the spinel phase and layered LCO are able to combine closely because of the coherent lattice structure between them. The spinel structure is well known to be more stable than the layered structure at high voltages, which is advantageous to the structural and electrochemical stability enhancement. On the other hand, the outer Li3PO4 species is not only a good Li+ conductor but also has superior chemical and thermal stability, even at high temperatures and voltages. Consequently, the modified LCO with LATP through the method developed above can deliver visibly improved cycling stability with 88.3% and 72.9% capacity retentions at 0.5C after 100 cycles at 25 and 45 ℃, respectively, while only 50.3% and 32.5% were achieved for bare LCO. Furthermore, a higher discharge capacity of 118.6 mAh g-1 was exhibited for the LATP@LCO-700 electrode at 5C, with only 68.5 mAh g-1 achieved for bare LCO, thereby confirming a remarkably modified rate capability.
Figure 3. Inorganic fast Li-ion conductor surface modification for lithium oxide cathode materials. (A) Schematic of LATP surface engineering for LCO. (B) Phase compositions of LCO and LATP at variable temperatures. (C) Schematic of surface layer growth mechanisms. Reproduced from Ref.[108] with permission from Wiley-VCH. (D) Influence of LBO coating on Li+ insertion reaction based on activation energies. Reproduced from Ref.[139] with permission from the Royal Society of Chemistry. (E) Cycling properties for NCA and NCA@LZP-1%. Reproduced from Ref.[34] with permission from the American Chemical Society. LCO@LBO: Li2B4O7-coated LiCoO2.
In addition, the solid electrolyte Li2B4O7[139] (LBO) has also been applied as a Li+-conductive coating layer for LCO to enhance its kinetic properties, and the working mechanism was investigated by electrochemical impedance spectroscopy. The calculated activation energies at different temperatures were used to explain the influence of the LBO coating on the discharge reaction [Figure 3D]. It was found that the amorphous LBO coating enables LCO to exhibit significantly lower activation energy for the desolvation process than bare LCO. In another study, a self-assembled fast ionic conducting LiZr2(PO4)3 (LZP) nanolayer was introduced for the surface modification of Ni-rich LiNi0.82Co0.15Al0.03O2 (NCA) via an electrostatic charge attraction[34]. It was noted that the NCA and LZP precursor particles have opposite charges according to zeta potential measurements, which allowed the LZP precursor particles to adhere homogeneously to the NCA surface and successfully construct a representative core-shell microstructure. As expected, NCA with 1 wt.% LZP (NCA@LZP-1%) could maintain the highest capacity of 120 mAh g-1 at 1C after 300 cycles with a voltage window of 2.7-4.3 V, while only after 150 cycles could the same capacity be reached for the pristine NCA [Figure 3E]. These findings clearly demonstrate the effectiveness of this fast ionic conductor with the charge attraction method.
Conductive polymers
Despite a large number of studies having proved that inorganic modification layers, such as metal oxides, fluorides, phosphates, and fast ionic conductors, play an important role in improving electrochemical performance, there are still some issues to be further solved, including uneven coverage, limited ion and electron transport, complex and expensive coating methods and coatings that can easily be removed under extreme conditions. In order to overcome the difficulties faced by the abovementioned inorganic surface coatings, conductive polymers, like PI, have attracted significant interest and have also been introduced as prominent coating materials to modify lithium oxide cathodes for advanced next-generation LIBs. PI has been employed as a novel surface coating material for high-voltage LCO and the effects of the PI encapsulating layer thickness on the electrochemical performance under high voltage, and interfacial exothermic reactions between the LCO cathode and liquid electrolyte were investigated[118]. As depicted in Figure 4A, with increasing PI layer thickness from 10 to 40 nm, although the interfacial side reactions can be effectively suppressed, Li+ transport is reduced, which results in poor rate and cycling performance. Therefore, LCO with a ~10 nm PI thin film can attain a well-balanced result between electrochemical performance and thermal stability.
Figure 4. Conductive polymer surface modification for lithium oxide cathode materials. (A) PI coating layers with different thicknesses and their effect on interfacial side reactions and ion transport. Reproduced from Ref.[118] with permission from Elsevier. (B) Schematic of specific synthesis of NCM@PPC and improved cycling stability. Reproduced from Ref.[121] with permission from Elsevier. (C) Comparison of surface film and charge-transfer resistance evolution during cycling. Diffusion coefficients calculated from GITT curves during (D) delithiation and (E) lithiation process. Reproduced from Ref.[124] with permission from the American Chemical Society. PI(0.3)-LCO, PI(0.5)-LCO and PI(1.0)-LCO: LCO with 0.3, 0.5 and 1.0 wt.% coating solutions, respectively; NCM811@PANI: PANI-modified NCM811; NCM811@PANI-PVP: PANI and PVP-modified NCM811; Rsf: surface film resistance; Rct: charge-transfer resistance; GITT: galvanostatic intermittent titration technique.
In addition, due to its functions of HF scavenging and polarity tuning, PVP is well designed to be combined with PI to form a multifunctional PI/PVP semi-interpenetrating polymer network on the LCO surface[119]. As expected, PI/PVP-modified LCO exhibits enhanced electrochemical performance and thermal stability under a high working voltage compared to single PI- or PVP-coated LCO and pristine LCO. Similarly, a PPC coating layer was in situ constructed on a Ni-rich NCM811 cathode material to form a sweater-like cross-linked network skeleton via a UV-induced photopolymerization self-assembly process[121], as shown in Figure 4B. The PPC-modified NCM (NCM@PPC) electrode exhibited a remarkable electrochemical stability enhancement in a hostile environment with a high cutoff voltage of 4.5 V and operating temperature of 60 ℃, even after long-term cycling of 500 cycles. This performance may be attributed to the enhanced electronic and ionic conductivity provided by the PPC co-polymer, particularly the cyano groups of citral nitrile, which can effectively ameliorate the problem of transition metal dissolution by coordination and ensure the formation of a continuous and uniform coating layer. Gan et al. reported a PVP surfactant-induced uniform PANI conductive coating layer for NCM811 surface modification, which can relieve the issues of particle rupture and irreversible side reactions due to the strong hydrogen bond and coordination interactions, thereby accelerating the interfacial dynamics, as shown in Figure 4C-E[124].
Other special surface coating materials
Some representative electrode materials, such as Li4V2Mn(PO4)4 and LiFePO4, can be adopted to modify lithium oxide cathodes with the aim of improving the electrochemical performance, particularly for long cycling stability under difficult conditions. Li3V2(PO4)3 is a well-known cathode material for LIBs due to its high average operating voltage (> 4.0 V) and specific capacity (197 mAh g-1), superior Li+ kinetics, and excellent structural and cyclic stability. Consequently, it is reasonable to conclude that a Li3V2(PO4)3-modified spinel LNMO cathode is capable of delivering the accelerated Li+ diffusion process, suppressed surface structural deterioration and interfacial side reactions, and depressed interfacial resistance[140], as depicted in Figure 5A. These are responsible for the satisfactory improvement in electrochemical performance; for example, the ever-increasing Rsf and Rct can be suppressed very well [Figure 5B], the capacity retention is as high as 87.8%, even after 500 cycles [Figure 5C], and the reversible specific capacity maintained at 90.4 mAh g-1, even at an extremely high rate of 20C. Moreover, the reduced dissolution of transition metal ions in the LNMO active material is clearly shown in Figure 5D. Likewise, Li4V2Mn(PO4)4 was introduced successfully to act as a coating layer on the surface of a Li-rich Li1.2Mn0.54Ni0.13Co0.13O2 cathode[130]. Through a series of experimental characterizations combined with theoretical calculations, it has been demonstrated that this surface modification not only reduces residual Li species, like LiOH and
Figure 5. Phosphate-based electrode surface modification for lithium oxide cathode materials. (A) Schematic of the underlying mechanism for a LVPO-modified LNMO particle. (B) Evolution of Rsf and Rct for pristine and modified LNMO during charge-discharge cycling. (C) Cycling performance for LNMO with or without LVPO. (D) Color changes of electrolytes with increasing soakage times for pristine and 1 wt.% LVPO-modified LNMO. Reproduced from Ref.[140] with permission from the American Chemical Society. LVPO:
Very recently, a simple and targeted masking modification strategy via LiFePO4@C nanoplates was proposed to function with the reduced active sites by means of the preferential adsorption of PO43- at transition metal sites, resulting in significant improvements in both electrochemical and thermal stability[131]. In addition to the representative electrode materials discussed above as coating layers, many carbon-based materials can also be utilized as surface modification layers on lithium oxide cathodes. It has been demonstrated that oxidized carbon nanotubes work as a coating layer on the spinel LNMO surface[141] and can suppress adverse side reactions and thus improve the rate and cycling capability.
In addition, the synergetic effects of multiple coating materials on lithium oxide cathode materials are also significant. For instance, a combination of both ionic and electronic conductive modification with
Electrolyte regulation
Surface modification by decorating the surface of active materials with various coating layers has significantly alleviated surface degradation for high-voltage oxide cathodes. Considering the existing significant interface issues, the design of novel electrolyte systems compatible with high-voltage cathode materials remains essential. It is well known that electrolytes are generally composed of lithium salts, like LiPF6, LiBF4, LiTFSI, and LiFSI, organic solvents, such as ethylene carbonate (EC), diethyl carbonate (DEC) and ethyl methyl carbonate (EMC), and additives, like fluoroethylene carbonate (FEC) and vinylene carbonate (VC)[143,144]. On the one hand, introducing novel functional additives into conventional carbonate-based electrolyte systems is popular. Table 6[33,145-163] summarizes a number of recently reported additives for high-voltage lithium oxide cathode materials. On the other hand, an alternative approach is to optimize conventional carbonate-based low concentration electrolytes (LCEs) by increasing the salt concentration to generate high concentration electrolytes (HCEs) or localized high-concentration electrolytes (LHCEs). In addition, other high-voltage electrolyte systems, such as ionic liquids and sulfone-, nitrile- and fluorinated carbonate-based electrolytes, have also been proposed and widely studied.
Summary of electrolyte additives for high-voltage lithium oxide cathode materials
| Electrolyte additive | Cathode material | Modification mechanism | Voltage range | Capacity retention | Ref. |
| TFPN | LCO | Form a protective layer to suppress the decomposition of electrolyte solvent and salt | 3.0-4.4 | 90.6%, 100th at 0.5C | [145] |
| ATCN | LCO | In situ construct a stable CEI film | 3.0-4.5 | 91%, 200th at 1C | [146] |
| TCEB | LCO | Form a CEI protective film | 2.8-4.5 | 78.2%, 200th at 1C | [147] |
| BA | NCM811 | Construct a robust CEI film and capture HF/H2O effectively | 3.0-4.5 | 84%, 200th at 1C | [148] |
| LiPO2F2 | NCM111 | Generate a steady low impedance CEI film | 3.0-4.5 | 48%, 1000th at 10C | [149] |
| DPDMS | NCM622 | Modify CEI layer through scavenging HF and PF5; promote thermal stability | 2.8-4.3 | 93.3%, 200th at 2C (55 ℃) | [150] |
| IPTS | NCM622 | Form a uniform and dense CEI film; react with water | 2.5-4.5 | 73.1%, 150th at 0.3C | [151] |
| TPB | NCM721 | Remove residual lithium species from Ni-rich cathode surface and create effective CEI layer | 3.0-4.3 | 88.6%, 100th at 1C | [152] |
| TAPi | NCM622 | Form an interfacial film to protect the electrode surface | 2.8-4.2 | 91.7%, 50th at 1C | [153] |
| TIB | NCM622 | Form a CEI to inhibit the electrolyte decomposition and dissolution of transition metal ions | 3.0-4.5 | 82.7%, 300th at 1C | [154] |
| AIP | NCM811 | Induce in situ polymerization of EC to fabricate a robust CEI | 2.7-4.3 | 97.8%, 200th at 1C | [155] |
| ETFB | LiNi0.7Co0.15Mn0.15O2 | Construct protective layers on both electrodes | 3.0-4.4 | 84.8%, 300th at 0.5C (45 ℃) | [156] |
| SA | NCM811 | Form a uniform and robust CEI layer | 2.8-4.3 | 93.8%, 400th at 1C | [157] |
| EGBE | Li1.2Mn0.54Ni0.13Co0.13O2 | Form a stable and robust CEI layer | 2.0-4.8 | 89%, 150th at 0.5C | [158] |
| BTMSC | Li1.2Mn0.55Ni0.15Co0.1O2 | Build a protective film on the cathode and eliminate HF | 2.0-4.8 | 72%, 200th at 0.5C | [159] |
| PFPDPP | LNMO | Stabilize LiPF6 salt; form a good CEI film | 3.0-5.0 | 71%, 300th at 2C | [160] |
| TMSOMs | Li1.2Ni0.16Co0.08Mn0.56O2 | Form an effective protection layer to remove fluoride species in electrolyte | 2.0-4.6 | Over 77%, 100th at 0.5C | [161] |
| SN | Li1.2Ni0.2Mn0.6O2 | Have good thermal stability and wide electrochemical oxidation window and form a CEI layer | 2.0-5.0 | ~89.5%, 50th at 0.1C | [162] |
| MA-C60 | Li1.17Ni0.17Mn0.5Co0.17O2 | Eliminate undesirable reactive substances and tune interfacial structures | 2.0-4.5 | 86%, 100th at 0.5C | [163] |
| LiNO3 and TPFPB/TPFPP | NCM811 | Form a robust SEI and amorphous CEI | 2.8-4.3 | 78.5%, 100th at 0.3C | [35] |
Overall, electrolyte additives have been intensively studied and draw considerable attention due to their unique effect on interfacial stability, even with very small concentrations. In particular, most additives can be preferentially oxidized to generate stable CEI/SEI layers to restrain the continuous electrolyte decomposition and further mitigate the dissolution of transition metal ions from active materials, thus leading to improved electrochemical performance. In contrast, some additives can also coordinate with transition metal ions and mitigate side reactions between the electrode and electrolyte. As demonstrated in Figure 6A, Kim et al. introduced ethyl 4,4,4-trifluorobutyrate (ETFB) into the baseline electrolyte 1.15 M LiPF6 in EC/EMC/DEC (2:5:3 in volume) as a bifunctional additive to stabilize the interfacial structure by establishing uniform and stable protective layers on the surface of a Ni-rich LiNi0.7Co0.15Mn0.15O2 cathode and graphite anode in full cells[156]. Specifically, ETFB delivers a higher highest occupied molecular orbital energy and can undergo oxidative decomposition to form the corresponding radical species and then react with EC molecules to induce the formation of a CEI layer with fluorine (-CF3-)-containing compounds on the surface of the NCM cathode. The ETFB-derived CEI is so robust that it can effectively ameliorate the intractable issues of continuous electrolyte decomposition, severe rupture of secondary particles, and significant dissolution of transition metal ions during repeated charge-discharge cycling.
Figure 6. Representative electrolyte additives for high-voltage lithium oxide cathode materials. (A) Schematic of ETFB-derived interfacial layers with favorable effects on both NCM cathode and graphite anode. Reproduced from Ref.[156] with permission from Elsevier. (B) Schematic of in situ constructed CEI on LCO cathode by ATCN additive. (C) Cycling performance of LCO/Li coin half-cells with and without ATCN in the base electrolyte for 200 cycles at 1C and 3.0-4.5 V. (D) Cycling stability of commercial LCO/graphite pouch cells with and without 0.2% ATCN at 0.5C and 3.0-4.45 V. Reproduced from Ref.[146] with permission from Elsevier.
Furthermore, ETFB can function as an electron acceptor to undergo reductive decomposition at the graphite anode due to its lower energy of lowest unoccupied molecular orbital than EC, EMC, and DEC. Its reactive intermediate species can induce the decomposition of EC molecules and further decomposition to generate fluorine (-CF2-)-containing compounds and LiF, which constitute a SEI layer. Therefore, the unwanted self-discharge of the charged graphite anode and the deposition of transition metal ions can be decreased by the ETFB-derived SEI on the surface of the anode. Consequently, the full cell based on the 1% ETFB-modified electrolyte is capable of exhibiting a remarkably enhanced capacity retention of 84.8% with a high CE of above 99.8%, even after 300 cycles at 45 ℃.
Recently, using a similar method, Ruan et al. used 5-acetylthiophene-2-carbonitrile (ATCN) to serve as a novel electrolyte additive, which can construct a CEI thin film with high stability and low impedance on LCO in situ[146]. Figure 6B shows the detailed mechanism of the in situ formed CEI on the LCO cathode by the ATCN additive. ATCN is oxidized to generate an acetyl cation (CH3CO+) and thiophenecarbonitrile radical (TCN∙). The former can react with HF, Li2O, and Li2CO3 to ultimately form stable CH3COOLi and LiF species as the inner layer of the CEI film. In contrast, the latter reacts to generate thiophene-containing organic polymers, which constitute the outer layer of the CEI film with excellent ionic and electronic conductivity. As a result, distinguished cycling stability and rate capability could be achieved for LCO under high voltage due to the unique in situ constructed artificial CEI film with both inorganic and organic components. In particular, the cycling performance of LCO/Li coin half cells with and without ATCN in the base electrolyte is displayed in Figure 6C, indicating that LCO with a 0.2% ATCN-containing electrolyte exhibited the highest capacity retention of 91% after 200 cycles at 1C and 3.0-4.5 V. Moreover, the excellent cycling stability of commercial LCO/graphite pouch cells with 0.2% ATCN was also shown at 0.5C and 3.00-4.45 V [Figure 6D]. These results prove that this novel electrolyte additive strategy has excellent application prospects.
In addition to the application of individual additives, there have been several studies featuring the simultaneous introducing of several additives into conventional electrolytes. For example, nitrile (suberonitrile or 1,3,6-hexanetricarbonitrile) and FEC co-additives have been reported to synergistically modify an electrolyte to achieve a high-voltage LCO cathode with high energy density[164]. It is well known that nitrile additives can be widely applied to widen the electrochemical window of electrolytes. It was proved that the cyano groups of nitriles can coordinate strongly with transition metal ions to suppress the side reactions between the cathode and electrolyte. Simultaneously, a FEC additive is considered to be helpful for the generation of a uniform CEI thin film. If only nitriles or FEC are introduced alone, there is still no significant improvement in the electrochemical performance.
In addition to introducing novel additives, some other electrolytes, including HCEs, LHCEs, ionic liquid electrolytes, and sulfone-, nitrile- and fluorinated carbonate-based electrolytes, have also been developed to improve the structural and interfacial stability for high-voltage lithium oxide cathodes in LIBs. Generally, HCEs and ionic liquids show high viscosity and cost. Sulfone- and nitrile-based electrolytes show superior oxidation stability but inferior compatibility with lithium metal or graphite anodes. Although fluorinated carbonate-based electrolytes display high thermal and electrochemical stability, they are high in cost. Typically, in recent years, an innovative concept of LHCEs has been proposed and widely developed. This is mainly due to the fact that LHCEs have the advantages of both HCEs and LCEs with an optimized Li+ solvation structure and low viscosity and cost. Accordingly, LHCEs are able to endow LIBs with lithium oxide cathodes that exhibit superior structural and electrochemical stability under high cutoff voltage. Generally, LHCEs can be obtained by adding a dilute solvent [like 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluropropyl ether (TTE)] into HCEs. For example, Zhang et al. designed an advanced LHCE made up of a LiFSI salt, 1,2-dimethoxyethane (DME) solvent, and TTE dilute solvent (1:1.2:3 in molar ratio) for a Ni-rich LiNi0.94Co0.06O2 cathode[165]. It was noted that the unique LHCE not only created a uniform LiF-rich CEI to significantly alleviate the structural degradation of the cathode, but also formed a robust SEI to protect the anode from corrosion from the electrolyte. Consequently, the LHCE endowed the Ni-rich cathode material with a remarkably improved electrochemical stability (81.4% capacity retention after 500 cycles) and excellent fast charging ability (209 mAh g-1 at 3C), thereby achieving high energy and power density for next-generation LIBs with wide working voltage and temperature windows.
Binder design
Electrolyte regulation has been proved to play a crucial role in optimizing structural and interfacial stability and electrochemical performance under high working voltages and wide operating temperature ranges for the practical applications of high energy density LIBs. Furthermore, other battery components, like binders, can also be optimized for high-voltage operation. Although binders make up a very small part of the overall battery components (less than 5%), they are still indispensable due to their prominent role in maintaining good connections between the active materials, current collector, and conductive agents. Significant progress has been made in novel functional binder design for next-generation high-performance LIBs. Table 7[33,166-178] lists a number of representative binders for high-voltage lithium oxide cathode materials, including their advantages or working mechanisms and electrochemical performance in LIBs. According to the literature, an ideal functional binder design should meet several principles: (1) high adhesion and cohesion through chemical or physical interactions, which can make electrode components combine closely, even during long-term cycling; (2) excellent chemical and electrochemical stability, which make the electrode work with wide electrochemical windows; (3) outstanding dispersive capability, which allows the electrode components homogeneous distribution; (4) superior electronic or ionic conductance, which is helpful to improve diffusion kinetics. In addition, some binders with polar groups are able to coordinate with transition metal ions to mitigate their dissolution and further enhance the electrochemical performance. Some binders with special groups, like phenol, are capable of scavenging free radicals. The following section reviews a number of aqueous and non-aqueous binders for high-voltage lithium oxide cathodes in LIBs.
Summary of selected novel binder designs for high-voltage lithium oxide cathode materials
| Binder | Cathode material | Voltage range | Advantages | Capacity retention | Rate capacity (mAh g-1) at C-rate | Ref. |
| DSL | LCO | 2.8-4.6 | Generate an artificial CEI and enhance the stability of Co-O bonds | 93.4%, 100th at 0.5C | - | [166] |
| SBR | LCO | 3.0-4.5 | High adhesion strength and flexibility | 93.9%, 50th at 1/8C | 150 at 4C | [167] |
| PAA | NCM811 | 3.0-4.3 | Decrease pH of the slurry and improve the electrode adhesion | 84.2%, 100th at 0.2C | 160 at 2C | [168] |
| BBP | NCM811 | 3.0-4.2 | Well-balanced amphiphilicity and bottlebrush-based structural uniqueness | 80.6%, 240th at 0.2C | 150 at 3C | [33] |
| PEO | NCM622 | 2.8-4.2 | High ionic conductance and flexibility, improve the adhesion and electrode integrity | 96.1%, 100th at 1C | 147.3 at 2C | [169] |
| PI-FTD | NCM811 | 2.7-4.4 | in situ build surface protective PI layer; non-flammable | 79%, 100th at 0.2C | ~82 at 10C | [170] |
| P(MVE-LMA) | LNMO | 3.5-5.0 | Superior adhesion and cohesion capabilities; interact with transition metal cations | 92%, 400th at 1C | 111.8 at 10C | [171] |
| γ-PGFO | LNMO | 3.5-5.0 | High-voltage resistant and favorable cathode interfacial stability property, superior coordination capability | 88.2%, 500th at 1C | 108.1 at 5C | [172] |
| CCTS/PEO | LNMO | 3.5-4.9 | Show a high electrochemical oxidation potential of above 5.0 V, improved mechanical properties | 81.4%, 200th at 0.5C | ~70 at 2C | [173] |
| LiPAA and Na-Alg | LNMO | 3.5-5.0 | Enhance cathode adhesion and cohesion; create an effective passivating CEI layer | 97.4%, 100th at 0.5C | - | [174] |
| Lignin | LNMO | 3.5-5.0 | Have numerous phenol groups; scavenge free radicals and generate compatible interfaces | 94.1%, 1000th at 1C | - | [175] |
| FPI | LMNC | 2.5-4.7 | Superior binding ability and form a robust surface structure | 89%, 100th at 0.2C (55 ℃) | - | [176] |
| XG | Li1.144Ni0.136Co0.136Mn0.544O2 | 2.0-4.8 | Have a unique double-helix superstructure with abundant charged functional groups | 98.4%, 200th at 0.1C | - | [177] |
| Al3+-doped Na-Alg | Li1.16Mn0.6Co0.12Ni0.12O2 | 2.0-4.8 | Form a coating layer on the surface of the primary particles | 99.9%, 150th at 0.5C | ~150 at 5C | [178] |
Aqueous binders
Aqueous binders, primarily carboxymethyl cellulose, have been widely applied in battery systems. However, when used in high-voltage lithium oxide electrode material systems, there are usually some issues, e.g., the surface structure of layered lithium oxide materials may be damaged in the water slurry because of the irreversible reaction with H2O molecules. Therefore, it is essential to reasonably design novel functional binders with a unique structure to withstand high voltages.
Recently, an innovative modification strategy has been developed to enhance the cycling stability of LCO under high cutoff voltages using a DSL binder instead of the traditional PVDF binder[166]. Figure 7A characterizes the different interactions between a LCO cathode and two binders and the effects on surface stability during repeated cycling. Due to strong hydrogen bonds, the DSL binder contributes to generating a uniform coating layer in situ, which can provide important protection against damage from electrolyte decomposition. In addition, the sulfate acid groups can be used to enhance the stability of Co-O bonds, which is beneficial in suppressing the phase transition under high voltage. As a result, LCO electrodes with PVDF and DSL binders (PVDF-LCO and DSL-LCO) can exhibit capacity retentions of 61.5% and 93.4% after 100 cycles at 0.5C, respectively, demonstrating a conspicuous improvement in cycling stability [Figure 7B].
Figure 7. Representative aqueous binders for high-voltage lithium oxide cathode materials. (A) Schematic of different interactions between LCO and PVDF/DSL binders and their effects on surface stability. (B) Cycling performance of LCO electrodes with PVDF and DSL binders at 0.5C and 2.8-4.6 V. (C) In situ X-ray diffraction measurements with (003) diffraction peak during the first charge-discharge cycle at 0.1 C. (D) Crystal structure under high voltage. (E) CV curves. (F) SEM images (cross-sectional view) after 50 cycles for two LCO electrodes. Reproduced from Ref.[166] with permission from Wiley-VCH. (G) Schematic of generation of the γ-PGFO binder with chemical structure. Reproduced from Ref.[172] with permission from Springer Nature. (H) Schematic of effects of P(MVE-LMA) and PVDF binders on LNMO electrode during cycling. Reproduced from Ref.[171] with permission from the Royal Society of Chemistry.
Furthermore, in situ X-ray diffraction measurements of PVDF-LCO and DSL-LCO electrodes were performed to reveal the crystal structural evolution of LCO with different binders. The distinctive (003) peak shifts above 4.55 V are marked by dashed squares in Figure 7C, and the DSL-LCO electrode delivers a smaller peak movement, confirming that the irreversible phase transformation of LCO from O3 to H1-3 under high voltage can be greatly suppressed in comparison with the PVDF-LCO electrode [Figure 7D]. These results are also consistent with the cyclic voltammetry curves in Figure 7E. Both of these electrodes demonstrate two couples of anodic and cathodic peaks at 3.9 and 4.5 V, which represent the benign phase transition between two O3 phases and the malignant phase transition from O3 to H1-3, respectively. Moreover, it can be observed that the peaks under a higher voltage are well suppressed for the DSL-LCO electrode. In addition, according to the cross-section SEM images of both the cycled PVDF-LCO and DSL-LCO electrodes in Figure 7F, the former shows apparent microcracks while the latter can maintain an intact morphology with a flat and smooth surface.
A unique binder design was also considered as a feasible strategy to improve the instability of electrode materials under high-voltage operation. A well-designed amino acid-based binder (γ-PGFO) was applied to achieve superior high-voltage performance for a spinel LNMO cathode[172]. As shown in Figure 7G, IH,1H,9H,9H-perfluoro-1,9-nonanediol functioning as a high-voltage resistant block with a well-matched energy level was introduced and grafted to a poly(γ-glutamic acid) backbone with a powerful coordination interaction with transition metal ions. Therefore, the synergistic effect from the unique binder can endow the LNMO cathode with outstanding electrochemical properties, including an enhanced capacity retention of 88.2% after 500 cycles at 1C and 3.5-5.0 V. In addition, similarly, the group of Cui[171] also reported a novel water-soluble binder [P(MVE-LMA)], which can deliver excellent adhesion properties with both active materials and an Al current collector. Furthermore, a stable CEI layer can be formed to effectively suppress the electrolyte decomposition and transition metal dissolution [Figure 7H]. However, in comparison, the LNMO cathode with a conventional PVDF binder goes through severe aggregation of electrode components, generation of an irregular CEI layer, severe electrolyte oxidative decomposition, and Mn dissolution from cathode materials.
Not-aqueous binders
According to recent reports and the above discussions, aqueous binders have made significant progress regarding the structural design and working mechanisms for high-performance lithium oxide cathodes in LIBs. In addition to aqueous binders, non-aqueous binders have also been widely investigated to obtain high-performance lithium oxide cathode materials under high working voltage. For example, FPI[176] was synthesized as an innovative high-voltage binder via the approach shown in Figure 8A. According to the chemical structure of FPI, PI with heterocyclic imide rings is well known to provide superior thermal stability, while the oxidation resisting -F or -CF3 groups are introduced to further improve the stability under high-voltage operation. The specific surface structure changes for Li1.13Mn0.463Ni0.203Co0.203O2 (LMNC) combined with PVDF and FPI binders (PVDF-LMNC and FPI-LMNC) are demonstrated in Figure 8B and C, respectively. Obviously, the PVDF-LMNC electrode inevitably suffers from a series of issues, including transition metal dissolution, oxygen loss, electrolyte decomposition, and particle microcracking during repeated cycling. In sharp contrast, for FPI-LMNC, there is a uniform thin film on the surface of the electrode active materials via -COO…metal (M) bonds, which help to restrain these harmful side reactions mentioned above, thus contributing to the electrochemical performance enhancement, even in harsh conditions.
Figure 8. Representative non-aqueous binders for high-voltage lithium oxide cathode materials. (A) Synthesis of FPI. Schematic of changes in surface structure for (B) PVDF-LMNC and (C) FPI-LMNC electrodes. Reproduced from Ref.[176] with permission from Wiley-VCH. (D) Chemical structure of PI-FTD binder and its interaction with NCM811 cathode surface. (E) Mechanism of crack formation for NCM811 electrodes with PVDF and PI-FTD during cycling. Comparison of electrochemical performance: (F) cycling performance; (G) energy density; (H) rate capability. Reproduced from Ref.[170] with permission from Elsevier.
This research team also introduced a novel functional binder (PI-FTD) into layered lithium ternary oxides system to improve their electrochemical performance for practical applications[170]. As illustrated in Figure 8D, the carboxylic acid group (-COOH) from the PI-FTD binder can be chemically grafted onto the surface of a NCM811 active material by covalently binding/combining with transition metal ions to build a PI-FTD coating layer in situ. The PI-FTD coating can function as a surface protective layer to ensure the integrity of active particles and avoid serious crack generation and electrolyte decomposition. However, for the NCM811 cathode combined with a traditional PVDF binder, a large number of cracks are created due to the stress caused by anisotropic lattice expansion and contraction and thus aggravate electrolyte penetration and decomposition during the repeated charge-discharge process with a high cutoff voltage, as exhibited in Figure 8E. Unsurprisingly, the NCM811 electrode with the PI-FTD binder delivered better electrochemical performance than that of the conventional NCM811 electrode with the PVDF binder. In particular, the NCM811 electrode with PI-FTD exhibited superior cycling stability with a dramatically increased capacity retention of 79% after 100 cycles with a voltage window of 2.7-4.4 V at 0.2 C, while only 30% of the initial capacity could be maintained for the NCM811 electrode with PVDF [Figure 8F]. The conspicuously improved energy density and rate capability are also displayed in Figure 8G and H.
Other modification strategies via special surface treatments
In addition to the aforementioned conventional surface modification strategies, including surface coating, electrolyte regulation, and binder design, emerging modification strategies based on specific surface treatments have also been proved to be facile and efficient for substantial electrochemical improvements in LIBs. Recently, Xiao and co-workers reported the introduction of LiBH4 and LiAlH4 to modify the LCO surface by means of simple chemical treatments[136,137]. In contrast to typical surface modification processes with additional high-temperature sintering, a one-step wet chemical treatment was designed to decorate LCO with LiBH4 or LiAlH4 in a tetrahydrofuran (THF) solution. The modified LCO demonstrated a remarkably enhanced rate and cycling stability under high voltage, which were largely attributed to the generation of a more robust CEI and more stable B-O and Al-O bonds.
In particular, for Li-rich cathode materials, a series of surface treatments with NH4HCO3, NH3•H2O, urea, or acid compounds are usually applied to modify the surface structure. Metal/oxygen vacancies and the spinel/rock salt phase will be artificially created on the cathode surface, which are conducive to electrochemical performance. Specifically, when modifying Li1.2Mn0.6Ni0.2O2 via a multifunctional urea treatment, the in situ construction of oxygen vacancies, the spinel structure, and a N-doped carbon layer can be obtained simultaneously[179]. It has been well proved that oxygen vacancies can induce the generation of a self-built charge field to facilitate Li+ transport and reversible oxygen evolution, the spinel phase with fast 3D Li+ diffusion paths can also enhance ionic conductivity. Simultaneously, the N-doped carbon layer is able to protect active particles from HF attack from electrolyte decomposition. Other surface treatments are similar regarding their underlying working mechanism.
Recently, Peng and co-workers[36] developed a facile interface engineering via an ultrasonic treatment with oleic acid (OA) for a pristine Li-rich Mn-based cathode material. Note that H+ from OA can exchange with Li+ in the lithium oxide material, resulting in the formation of not only lithium defects but also a uniform organic coating layer on the cathode material surface by OA polymerization. The following calcination process further induces the generation of transition metal and oxygen vacancies, as well as surface reconstruction layer, including spinel phase Li4Mn5O12 and a carbon coating layer. It is well established that both cation/anion defects and in situ surface reconstruction play a prominent role in improving the structural and electrochemical stability of lithium oxide cathode materials, as mentioned above. Additionally, the innovative and scalable strategy can be applied to other kinds of layered cathode materials. As a result, superior electrochemical performance, including improved initial CE, ameliorative voltage and capacity decay during cycles, as well as outstanding rate capability, can be achieved.
CONCLUSIONS AND OUTLOOK
In this review, we have systematically discussed four representative oxide cathode materials, namely, layered lithium cobalt oxides, lithium ternary oxides, Li-rich oxides, and spinel lithium (nickel) manganese oxides. Generally, these materials exhibit diverse electrochemical behavior based on the intrinsically distinct crystal structures. In order to achieve high energy density, working at a raised cutoff voltage has been an important trend. However, these cathodes often suffer from unsatisfactory structural and electrochemical stability simultaneously, which is mainly due to the resulting fragile surface and interface under a high operating voltage. In the process of summarizing recent research progress on high-voltage lithium oxide cathode materials, a series of universal surface/interface modification strategies have been considered, including surface coating, electrolyte regulation, binder design, and special surface treatments. Their specific mechanisms of electrochemical improvement have also been investigated in detail to obtain a better understanding of high-voltage oxide cathode materials with high energy density.
Primarily, surface coating is the most efficient and simple modification, which has been proved to effectively improve the stability of not only the surface of active materials but also the interface between the electrode and electrolyte. In particular, coating materials (like fluorides, phosphates, polymers, and so on), methods (like wet-chemical, coprecipitation, sol-gel, ALD, and so on), and working mechanisms (like physically insulating electrodes from electrolytes, scavenging HF, constructing stable electrode/electrolyte interphase, and so on) have been summarized and discussed in detail. Note that synergetic surface modifications with several coating materials have also been attempted and can further promote the electrochemical performance improvement for high-performance LIBs with lithium oxide cathodes under high voltages. Simultaneously, some novel coating species and techniques of surface modification should be developed to achieve a better balance between cost and performance for practical applications in the future.
Considering the interfacial instability from side reactions between the electrode and electrolyte accompanied by electrolyte decomposition and structural degradation, electrolyte regulation is well known to be applied as a highly efficient modification strategy. Generally, studies on high-voltage electrolyte systems focus on two aspects. The first is introducing functional additives into traditional electrolyte systems. When charging to a high working voltage, the electrolyte additives will be preferentially oxidized to help generate a more stable CEI/SEI, inhibit the subsequent electrolyte decomposition and suppress the detrimental structure transformation and parasitic side reactions, thus causing the improved electrochemical performance. The second is designing novel electrolyte systems to meet the ever-increasing demand for high-voltage operation, which has also proved to be feasible. It is noteworthy that LHCEs take into full account several factors, including ionic conductivity, viscosity, oxidation stability, and cost, which are able to significantly improve significant interfacial issues and enhance electrochemical performance.
Furthermore, a novel functional binder design has also been developed as a feasible modification strategy recently, which is expected to play a significant part in consolidating the electrode itself and therefore enhancing the comprehensive electrochemical performance. At present, extensive research is still devoted to optimizing binder structure and uncovering the underlying working mechanism for high-voltage lithium oxide cathode materials. In particular, novel aqueous binders are usually cost-efficient and eco-friendly. Additionally, some binders with superior high-voltage tolerant capability are beneficial to the structural and electrochemical performance of advanced LIBs with lithium oxide cathodes under high-voltage operation. In contrast, some binders have the ability to coordinate with transition metal ions of active materials, which can reduce the dissolution of transition metal ions and thus lead to improved energy storage capability.
Finally, special surface treatments have been regarded as another widely reported modification strategy in recent years. Generally, surface treatments with special chemical agents like acids have the ability to change the surface structure in a certain content and obtain improved electrochemical performance for lithium oxide materials. In particular, for Li-rich oxide cathode materials, surface treatments through acids or amine are applied to realize surface reconduction and specific oxygen vacancy formation, further are beneficial to relieve a series of harmful side reactions during cycling and realize high-performance LIBs.
So far, there is no doubt that significant improvements in structural and electrochemical stability with the high-voltage operation have been achieved for commonly used lithium oxide cathode materials. Nevertheless, consistent efforts are still required for next-generation LIBs with both high energy density and long cycle life. As a consequence, definite development directions in the future can be proposed. These mainly involve the following aspects: (1) In the perspective of cathode materials, it is indispensable to optimize the existing oxides and develop innovative active materials with high lithium storage performance and stable crystal structure. For example, considering the limited capacity, developing novel cobalt-free and nickel-rich oxide materials like LiNi0.9Mn0.1O2 is urgent and attractive. This is able to not only improve the reversible capacity but also reduce cost. However, poor rate and cyclic capability need to be further enhanced by various modification strategies. In addition, layered Li-rich oxide materials can deliver ultrahigh specific capacity with cation and anion redox, which should be further developed for next-generation LIBs with high energy and power density during cycling. (2) In order to successfully achieve advanced LIBs with superior electrochemical performance under the growing cutoff voltage, other battery compositions like electrolyte, separator, and binder should be optimized to ensure high compatibility with cathode and anode. High-voltage electrolyte systems such as LHCEs and novel functional binders like polymers should be designed in the future. (3) It is expected that rational combinations of multiple modification strategy are promising to further upgrade the electrochemical performance of next-generation LIBs. Meanwhile, the simplified and environmental modification process will be more conducive to large-scale applications in the future. (4) Advanced characterization techniques are also vitally important. More in-depth theoretical and experimental studies will be carried out to pursue more insight into the underlying mechanism of performance deterioration for various lithium oxide cathode materials under high-voltage operation during cycling. Subsequently, the corresponding performance improvements will be more targeted and efficient. In particular, recently, artificial intelligence, including machine learning, has been devoted to materials science, which can predict, design, and research new energy materials and battery systems with vast development potential.
DECLARATIONS
Authors’ contributionsConceived the manuscript: Heng YL, Wu XL
Wrote and reviewed the manuscript: Heng YL, Wu XL
Contributed to the discussion of the manuscript: Heng YL, Gu ZY, Guo JZ, Wang XT, Zhao XX, Wu XL
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was financially supported by the National Natural Science Foundation of China (Grant Nos. 91963118 and 52102213), the Science Technology Program of Jilin Province (Grant No. 20200201066JC), and the 111 Project (No. B13013).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Goodenough JB, Park KS. The Li-ion rechargeable battery: a perspective. J Am Chem Soc 2013;135:1167-76.
2. Li C, Zhang Z, Chen Y, et al. Architecting braided porous carbon fibers based on high-density catalytic crystal planes to achieve highly reversible sodium-ion storage. Adv Sci (Weinh) 2022:e2104780.
3. Zhang Z, Chen Y, Sun S, et al. Recent progress on three-dimensional nanoarchitecture anode materials for lithium/sodium storage. J Mater Sci Technol 2022;119:167-81.
4. Pimlott JL, Street RJ, Down MP, Banks CE. Electrochemical overview: a summary of ACoxMnyNizO2 and metal oxides as versatile cathode materials for metal-ion batteries. Adv Funct Materials 2021;31:2107761.
5. Zhao H, Lam WA, Sheng L, et al. Cobalt-free cathode materials: families and their prospects. Adv Energy Mater 2022;12:2103894.
6. Lee W, Muhammad S, Sergey C, et al. Advances in the cathode materials for lithium rechargeable batteries. Angew Chem Int Ed Engl 2020;59:2578-605.
7. Chaudhary M, Tyagi S, Gupta RK, Singh BP, Singhal R. Surface modification of cathode materials for energy storage devices: a review. Surf Coat Technol 2021;412:127009.
8. Kim J, Zhang X, Zhang J, Manthiram A, Meng YS, Xu W. A review on the stability and surface modification of layered transition-metal oxide cathodes. Mater Today 2021;46:155-82.
9. Xiao B, Omenya F, Reed D, Li X. A glance of the layered transition metal oxide cathodes in sodium and lithium-ion batteries: difference and similarities. Nanotechnology 2021;32:422501.
10. Kalluri S, Yoon M, Jo M, et al. Surface engineering strategies of layered LiCoO2 cathode material to realize high-energy and high-voltage Li-ion cells. Adv Energy Mater 2017;7:1601507.
13. Wang X, Bai Y, Wang X, Wu C. High-voltage layered ternary oxide cathode materials: failure mechanisms and modification methods†. Chin J Chem 2020;38:1847-69.
14. Lv H, Li C, Zhao Z, Wu B, Mu D. A review: modification strategies of nickel-rich layer structure cathode (Ni ≥ 0.8) materials for lithium ion power batteries. J Energy Chem 2021;60:435-50.
17. He W, Guo W, Wu H, et al. Challenges and recent advances in high capacity Li-rich cathode materials for high energy density lithium-ion batteries. Adv Mater 2021;33:e2005937.
18. Liu J, Wang J, Ni Y, Zhang K, Cheng F, Chen J. Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries. Mater Today 2021;43:132-65.
19. Zheng J, Yan P, Zhang J, et al. Suppressed oxygen extraction and degradation of LiNixMnyCozO2 cathodes at high charge cut-off voltages. Nano Res 2017;10:4221-31.
20. Kong W, Zhang J, Wong D, et al. Tailoring Co3d and O2p band centers to inhibit oxygen escape for stable 4.6 V LiCoO2 cathodes. Angew Chem Int Ed Engl 2021;60:27102-12.
21. Aishova A, Park G, Yoon CS, Sun Y. Cobalt-free high-capacity ni-rich layered Li[Ni0.9Mn0.1]O2 cathode. Adv Energy Mater 2020;10:1903179.
22. Takahashi I, Kiuchi H, Ohma A, Fukunaga T, Matsubara E. Cathode electrolyte interphase formation and electrolyte oxidation mechanism for Ni-rich cathode materials. J Phys Chem C 2020;124:9243-8.
23. Zhang J, Li Q, Wang Y, Zheng J, Yu X, Li H. Dynamic evolution of cathode electrolyte interphase (CEI) on high voltage LiCoO2 cathode and its interaction with Li anode. Energy Storage Mater 2018;14:1-7.
24. Hou P, Yin J, Ding M, Huang J, Xu X. Surface/Interfacial structure and chemistry of high-energy nickel-rich layered oxide cathodes: advances and perspectives. Small 2017;13:1701802.
25. Su Y, Zhao J, Chen L, et al. Interfacial degradation and optimization of Li-rich cathode materials†. Chin J Chem 2021;39:402-20.
26. Pham HQ, Hwang E, Kwon Y, Song S. Toward 5 V lithium-ion battery: exploring the limit of charge cut-off voltage of Li-rich layered oxide cathode and high-voltage interfacial processes. Adv Mater Interfaces 2017;4:1700483.
27. Li Q, Ning, Wong D, et al. Improving the oxygen redox reversibility of Li-rich battery cathode materials via Coulombic repulsive interactions strategy. Nat Commun 2022;13:1123.
28. Zhu Z, Wang H, Li Y, et al. A surface Se-substituted LiCo[O2-δ Seδ] cathode with ultrastable high-voltage cycling in pouch full-cells. Adv Mater 2020;32:e2005182.
29. Jayawardana C, Rodrigo N, Parimalam B, Lucht BL. Role of electrolyte oxidation and difluorophosphoric acid generation in crossover and capacity fade in lithium ion batteries. ACS Energy Lett 2021;6:3788-92.
30. Liu M, Vatamanu J, Chen X, Xing L, Xu K, Li W. Hydrolysis of LiPF6 -containing electrolyte at high voltage. ACS Energy Lett 2021;6:2096-102.
31. Su C, He M, Amine R, et al. Unveiling decaying mechanism through quantitative structure-activity relationship in electrolytes for lithium-ion batteries. Nano Energy 2021;83:105843.
32. Zhao J, Zhang X, Liang Y, et al. Interphase engineering by electrolyte additives for lithium-rich layered oxides: advances and perspectives. ACS Energy Lett 2021;6:2552-64.
33. Kim N, Moon J, Ryou M, et al. Amphiphilic bottlebrush polymeric binders for high-mass-loading cathodes in lithium-ion batteries. Adv Energy Mater 2022;12:2102109.
34. Zhang J, Zhang J, Ou X, Wang C, Peng C, Zhang B. Enhancing high-voltage performance of Ni-rich cathode by surface modification of self-assembled NASICON fast ionic conductor LiZr2(PO4)3. ACS Appl Mater Interfaces 2019;11:15507-16.
35. Li S, Zhang W, Wu Q, et al. Synergistic dual-additive electrolyte enables practical lithium-metal batteries. Angew Chem Int Ed Engl 2020;59:14935-41.
36. Guo W, Zhang C, Zhang Y, et al. A universal strategy toward the precise regulation of initial coulombic efficiency of Li-rich Mn-based cathode materials. Adv Mater 2021;33:e2103173.
37. Wang X, Wang X, Lu Y. Realizing high voltage lithium cobalt oxide in lithium-ion batteries. Ind Eng Chem Res 2019;58:10119-39.
38. Lyu Y, Wu X, Wang K, et al. An overview on the advances of LiCoO2 cathodes for lithium-ion batteries. Adv Energy Mater 2021;11:2000982.
39. Wang L, Ma J, Wang C, et al. A novel bifunctional self-stabilized strategy enabling 4.6 V LiCoO2 with excellent long-term cyclability and high-rate capability. Adv Sci (Weinh) 2019;6:1900355.
40. Li J, Lin C, Weng M, et al. Structural origin of the high-voltage instability of lithium cobalt oxide. Nat Nanotechnol 2021;16:599-605.
41. Cheng T, Ma Z, Qian R, et al. Achieving stable cycling of LiCoO2 at 4.6 V by multilayer surface modification. Adv Funct Mater 2021;31:2001974.
42. Zhang J, Tan X, Guo L, et al. Controllable formation of lithium carbonate surface phase during synthesis of nickel-rich LiNi0.9Mn0.1O2 in air and its protection role in electrochemical reaction. J Alloys Compd 2019;771:42-50.
43. Li W, Zhang X, Si J, Yang J, Sun X. TiO2-coated LiNi0.9Co0.08Al0.02O2 cathode materials with enhanced cycle performance for Li-ion batteries. Rare Met 2021;40:1719-26.
44. Wang L, Wang J, Wang L, Zhang M, Wang R, Zhan C. A critical review on nickel-based cathodes in rechargeable batteries. Int J Miner Metall Mater 2022;29:925-41.
45. Cheng F, Zhang X, Qiu Y, et al. Tailoring electrolyte to enable high-rate and super-stable Ni-rich NCM cathode materials for Li-ion batteries. Nano Energy 2021;88:106301.
46. Huang Y, Dong Y, Li S, et al. Lithium manganese spinel cathodes for lithium-ion batteries. Adv Energy Mater 2021;11:2000997.
47. Zuo C, Hu Z, Qi R, et al. Double the capacity of manganese spinel for lithium-ion storage by suppression of cooperative jahn-teller distortion. Adv Energy Mater 2020;10:2000363.
48. Liang G, Peterson VK, See KW, Guo Z, Pang WK. Developing high-voltage spinel LiNi0.5Mn1.5O4 cathodes for high-energy-density lithium-ion batteries: current achievements and future prospects. J Mater Chem A 2020;8:15373-98.
49. Gou X, Hao Z, Hao Z, et al. In situ surface self-reconstruction strategies in Li-rich Mn-based layered cathodes for energy-dense Li-ion batteries. Adv Funct Mater 2022;32:2112088.
50. Fan Y, Zhang W, Zhao Y, Guo Z, Cai Q. Fundamental understanding and practical challenges of lithium-rich oxide cathode materials: layered and disordered-rocksalt structure. Energy Storage Mater 2021;40:51-71.
51. Kim SY, Park CS, Hosseini S, Lampert J, Kim YJ, Nazar LF. Inhibiting oxygen release from Li-rich, Mn-rich layered oxides at the surface with a solution processable oxygen scavenger polymer. Adv Energy Mater 2021;11:2100552.
52. Li Z, Peng Z, Sun R, et al. Super Na+ half/full batteries and ultrafast Na+ diffusion kinetics of cobalt-nickel selenide from assembling Co0.5Ni0.5Se2 @ NC nanosheets into cross-stacked architecture. Chin J Chem 2021;39:2599-606.
53. Lian J, Wu Y, Guo Y, et al. Design of hierarchical and mesoporous FeF3/rGO hybrids as cathodes for superior lithium-ion batteries. Chin Chem Lett 2021; doi: 10.1016/j.cclet.2021.12.014.
54. Sun R, Dong S, Xu F, et al. Co-intercalation strategy of constructing partial cation substitution of ammonium vanadate {(NH4)2V6O16} for stable zinc ion storage. Dalton Trans 2022;51:7607-12.
55. Sun R, Qin Z, Li Z, Fan H, Lu S. Binary zinc-cobalt metal-organic framework derived mesoporous ZnCo2O4@NC polyhedron as a high-performance lithium-ion battery anode. Dalton Trans 2020;49:14237-42.
56. Sun R, Qin Z, Liu X, et al. Intercalation mechanism of the ammonium vanadate (NH4V4O10) 3D decussate superstructure as the cathode for high-performance aqueous zinc-ion batteries. ACS Sustainable Chem Eng 2021;9:11769-77.
57. Wang J, Du C, Xue Y, et al. MXenes as a versatile platform for reactive surface modification and superior sodium-ion storages. Exploration 2021;1:20210024.
58. Zhu H, Li Z, Xu F, et al. Ni3Se4@CoSe2 hetero-nanocrystals encapsulated into CNT-porous carbon interpenetrating frameworks for high-performance sodium ion battery. J Colloid Interface Sci 2022;611:718-25.
59. Cho M, Song SH, Hong S, et al. Critical role of Ti4+ in stabilizing high-voltage redox reactions in Li-rich layered material. Small 2021;17:e2100840.
60. Sun HH, Kim UH, Park JH, et al. Transition metal-doped Ni-rich layered cathode materials for durable Li-ion batteries. Nat Commun 2021;12:6552.
61. Zhang J, Li Q, Ouyang C, et al. Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V. Nat Energy 2019;4:594-603.
62. Zhou A, Liu Q, Wang Y, et al. Al2O3 surface coating on LiCoO2 through a facile and scalable wet-chemical method towards high-energy cathode materials withstanding high cutoff voltages. J Mater Chem A 2017;5:24361-70.
63. Hu D, Du F, Cao H, et al. An effective strategy to control thickness of Al2O3 coating layer on nickel-rich cathode materials. J Electroanal Chem 2021;880:114910.
64. Østli ER, Tesfamhret Y, Wenner S, et al. Limitations of ultrathin Al2O3 coatings on LNMO cathodes. ACS Omega 2021;6:30644-55.
65. Zou T, Qi W, Liu X, et al. Improvement of the electrochemical performance of Li1.2Ni0.13Co0.13Mn0.54O2 cathode material by Al2O3 surface coating. J Electro Chem 2020;859:113845.
66. Lee S, Park G, Sim S, Jin B, Kim H. Improved electrochemical performances of LiNi0.8Co0.1Mn0.1O2 cathode via SiO2 coating. J Alloys Compd 2019;791:193-9.
67. Nisar U, Al-hail SAJA, Petla RK, et al. Understanding the origin of the ultrahigh rate performance of a SiO2 -Modified LiNi0.5Mn1.5O4 cathode for lithium-ion batteries. ACS Appl Energy Mater 2019;2:7263-71.
68. Zhai X, Zhang P, Huang H, et al. Surface grafting SiO2 on lithium-rich layered oxide cathode material for improving structural stability. J Electrochem Soc 2021;168:060528.
69. Zhou A, Lu Y, Wang Q, et al. Sputtering TiO2 on LiCoO2 composite electrodes as a simple and effective coating to enhance high-voltage cathode performance. J Power Sources 2017;346:24-30.
70. Zhao S, Zhu Y, Qian Y, et al. Annealing effects of TiO2 coating on cycling performance of Ni-rich cathode material LiNi0.8Co0.1Mn0.1O2 for lithium-ion battery. Mater Lett 2020;265:127418.
71. Zhang C, Liu X, Su Q, Wu J, Huang T, Yu A. Enhancing Electrochemical Performance of LiMn2O4 cathode material at elevated temperature by uniform nanosized TiO2 coating. ACS Sustainable Chem Eng 2017;5:640-7.
72. Liu J, Wu Z, Yu M, et al. Building homogenous Li2 TiO3 coating layer on primary particles to stabilize Li-rich Mn-based cathode materials. Small 2022;18:e2106337.
73. Kong J, Ren C, Tai G, et al. Ultrathin ZnO coating for improved electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material. J Power Sources 2014;266:433-9.
74. Wang F, Hong R, Lu X, et al. Improvement of long-term cycling performance of high-nickel cathode materials by ZnO coating. Nanotechnol Rev 2021;10:210-20.
75. Nie K, Sun X, Wang J, et al. Realizing long-term cycling stability and superior rate performance of 4.5 V-LiCoO2 by aluminum doped zinc oxide coating achieved by a simple wet-mixing method. J Power Sources 2020;470:228423.
76. Hudaya C, Park JH, Lee JK, Choi W. SnO2-coated LiCoO2 cathode material for high-voltage applications in lithium-ion batteries. Solid State Ionics 2014;256:89-92.
77. Xie Z, Zhang Y, Yuan A, Xu J. Effects of lithium excess and SnO2 surface coating on the electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode material for Li-ion batteries. J Alloys Compd 2019;787:429-39.
78. Chen C, Geng T, Du C, et al. Oxygen vacancies in SnO2 surface coating to enhance the activation of layered Li-rich Li1.2Mn0.54Ni0.13Co0.13O2 cathode material for Li-ion batteries. J Power Sources 2016;331:91-9.
79. Hao Q, Xu C, Jia S, Zhao X. Improving the cycling stability of LiCoO2 at 4.5 V through surface modification by Fe2O3 coating. Electrochim Acta 2013;113:439-45.
80. Huang Y, Huang Y, Hu X. Enhanced electrochemical performance of LiNi0.8Co0.15Al0.05O2 by nanoscale surface modification with Co3O4. Electrochim Acta 2017;231:294-9.
81. Liu K, Yang G, Dong Y, Shi T, Chen L. Enhanced cycling stability and rate performance of Li[Ni0.5Co0.2Mn0.3]O2 by CeO2 coating at high cut-off voltage. J Power Sources 2015;281:370-7.
82. Wu ZH, Shih JY, Li YJ, et al. MoO3 nanoparticle coatings on high-voltage 5 V LiNi0.5Mn1.5O4 cathode materials for improving lithium-ion battery performance. Nanomaterials (Basel) 2022;12:409.
83. Huang J, Fang X, Wu Y, et al. Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2 by surface modification with lithium-active MoO3. J Electro Chem 2018;823:359-67.
84. Zhu J, Cao G, Li Y, et al. Nd2O3 encapsulation-assisted surface passivation of Ni-rich LiNi0.8Co0.1Mn0.1O2 active material and its electrochemical performance. Electrochim Acta 2019;325:134889.
85. Jung SH, Kim DH, Brüner P, et al. Extremely conductive RuO2-coated LiNi0.5Mn1.5O4 for lithium-ion batteries. Electrochim Acta 2017;232:236-43.
86. Sattar T, Sim S, Jin B, Kim H. Improving the cycle stability and rate performance of LiNi0.91Co0.06Mn0.03O2 Ni-rich cathode material by La2O3 coating for Lithium-ion batteries. Curr Appl Phys 2022;36:176-82.
87. Liu X, Kou L, Shi T, Liu K, Chen L. Excellent high rate capability and high voltage cycling stability of Y2O3-coated LiNi0.5Co0.2Mn0.3O2. J Power Sources 2014;267:874-80.
88. Yao L, Liang F, Jin J, Chowdari BV, Yang J, Wen Z. Improved electrochemical property of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode via in-situ ZrO2 coating for high energy density lithium ion batteries. Chem Eng J 2020;389:124403.
89. Shapira A, Tiurin O, Solomatin N, Auinat M, Meitav A, Ein-eli Y. Robust AlF3 atomic layer deposition protective coating on LiMn1.5 Ni0.5 O4 particles: an advanced Li-ion battery cathode material powder. ACS Appl Energy Mater 2018;1:6809-23.
90. Zhao B, Xie J, Zhuang H, et al. Improved low-temperature performance of surface modified lithium-rich Li1.2Ni0.13Co0.13Mn0.54O2 cathode materials for lithium ion batteries. Solid State Ionics 2020;347:115245.
91. Dai S, Yan G, Wang L, et al. Enhanced electrochemical performance and thermal properties of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material via CaF2 coating. J Electro Chem 2019;847:113197.
92. Wei J, Ji Y, Liang D, Chen B, Jiang C, Li X. Anticorrosive nanosized LiF thin film coating for achieving long-cycling stability of LiCoO2 at high voltages. Ceram Int 2022;48:10288-98.
93. Tiurin O, Solomatin N, Auinat M, Ein-eli Y. Atomic layer deposition (ALD) of lithium fluoride (LiF) protective film on Li-ion battery LiMn1.5Ni0.5O4 cathode powder material. J Power Sources 2020;448:227373.
94. Zhao T, Li L, Chen R, et al. Design of surface protective layer of LiF/FeF3 nanoparticles in Li-rich cathode for high-capacity Li-ion batteries. Nano Energy 2015;15:164-76.
95. Li Y, Zhang Q, Xu T, et al. LaF3 nanolayer surface modified spinel LiNi0.5Mn1.5O4 cathode material for advanced lithium-ion batteries. Ceram Int 2018;44:4058-66.
96. Zhou A, Xu J, Dai X, et al. Improved high-voltage and high-temperature electrochemical performances of LiCoO2 cathode by electrode sputter-coating with Li3PO4. J Power Sources 2016;322:10-6.
97. Lee S, Kim M, Jeong JH, et al. Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted sol-gel method: improved thermal stability and high-voltage performance. J Power Sources 2017;360:206-14.
98. Bian X, Fu Q, Bie X, et al. Improved electrochemical performance and thermal stability of Li-excess Li1.18Co0.15Ni0.15Mn0.52O2 cathode material by Li3PO4 surface coating. Electrochim Acta 2015;174:875-84.
99. Li W, Yang L, Li Y, et al. Ultra-thin AlPO4 layer coated LiNi0.7Co0.15Mn0.15O2 cathodes with enhanced high-voltage and high-temperature performance for lithium-ion half/full batteries. Front Chem 2020;8:597.
100. Ma X, Wang C, Han X, Sun J. Effect of AlPO4 coating on the electrochemical properties of LiNi0.8Co0.2O2 cathode material. J Alloy Compd 2008;453:352-5.
101. Wu Y, Ming H, Li M, et al. New organic complex for lithium layered oxide modification: ultrathin coating, high-voltage, and safety performances. ACS Energy Lett 2019;4:656-65.
102. Wu F, Zhang X, Zhao T, Li L, Xie M, Chen R. Multifunctional AlPO4 coating for improving electrochemical properties of low-cost Li[Li0.2Fe0.1Ni0.15Mn0.55]O2 cathode materials for lithium-ion batteries. ACS Appl Mater Interfaces 2015;7:3773-81.
103. Chen Z, Kim G, Bresser D, et al. MnPO4-coated Li(Ni0.4Co0.2Mn0.4)O2 for lithium(-ion) batteries with outstanding cycling stability and enhanced lithiation kinetics. Adv Energy Mater 2018;8:1801573.
104. Kim KC, Jegal J, Bak S, Roh KC, Kim K. Improved high-voltage performance of FePO4-coated LiCoO2 by microwave-assisted hydrothermal method. Electrochem Commun 2014;43:113-6.
105. Wang Z, Liu E, He C, Shi C, Li J, Zhao N. Effect of amorphous FePO4 coating on structure and electrochemical performance of Li1.2Ni0.13Co0.13Mn0.54O2 as cathode material for Li-ion batteries. J Power Sources 2013;236:25-32.
106. Lee D, Scrosati B, Sun Y. Ni3(PO4)2-coated Li[Ni0.8Co0.15Al0.05]O2 lithium battery electrode with improved cycling performance at
107. Xu T, Li Y, Wang D, et al. Enhanced electrochemical performance of LiNi0.5 Mn1.5 O4 cathode material by YPO4 surface modification. ACS Sustainable Chem Eng 2018;6:5818-25.
108. Wang Y, Zhang Q, Xue Z, et al. An in situ formed surface coating layer enabling LiCoO 2 with stable 4.6 V high-voltage cycle performances. Adv Energy Mater 2020;10:2001413.
109. Yang Q, Huang J, Li Y, et al. Surface-protected LiCoO2 with ultrathin solid oxide electrolyte film for high-voltage lithium ion batteries and lithium polymer batteries. J Power Sources 2018;388:65-70.
110. Li Z, Li A, Zhang H, et al. Multi-scale stabilization of high-voltage LiCoO2 enabled by nanoscale solid electrolyte coating. Energy Storage Mater 2020;29:71-7.
111. Liu Y, Fan X, Huang X, et al. Electrochemical performance of Li1.2Ni0.2Mn0.6O2 coated with a facilely synthesized Li1.3Al0.3Ti1.7(PO4)3. J Power Sources 2018;403:27-37.
112. Jamil S, Ran Q, Yang L, et al. Improved high-voltage performance of LiNi0.87Co0.1Al0.03O2 by Li-conductor coating. Chem Eng J 2021;407:126442.
113. Deng JC, Xu YL, Li L, Feng TY, Li L. Microporous LiAlSiO4 with high ionic conductivity working as a coating material and water adsorbent for LiNi0.5Mn1.5O4 cathode. J Mater Chem A 2016;4:6561-8.
114. Yang S, Wei H, Tang L, et al. Fast Li-ion conductor Li1+yTi2-yAly(PO4)3 modified Li1.2[Mn0.54Ni0.13Co0.13]O2 as high performance cathode material for Li-ion battery. Ceram Int 2021;47:18397-404.
115. Deng Y, Zhao S, Xu Y, Nan C. Effect of temperature of Li2O-Al2O3-TiO2-P2O5 solid-state electrolyte coating process on the performance of LiNi0.5Mn1.5O4 cathode materials. J Power Sources 2015;296:261-7.
116. Su Y, Chen G, Chen L, et al. Roles of fast-ion conductor LiTaO3 modifying Ni-rich cathode material for Li-ion batteries. ChemSusChem 2021;14:1955-61.
117. Zhang J, Cao Y, Ou X, et al. Constituting the NASICON type solid electrolyte coated material forming anti-high voltage system to enhance the high cut-off voltage performance of LiNi0.6Co0.2Mn0.2O2 via charge attracts electrostatic assembly. J Power Sources 2019;436:226722.
118. Park J, Cho J, Lee E, Kim J, Lee S. Thickness-tunable polyimide nanoencapsulating layers and their influence on cell performance/thermal stability of high-voltage LiCoO2 cathode materials for lithium-ion batteries. J Power Sources 2013;244:442-9.
119. Kim JM, Park JH, Lee CK, Lee SY. Multifunctional semi-interpenetrating polymer network-nanoencapsulated cathode materials for high-performance lithium-ion batteries. Sci Rep 2014;4:4602.
120. Wang H, Lin J, Zhang X, et al. Improved electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode materials induced by a facile polymer coating for lithium-ion batteries. ACS Appl Energy Mater 2021;4:6205-13.
121. Lin K, Yang S, Shi Z, Fan Q, Liu Z, Liu L. Knitting a sweater with UV-induced in situ polymerization of poly(pyrrole-co-citral nitrile) on Ni-rich layer oxide cathode materials for lithium ion batteries. J Power Sources 2022;520:230768.
122. Sun Q, Hu G, Peng Z, et al. Achieving a bifunctional conformal coating on nickel-rich cathode LiNi0.8Co0.1Mn0.1O2 with half-cyclized polyacrylonitrile. Electrochim Acta 2021;386:138440.
123. Cao Y, Qi X, Hu K, et al. Conductive polymers encapsulation to enhance electrochemical performance of Ni-rich cathode materials for Li-ion batteries. ACS Appl Mater Interfaces 2018;10:18270-80.
124. Gan Q, Qin N, Zhu Y, et al. Polyvinylpyrrolidone-induced uniform surface-conductive polymer coating endows Ni-Rich LiNi0.8Co0.1Mn0.1O2 with enhanced cyclability for lithium-ion batteries. ACS Appl Mater Interfaces 2019;11:12594-604.
125. Gao X, Deng Y, Wexler D, et al. Improving the electrochemical performance of the LiNi0.5Mn1.5O4 spinel by polypyrrole coating as a cathode material for the lithium-ion battery. J Mater Chem A 2015;3:404-11.
126. Zhang J, Lu Q, Fang J, Wang J, Yang J, NuLi Y. Polyimide encapsulated lithium-rich cathode material for high voltage lithium-ion battery. ACS Appl Mater Interfaces 2014;6:17965-73.
127. Yang X, Wang C, Yan P, et al. Pushing lithium cobalt oxides to 4.7 V by lattice-matched interfacial engineering. Adv Energy Mater 2022;2200197.
128. Hu G, Cao J, Peng Z, Cao Y, Du K. Enhanced high-voltage properties of LiCoO2 coated with Li[Li0.2Mn0.6Ni0.2]O2. Electrochim Acta 2014;149:49-55.
129. Liu Y, Huang X, Qiao Q, Wang Y, Ye S, Gao X. Li3V2(PO4)3-coated Li1.17Ni0.2Co0.05Mn0.58O2 as the cathode materials with high rate capability for Lithium ion batteries. Electrochim Acta 2014;147:696-703.
130. Yang S, Wang P, Wei H, et al. Li4V2Mn(PO4)4-stablized Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode materials for lithium ion batteries. Nano Energy 2019;63:103889.
131. Hu Q, He Y, Ren D, et al. Targeted masking enables stable cycling of LiNi0.6Co0.2Mn0.2O2 at 4.6 V. Nano Energy 2022;96:107123.
132. Jerng SE, Chang B, Shin H, et al. Pyrazine-linked 2D covalent organic frameworks as coating material for high-nickel layered oxide cathodes in lithium-ion batteries. ACS Appl Mater Interfaces 2020;12:10597-606.
133. Zheng X, Liu W, Qu Q, Shi Q, Zheng H, Huang Y. Effectively stabilizing 5 V spinel LiNi0.5Mn1.5O4 cathode in organic electrolyte by polyvinylidene fluoride coating. Appl Surf Sci 2018;455:349-56.
134. Yu F, Que L, Xu C, et al. Dual conductive surface engineering of Li-Rich oxides cathode for superior high-energy-density Li-Ion batteries. Nano Energy 2019;59:527-36.
135. Gao M, Yan C, Shao Q, et al. A novel perovskite electron-ion conductive coating to simultaneously enhance cycling stability and rate capability of Li1.2Ni0.13Co0.13Mn0.54O2 cathode material for lithium-ion batteries. Small 2021;17:e2008132.
136. Pu W, Meng Y, Wang Y, et al. Investigation of the LiBH4 modification effect on cycling stability and high-rate capacity of LiCoO2 cathodes. ACS Appl Energy Mater 2021;4:6933-41.
137. Wang P, Meng Y, Wang Y, et al. Oxygen framework reconstruction by LiAlH4 treatment enabling stable cycling of high-voltage LiCoO2. Energy Storage Mater 2022;44:487-96.
138. Maiti S, Konar R, Sclar H, et al. Stabilizing high-voltage lithium-ion battery cathodes using functional coatings of 2D tungsten diselenide. ACS Energy Lett 2022;7:1383-91.
139. Charles-blin Y, Nemoto K, Zettsu N, Teshima K. Effects of a solid electrolyte coating on the discharge kinetics of a LiCoO2 electrode: mechanism and potential applications. J Mater Chem A 2020;8:20979-86.
140. Zhao R, Li L, Xu T, et al. One-step integrated surface modification to build a stable interface on high-voltage cathode for lithium-ion batteries. ACS Appl Mater Interfaces 2019;11:16233-42.
141. Hwang T, Lee JK, Mun J, Choi W. Surface-modified carbon nanotube coating on high-voltage LiNi0.5Mn1.5O4 cathodes for lithium ion batteries. J Power Sources 2016;322:40-8.
142. Lai X, Hu G, Peng Z, et al. Surface structure decoration of high capacity Li1.2Mn0.54Ni0.13Co0.13O2 cathode by mixed conductive coating of Li1.4Al0.4Ti1.6(PO4)3 and polyaniline for lithium-ion batteries. J Power Sources 2019;431:144-52.
143. Tong B, Song Z, Wan H, et al. Sulfur-containing compounds as electrolyte additives for lithium-ion batteries. InfoMat 2021;3:1364-92.
144. Zhang SS. Design aspects of electrolytes for fast charge of Li-ion batteries. InfoMat 2021;3:125-30.
145. Sun Y, Huang J, Xiang H, Liang X, Feng Y, Yu Y. 2-(Trifluoroacetyl) thiophene as an electrolyte additive for high-voltage lithium-ion batteries using LiCoO2 cathode. J Mater Sci Techno 2020;55:198-202.
146. Ruan D, Chen M, Wen X, et al. In situ constructing a stable interface film on high-voltage LiCoO2 cathode via a novel electrolyte additive. Nano Energy 2021;90:106535.
147. Zhang Z, Liu F, Huang Z, et al. Enhancing the electrochemical performance of a high-voltage LiCoO 2 cathode with a bifunctional electrolyte additive. ACS Appl Energy Mater 2021;4:12954-64.
148. Jiang S, Wu H, Yin J, et al. Benzoic anhydride as a bifunctional electrolyte additive for hydrogen fluoride capture and robust film construction over high-voltage Li-ion batteries. ChemSusChem 2021;14:2067-75.
149. Chen J, Xing L, Yang X, Liu X, Li T, Li W. Outstanding electrochemical performance of high-voltage LiNi1/3Co1/3Mn1/3O2 cathode achieved by application of LiPO2F2 electrolyte additive. Electrochim Acta 2018;290:568-76.
150. Deng B, Wang H, Ge W, et al. Investigating the influence of high temperatures on the cycling stability of a LiNi0.6 Co0.2Mn0.2O2 cathode using an innovative electrolyte additive. Electrochim Acta 2017;236:61-71.
151. Wang S, Chen S, Gao W, Liu L, Zhang S. A new additive 3-Isocyanatopropyltriethoxysilane to improve electrochemical performance of Li/NCM622 half-cell at high voltage. J Power Sources 2019;423:90-7.
152. Yim T, Jang SH, Han Y. Triphenyl borate as a bi-functional additive to improve surface stability of Ni-rich cathode material. J Power Sources 2017;372:24-30.
153. Lin Y, Zhang H, Yue X, Yu L, Fan W. Triallyl phosphite as an electrolyte additive to improve performance at elevated temperature of LiNi0.6Co0.2Mn0.2O2/graphite cells. J Electro Chem 2019;832:408-16.
154. Qin Z, Hong S, Hong B, Duan B, Lai Y, Feng J. Triisopropyl borate as an electrolyte additive for improving the high voltage stability of LiNi0.6Co0.2Mn0.2O2 cathode. J Electro Chem 2019;854:113506.
155. Yang J, Liu X, Wang Y, et al. Electrolytes polymerization-induced cathode-electrolyte-interphase for high voltage lithium-ion batteries. Adv Energy Mater 2021;11:2101956.
156. Kim K, Kim Y, Park S, et al. Dual-function ethyl 4,4,4-trifluorobutyrate additive for high-performance Ni-rich cathodes and stable graphite anodes. J Power Sources 2018;396:276-87.
157. Shi C, Shen C, Peng X, et al. A special enabler for boosting cyclic life and rate capability of LiNi0.8Co0.1Mn0.1O2: green and simple additive. Nano Energy 2019;65:104084.
158. Hong P, Xu M, Zheng X, et al. Effect of ethylene glycol bis (propionitrile) ether (EGBE) on the performance and interfacial chemistry of lithium-rich layered oxide cathode. J Power Sources 2016;329:216-24.
159. Lan J, Zheng Q, Zhou H, et al. Stabilizing a high-voltage lithium-rich layered oxide cathode with a novel electrolyte additive. ACS Appl Mater Interfaces 2019;11:28841-50.
160. Bolloju S, Chiou C, Vikramaditya T, Lee J. (Pentafluorophenyl)diphenylphosphine as a dual-functional electrolyte additive for LiNi0.5Mn1.5O4 cathodes in high-voltage lithium-ion batteries. Electrochim Acta 2019;299:663-71.
161. Lim SH, Cho W, Kim Y, Yim T. Insight into the electrochemical behaviors of 5V-class high-voltage batteries composed of lithium-rich layered oxide with multifunctional additive. J Power Sources 2016;336:465-74.
162. Chen R, Liu F, Chen Y, et al. An investigation of functionalized electrolyte using succinonitrile additive for high voltage lithium-ion batteries. J Power Sources 2016;306:70-7.
163. Han J, Hwang C, Kim SH, et al. An antiaging electrolyte additive for high-energy-density lithium-ion batteries. Adv Energy Mater 2020;10:2000563.
164. Yang X, Lin M, Zheng G, et al. Enabling stable high-voltage LiCoO2 operation by using synergetic interfacial modification strategy. Adv Funct Mater 2020;30:2004664.
165. Zhang X, Zou L, Cui Z, et al. Stabilizing ultrahigh-nickel layered oxide cathodes for high-voltage lithium metal batteries. Materials Today 2021;44:15-24.
166. Huang H, Li Z, Gu S, et al. Dextran sulfate lithium as versatile binder to stabilize high-voltage LiCoO2 to 4.6 V. Adv Energy Mater 2021;11:2101864.
167. Isozumi H, Horiba T, Kubota K, et al. Application of modified styrene-butadiene-rubber-based latex binder to high-voltage operating LiCoO2 composite electrodes for lithium-ion batteries. J Power Sources 2020;468:228332.
168. Kuo J, Li C. Water-Based Process to the Preparation of Nickel-Rich Li(Ni0.8Co0.1Mn0.1)O2 Cathode. J Electrochem Soc 2020;167:100504.
169. Mo J, Zhang D, Sun M, et al. Polyethylene oxide as a multifunctional binder for high-performance ternary layered cathodes. Polymers (Basel) 2021;13:3992.
170. Pham HQ, Lee J, Jung HM, Song S. Non-flammable LiNi0.8Co0.1Mn0.1O2 cathode via functional binder; stabilizing high-voltage interface and performance for safer and high-energy lithium rechargeable batteries. Electrochim Acta 2019;317:711-21.
171. Dong T, Zhang H, Ma Y, et al. A well-designed water-soluble binder enlightening the 5 V-class LiNi0.5Mn1.5O4 cathodes. J Mater Chem A 2019;7:24594-601.
172. Ma Y, Wang C, Ma J, et al. Interfacial chemistry of γ-glutamic acid derived block polymer binder directing the interfacial compatibility of high voltage LiNi0.5Mn1.5O4 electrode. Sci China Chem 2021;64:92-100.
173. Zhong H, Lu J, He A, Sun M, He J, Zhang L. Carboxymethyl chitosan/poly(ethylene oxide) water soluble binder: challenging application for 5 V LiNi0.5Mn1.5O4 cathode. J Mater Sci Techno 2017;33:763-7.
174. Rao L, Jiao X, Yu CY, et al. Multifunctional composite binder for thick high-voltage cathodes in lithium-ion batteries. ACS Appl Mater Interfaces 2022;14:861-72.
175. Ma Y, Chen K, Ma J, et al. A biomass based free radical scavenger binder endowing a compatible cathode interface for 5 V lithium-ion batteries. Energy Environ Sci 2019;12:273-80.
176. Pham HQ, Kim G, Jung HM, Song S. Fluorinated polyimide as a novel high-voltage binder for high-capacity cathode of lithium-ion batteries. Adv Funct Mater 2018;28:1704690.
177. Zhang G, Qiu B, Xia Y, et al. Double-helix-superstructure aqueous binder to boost excellent electrochemical performance in Li-rich layered oxide cathode. J Power Sources 2019;420:29-37.
178. Zhang S, Deng Y, Wu Q, et al. Sodium-alginate-based binders for lithium-rich cathode materials in lithium-ion batteries to suppress voltage and capacity fading. Chem Electro Chem 2018;5:1321-9.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Heng YL, Gu ZY, Guo JZ, Wang XT, Zhao XX, Wu XL. Research progress on the surface/interface modification of high-voltage lithium oxide cathode materials. Energy Mater 2022;2:200017. http://dx.doi.org/10.20517/energymater.2022.18
AMA Style
Heng YL, Gu ZY, Guo JZ, Wang XT, Zhao XX, Wu XL. Research progress on the surface/interface modification of high-voltage lithium oxide cathode materials. Energy Materials. 2022; 2(3): 200017. http://dx.doi.org/10.20517/energymater.2022.18
Chicago/Turabian Style
Heng, Yong-Li, Zhen-Yi Gu, Jin-Zhi Guo, Xiao-Tong Wang, Xin-Xin Zhao, Xing-Long Wu. 2022. "Research progress on the surface/interface modification of high-voltage lithium oxide cathode materials" Energy Materials. 2, no.3: 200017. http://dx.doi.org/10.20517/energymater.2022.18
ACS Style
Heng, Y.L.; Gu Z.Y.; Guo J.Z.; Wang X.T.; Zhao X.X.; Wu X.L. Research progress on the surface/interface modification of high-voltage lithium oxide cathode materials. Energy Mater. 2022, 2, 200017. http://dx.doi.org/10.20517/energymater.2022.18
About This Article
Copyright
Data & Comments
Data
 Cite This Article 22 clicks
Cite This Article 22 clicks



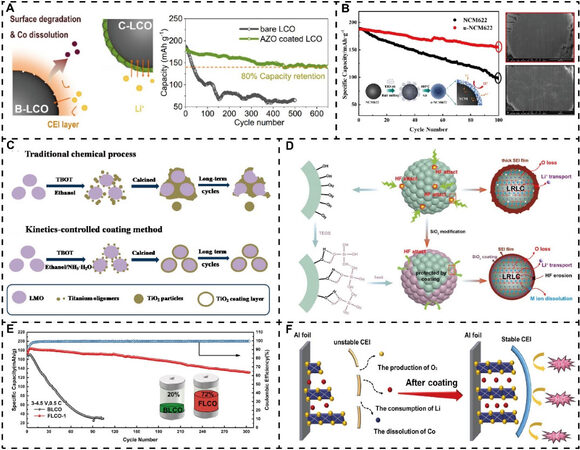
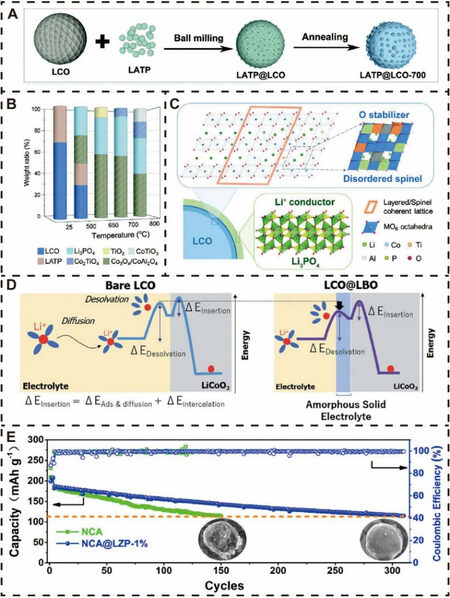
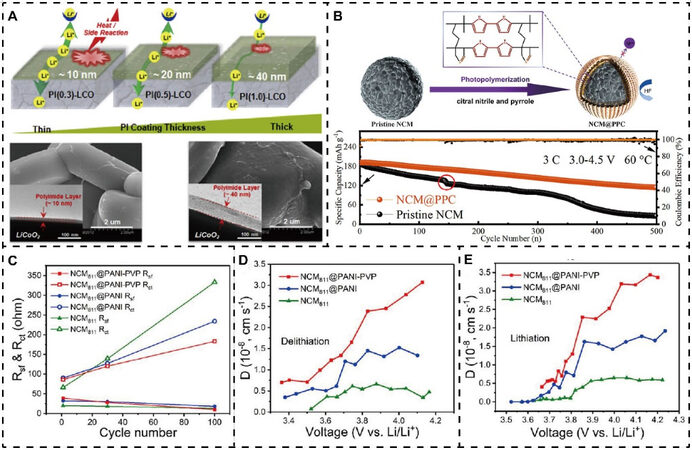
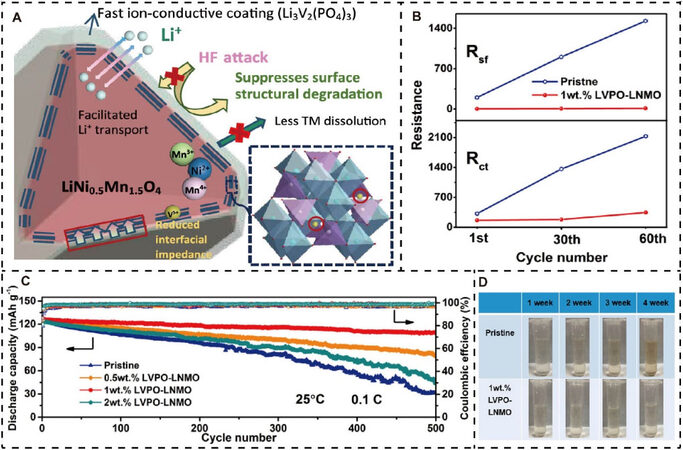
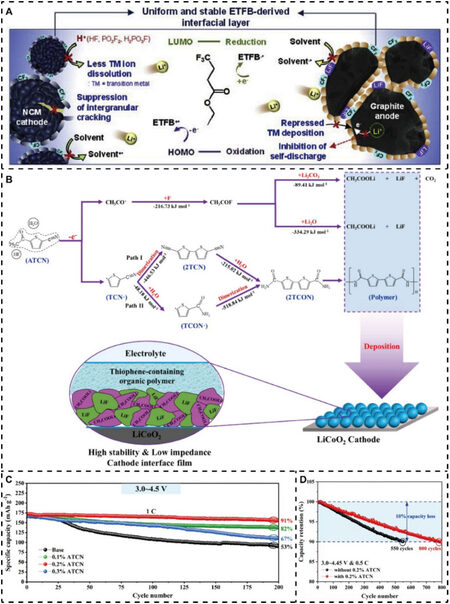
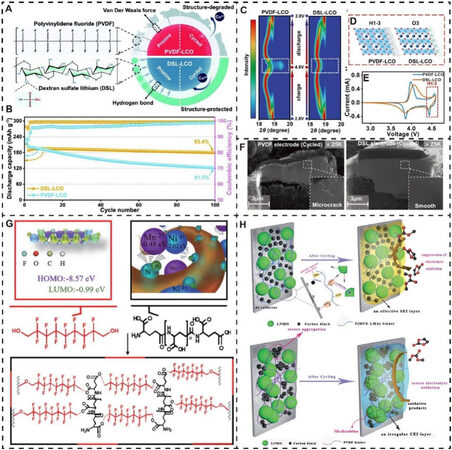
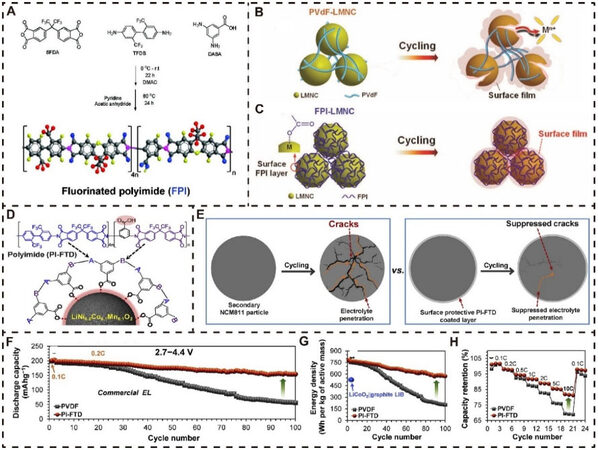











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.