A bimetallic-activated MnO2 self-assembly electrode with a dual heterojunction structure for high-performance rechargeable zinc-air batteries
Abstract
A major challenge in developing zinc-air batteries (ZABs) is to exploit suitable cathodes to efficiently accelerate the key electrocatalytic processes involved. Herein, a bifunctional oxygen catalytic self-supported MnO2-based electrode is designed that displays superior oxygen reduction and evolution reaction performance over noble metal electrodes with a total overpotential of 0.69 V. In addition, the as-synthesized NiCo2O4@MnO2/carbon nanotube (CNT)-Ni foam self-supported electrode can be directly used as an oxygen electrode without externally adding carbon or a binder and shows reasonable battery performance with a high peak power density of 226 mW cm-2 and a long-term charge-discharge cycling lifetime (5 mA for 160 h). As expected, the rapid oxygen catalytic intrinsic kinetics and high battery performance of the NiCo2O4@MnO2/CNTs-Ni foam electrode originates from the unique three-dimensional hierarchical structure, which effectively promotes mass transfer. Furthermore, the CNTs combined with Ni foam form a unique “meridian” conductive structure that enables rapid electron conduction. Finally, the abundant Mn3+ active sites activated by bimetallic ions shorten the oxygen catalytic reaction distance between the active sites and reactant and reduce the surface activity of MnO2 for the O, OH, and OOH species. This work not only offers a high-performance bifunctional self-supported electrode for ZABs but also opens new insights into the activation of Mn-based electrodes.
Keywords
INTRODUCTION
Zinc-air batteries (ZABs) have attracted significant interest due to their strong competitiveness in terms of cost, safety, and energy density[1-6]. However, the sluggish dynamics of the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) occurring at the cathode severely limit the charge-discharge performance and efficiency of ZABs. Although substantial work has been carried out on the synthesis of noble metal powder catalysts (e.g., Pt/C and IrO2) for the ORR and OER, the scarcity and unsatisfactory bifunctionality of noble metals severely reduce the possibility of their large-scale applications[1,7]. Thus, it is particularly necessary to develop low-cost and highly efficient non-precious metal bifunctional catalysts for the ORR and OER.
α-MnO2-based catalysts remain the most promising non-noble oxygen catalytic reaction candidates in alkaline electrolytes, owing to their unique layered and 2 × 2 tunnel structures[3,8,9]. Generally, the basic structural lattice framework of α-MnO2 is a corner-/edge-sharing [MnO6] octahedral unit and a Mn oxidation state of 4+[10]. In O2 catalytic reactions, Mn3+, cyclically produced by the Mn4+reduction, serves as the active site to form the O2 intermediate by combining with O2, which is the rate-determining step[11,12]. However, the formed Mn3+ still occupies the octahedral sites of the [MnO6] units and exists with unpaired single electrons at the high spin electronic energy eg band (Mn3+, t2g3, eg1), which shows extremely low thermodynamic stability[12]. Accordingly, obtaining abundant and stable Mn3+ is of great significance for designing high-performance Mn-based bifunctional catalysts. In recent decades, several valuable strategies have been proposed to further enhance the performance of α-MnO2-based catalysts, such as coupling other transition metal oxides to construct unique heterojunction structures[13-15]. For example, a Co3O4-MnO2/carbon nanotube (CNT) hybrid exhibited a significant increase in Mn3+ density and a concomitant decrease in electron energy due to the bonding of the bottom Co atoms to the surface O on the MnO2 surface[13]. As a result, the Co3O4-MnO2/CNT hybrid showed good ORR and OER performance. However, there are still two major issues for Mn-based catalysts manipulated by the unary spinel: (1) the electronic configuration of the Co-Mn heterojunction needs to be further modulated[16]; and (2) the inherent limitations of powder catalysts still need to be overcome (e.g., significant dead volume, an undesirable three-phase reaction interface and uncontrolled microstructures).
Some studies reported that partially replacing the Co element of Co3O4 in Co3O4-MnO2/CNT hybrids with nickel can reduce the particle size and increase the Co-Mn heterojunction density, which contributes to the activity and the long-term durability of the catalyst[14,17,18]. In particular, the interaction force between the low-spin Co3+ in the octahedral site and the oxygen intermediate can be weakened by the partial substitution of Ni atoms[19,20]. Thus, the bond length of Co-Mn-O can be further modulated by coupling the NiCo2O4 binary spinel instead of Co3O4[21]. In contrast, achieving the self-support of active species on conductive substrates can effectively avoid the limitations of powder catalysts[22,23]. On this basis, it has become more attractive to synthesize high-performance NiCo2O4/MnO2 self-supported bifunctional electrodes. The advantages of such a new self-supported bifunctional electrode include: (1) the construction of a high electron transfer pathway via in situ growth of the conductive substrates with electroactive species. The in situ self-fabrication procedure assures good conductivity and high mechanical stability of the electrode, which does not require the post-costing process and additional organic binders[24]; (2) a possibly rapid intrinsic kinetics and long-term durability due to the stable double heterojunction structure (NiCoOx-MnO2 and MnO2 substrate) and the activation of Mn sites[14]; and (3) rapid mass transfer (e.g., the ion diffusion and the emission of the reaction products) at the three-phase reaction interface, which should be attributed to the formation of the open space within a three-dimensional (3D) nanostructured array network[25]. Notably, a bimetallic-activated MnO2 self-supported electrode has not yet been explored in ZABs.
Hence, inspired by the aforementioned considerations, we design a useful strategy to construct a bimetallic-activated MnO2-based electrode by a self-assembly process. During this process, NiCo2O4-bound CNTs (a nanosheet) along the self-assembled MnO2 arrays on the surface of the nickel foam form a unique tremella-like 3D hierarchical structure with an abundant heterojunction (NiCo2O4@MnO2/CNTs-Ni foam). The unique 3D hierarchical structure effectively promotes electrolyte penetration, reactant (O2 and OH-) diffusion, and product emission, consequently facilitating the ORR and OER kinetics. In particular, the intrinsic activity of oxygen catalysis is further enhanced by the strategy of introducing nickel foam to form abundant Mn3+ active sites and utilizing bimetallic activation of the active sites to reduce the surface activity of MnO2 for the O, OH, and OOH species. As expected, the as-prepared NiCo2O4@MnO2/CNTs-Ni foam self-supporting electrode exhibits good OER and ORR performance with an ultralow total overpotential of 0.69 V. The NiCo2O4@MnO2/CNTs-Ni foam-based ZABs show a high peak power density of 226 mW cm-2 at 0.74 V, which is superior to Pt/C-RuO2 (93 mW cm-2 at 0.47 V). Additionally, the NiCo2O4@MnO2/CNTs-Ni foam electrode also displays long-term charge-discharge cycling stability, which should be attributed to the stable 3D hierarchical hollow hybrid structure and the active sites. This study provides a simple strategy to construct 3D hierarchical hollow hybrids and affords implications for the interface engineering field of high-performance catalysts.
RESULTS AND DISCUSSION
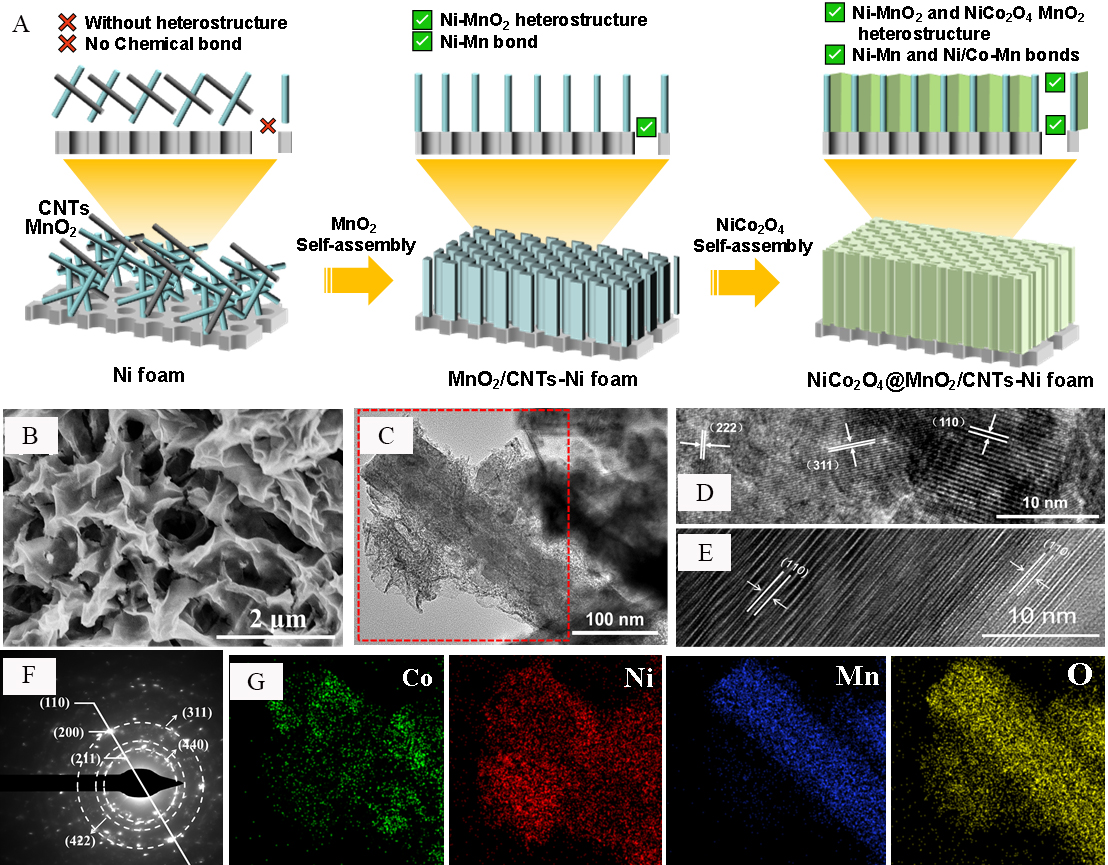
As shown in Figure 1A, the NiCo2O4@MnO2/CNTs-Ni foam electrode was synthesized via a facile hydrothermal coupled calcination approach. The SEM image in Figure 1B illustrates that the NiCo2O4@MnO2/CNTs-Ni foam synthesized at 160 °C for 6 h has a tremella-like 3D hierarchical structure with a pore size of ~0.4 μm. This hollow porous structure is believed to not only be beneficial for buffering the volume change induced by charge-discharge cycling but also to accelerate the penetration of the electrolyte, the diffusion of OH to the electrode surface, and the rapid dissociation of the reaction products. However, when the reaction time is less than 6 h, it is difficult to form this tremella-like 3D hierarchical structure, and when the reaction time is higher than 6 h, the structure is destroyed [Supplementary Figure 1]. This is mainly due to the thermodynamic conditions during the synthesis. When the reaction time is too short, its thermodynamic environment cannot perform the process of MnO2 self-assembly, and when the reaction time is too long, the excess energy will dissolve to form nanosheets.
Figure 1. (A) Schematic of NiCo2O4@MnO2/CNTs-Ni foam synthesis. (B) Scanning electron microscope (SEM) image of NiCo2O4@MnO2/CNTs-Ni foam. (C) Transmission electron microscope (TEM) image of NiCo2O4@MnO2/CNTs-Ni foam. (D) and (E) High-resolution transmission electron microscopy (HRTEM) images of NiCo2O4@MnO2/CNTs-Ni foam. (F) Selected area electron diffraction (SAED) image of NiCo2O4@MnO2/CNTs-Ni foam. (G) Elemental (Co, Ni, Mn and O) mapping of the area within the red dotted box in Figure 1C.
The structural information for the catalysts was obtained by TEM. As shown in Figure 1C, the NiCo2O4/CNT nanolayered structure can be well synthesized using the second hydrothermal self-assembly process at 160 °C for 6 h. Considerable NiCo2O4/CNT nanolayered structures with an average thickness of 30 nm wrap around the MnO2 nanotubes. MnO2 nanotubes were introduced into the NiCo2O4/CNT hybrid catalytic system and served as its skeleton. The distribution of the individual phases was studied by HRTEM analysis. The lattice spacings in Figure 1D and E are 0.69, 0.22, and 0.24 nm, corresponding to the (110) plane of a-MnO2 and the (222) and (311) planes of NiCo2O4, respectively. The SAED pattern [Figure 1F] results are consistent with those of the XRD analysis [Supplementary Figure 2], indicating a nickel-cobalt bimetallic composite of oxides and α-MnO2. The XRD characteristic peaks in Supplementary Figure 2 are contributed by α-MnO2 and NiCo2O4. This result proves that the Ni/Co bimetallic oxide synthesized by this method is NiCo2O4. Co, Ni, O, and Mn are uniformly distributed, as confirmed by the TEM elemental mappings shown in Figure 1G.
In order to gain a deeper understanding of the self-assembly process of the NiCo2O4@MnO2/CNTs-Ni foam, the SEM images of NiCo2O4, the Co3O4@MnO2-CNT@Ni foam, Co3O4/MnO2-CNTs, and MnO2 were obtained. As shown in Supplementary Figure 3, the length of the pure MnO2 tubes approaches 500 nm with a width of 50 to 100 nm. For the NiCo2O4 composites prepared at the same synthesis conditions without MnO2 and Ni foam, the diameter of the NiCo2O4 spheres is ~500 nm and they are huddled together tightly. When doped only with Co ions (Co3O4/MnO2-CNTs) without Ni foam, the Co3O4 particles are sparsely arranged on the surface of MnO2. In contrast, for Co and Ni ions (NiCo2O4/MnO2-CNTs) without Ni foam, the NiCo2O4 nanoparticles wrap on the surface of the MnO2 nanotubes densely and uniformly. The size of the NiCo2O4 particles (25 nm) on the MnO2 nanotubes is 25 times smaller than that of pure NiCo2O4 (500 nm). Smaller particle sizes tend to provide better structural stability and larger surface areas, thereby further facilitating the electrode activity[26]. Although the catalyst has been improved with regards to size and dispersibility, deficiency of the active sites still exists caused by random gatherings of the nanoparticles. When the Ni foam is introduced to the catalyst synthesis process, these nanoparticles self-assemble with CNTs along the MnO2 nanotubes to build a unique 3D network structure. In addition, the Co3O4@MnO2/CNTs-Ni foam [Supplementary Figure 3] also shows a similar tremella-like 3D hierarchical hollow hybrid structure as the nickel-cobalt bimetallic oxide composite.
The above findings reveal the importance of the Ni substrate, which can significantly reduce the size of the metal oxides by forming a steric hindrance effect during the self-assembling process, as well as avoiding the agglomeration of metal oxides and forming an abundant metal oxide-MnO2 heterojunction structure[27,28]. Ni foam can also provide growth sites for the self-assembly of MnO2, avoid the agglomeration of MnO2 and form the Ni foam-MnO2 heterojunction structure. Furthermore, it can induce the evolution of the nanoparticles on the surface of MnO2 into two-dimensional nanosheets, thereby realizing the rapid transformation of the metal oxide-MnO2 heterojunction in structure and density. This dense heterojunction structure forms an abundant three-phase reaction interface.
The XPS survey scan of the NiCo2O4@MnO2/CNTs-Ni foam confirms the presence of Mn, Co, Ni, C, and O elements [Figure 2A]. As shown in Figure 2B, for the spectrum of Mn 2p, the characteristic peaks of 640-650 eV and 650-660 eV belong to Mn 2p3/2 and Mn 2p1/2, respectively. The Mn 2p3/2 spectrum is deconvoluted into three peaks. The peaks located at 645.2, 641.4, and 638.2 eV are attributed to Mn4+, Mn3+, and Mn2+, respectively. Mn3+ is widely regarded as the active center for the oxygen-catalyzed reaction. Notably, the proportion of Mn3+ in the NiCo2O4@MnO2/CNTs-Ni foam is 55.8%, which is higher than for pure MnO2 (0%, Supplementary Figure 4A), the NiCo2O4@MnO2/CNTs-Ni foam catalyst (0%, Supplementary Figure 4B) and the Co3O4@MnO2-CNT@Ni foam (36.4%, Supplementary Figure 4C). Therefore, for the Ni-based self-supporting electrode, the introduction of Ni foam is beneficial to the existence of Mn ions in the form of Mn3+, which will effectively increase the active site density of the catalytic system.
Figure 2. (A) Overall XPS spectrum of NiCo2O4@MnO2/CNTs-Ni foam. High-resolution curves of (B) Mn 2p, (C) Co 2p, (D) Ni 2p, (E) C 1 s and (F) O 1 s regions.
In addition, compared with the normal Mn 2p peaks (641.8 eV for Mn 2p3/2 and 653.5 eV for Mn 2p1/2), the main Mn 2p peaks (641.4 eV and 652.9 eV) in the NiCo2O4@MnO2/CNTs-Ni foam shift to a lower binding energy. The binding energy shift should be caused by the transfer of electrons from NiCo2O4 to the MnO2 surface and the heterojunction formation of NiCo2O4-MnO2. The electrons obtained from NiCo2O4 activate the active site on the surface of MnO2 by moving the Mn electron cloud to a lower energy, thereby further enhancing the ORR and OER activities[29,30]. Specifically, in comparison to the Co3O4@MnO2-CNT@Ni foam, the NiCo2O4@MnO2/CNTs-Ni foam modified by Ni ions showed a stronger active center activation effect on MnO2 by changing the strength of the Ni-Co-O-Mn bond[14]. The Ni-Co-O-Mn bond can be significantly controlled by substituting Co2+ at tetrahedral sites with Ni ions[18]. Overall, the change in the Mn state may be due to the abundant Ni ions provided by the Ni foam and nitrate (especially Ni foam).
Figure 2C shows the Co 2p spectrum, with two peaks observed for Co 2p1/2 and Co 2p3/2 at ~790-800 eV and ~775-785 eV, respectively[31]. For the unfolded spectra, the peaks located at 798.9 and 795.8 eV are considered as Co3+ and Co2+, respectively, with the remaining two peaks at 783.5 and 780.1 eV also corresponding to Co3+ and Co2+. In addition, the Ni 2p spectra can be deconvoluted into 2 paired peaks of Ni2+ (874.0 and 855.9 eV)/Ni3+ (872.1 and 854.0 eV) with an energy separation of 18.1 eV, as well as two shakeup satellites (879.3 and 861.2 eV) [Figure 2D][32]. In addition, the intense peak of the C 1s spectrum
For the ORR and OER, the bimetallic activation effect caused by the construction of the abundant heterojunction of the self-supported electrode significantly decreases the hydrogen bonding with OH and OOH. In addition, the rich Mn3+ ions as the active sites further accelerate its intrinsic dynamics. In particular, the tremella-like 3D hierarchical structure builds a rich three-phase reaction interface, which improves the mass transfer. Finally, the coupling of CNTs and Ni foam form a unique "meridian" conductive structure that enables rapid electron transfer.
The activity and stability of the oxygen electrocatalyst were tested via cyclic voltammetry and linear sweep voltammetry (LSV) in O2 saturated 0.1 M KOH. As expected, the LSV curves of the OER indicate that the NiCo2O4@MnO2/CNTs-Ni foam exhibits a markedly decreased overpotential (η, η = Ej = 10 -1.23 V, Ej = 10: the OER potential at 10 mA cm-2) for the OER of 270 mV in comparison to the Co3O4@MnO2/CNTs-Ni foam (480 mV), NiCo2O4/MnO2-CNTs (510 mV) and NiCo2O4 (590 mV) [Figure 3A]. This overpotential is also 300 mV lower than that of commercial Pt/C-RuO2 catalysts [Supplementary Figure 5A]. This finding demonstrates that the self-supporting electrodes exhibit better OER activity than the powder catalyst, which can be attributed to the 3D hierarchical hollow hybrid structure created by the Ni foam base and the binder-free advantage for the self-supporting electrode[37]. Furthermore, the bimetallic-activated MnO2-based sample also shows a higher OER activity than the single metal-activated MnO2-based catalyst and pure MnO2, indicating that a metal oxide anchored on the MnO2 surface is an effective method to improve the catalytic activity.
Figure 3. (A) OER curves, (B) Tafel slopes, (C) ORR curves, (D) RDE curves at 400-1600 rpm (insert: K-L plots), € EIS curves, and (F) potential gap (ΔE) of ORR and OER for Pt/C-RuO2, NiCo2O4-CNTs, NiCo2O4/MnO2-CNTs, Co3O4@MnO2/CNTs-Ni foam, and NiCo2O4@MnO2/CNTs-Ni foam. (G) Schematic reaction mechanism of ORR and OER electrocatalyzed by NiCo2O4@MnO2/CNTs-Ni foam.
The Tafel slope was also calculated to reveal the reaction kinetics for the OER. As shown in Figure 3B, the small slope (105 mV dec-1) further indicates the rapid OER kinetics of the NiCo2O4@MnO2/CNTs-Ni foam, which is faster than the Co3O4@MnO2/CNTs-Ni foam (235 mV dec-1), NiCo2O4/MnO2-CNTs
The electron transfer number of the NiCo2O4@MnO2/CNTs-Ni foam was then calculated using Koutecky-Levich (K-L) plots. The LSV measurement of the sample powder was also carried out at 400-2025 rpm [Figure 3D], and the electron transfer number of NiCo2O4/MnO2-CNTs is in the range of 3.92-3.99, indicating a four-electron dominant transfer ORR pathway (O2 directly forms OH-). In addition, the semicircle of the Nyquist plot at low frequency corresponds to the Rct, i.e., the charge transfer resistance[39]. The Rct of the NiCo2O4@MnO2/CNTs-Ni foam is closer to the Co3O4/MnO2-CNTs@NF and lower than NiCo2O4/MnO2-CNTs, NiCo2O4, and MnO2, suggesting that the tremella-like 3D hierarchical structure and dual heterojunction structure of the NiCo2O4@MnO2/CNTs-Ni foam are beneficial to the rapid electron transfer, ion diffusion and product emission [Figure 3E]. Accordingly, the high ORR and OER activities should be due to the unique 3D hierarchical structure effectively promoting electrolyte penetration, O2/OH- diffusion, and product emission. Furthermore, the abundant Mn3+ active sites are activated by bimetallic ions, which shorten the oxygen catalytic reactions distance between the active sites and reactant and reduce the surface activity of MnO2 for the O, OH, and OOH species[13-14,26]. Finally, the CNTs combined with Ni foam form a novel “meridian” conductive structure that enables rapid electron conduction. As a result, the NiCo2O4@MnO2/CNTs-Ni foam electrode exhibits the lowest potential gap of ORR and OER
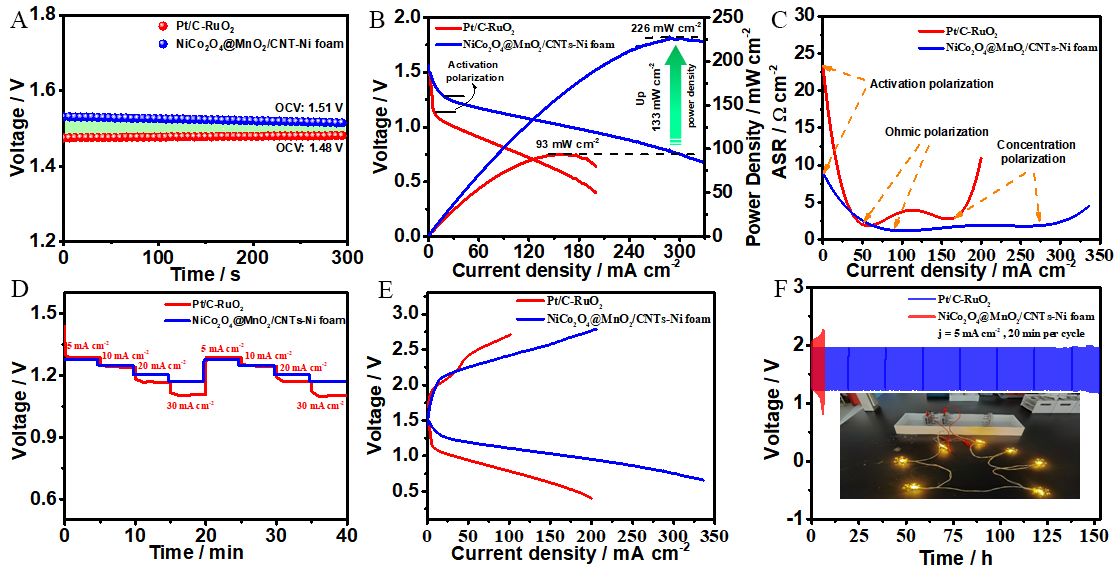
To examine the practical applications of the NiCo2O4@MnO2/CNTs-Ni foam electrode, ZABs were assembled with a zinc plate as the anode and the NiCo2O4@MnO2/CNTs-Ni foam self-supporting electrode as the air cathode. A ZAB with Pt/C-RuO2 was tested as a contrast. The open-circuit voltage (OCV) of the NiCo2O4@MnO2/CNTs-Ni foam battery was 1.51 V, which is superior to the Pt/C-RuO2 counterpart
Figure 4. ZAB using Pt/C-RuO2 and NiCo2O4@MnO2/CNTs-Ni foam. (A) OCV curves. (B) Polarization curves and corresponding power density plots. (C) Total ASR curves. (d) Different rate discharge cycling curves at different current densities (5-30 mA cm-2). (E) Charge and discharge polarization curves. (F) Charge-discharge cycles at 5 mA cm-2 (insert: a small bulb powered by a ZAB in series).
polarization than Pt/C-RuO2 [Figure 4C]. In addition, the zinc-air battery with NiCo2O4@MnO2/CNTs-Ni foam cathode shows a stable discharge performance with a high specific capacity of 814 mAh g-1
To further evaluate the charge-discharge potential and cycling stability, the galvanodynamic method and discharge-charge cycling testing were performed, respectively. As shown in Figure 4E, the NiCo2O4@MnO2/CNTs-Ni foam shows a lower charge-discharge potential at a larger current density than Pt/C-IrO2. The ZAB based on the NiCo2O4@MnO2/CNTs-Ni foam also displayed better cycling stability (165 h) with a durable and small charge-discharge voltage gap of 0.72 V than Pt/C-RuO2 (6 h, 0.82 V) [Figure 4F]. For the NiCo2O4@MnO2/CNTs-Ni foam, the voltage gap of the ZAB based on the NiCo2O4@MnO2/CNTs-Ni foam increased from 0.72 to 0.77 V after a continuous cycling operation for 150 h, showing that good long-term charge-discharge cycling performance. In comparison, the Pt/C-RuO2 electrode showed a fast performance degradation after 7 h. Finally, the morphology and electron structure of the NiCo2O4@MnO2/CNTs-Ni foam were obtained by using SEM and XPS after the long-term stability testing; the result proves the self-supporting 3D hierarchical hollow hybrid structure and the stable active sites greatly improve the catalyst lifetime [Supplementary Figure 7]. The ZAB based on the NiCo2O4@MnO2/CNTs-Ni foam air electrode shows better primary/rechargeable battery performance with higher power density and smaller charge-discharge voltage gap than the most recently reported NiCo2O4/MnO2-based bifunctional electrodes [Supplementary Table 1]. Simultaneously, the ZAB in series can power multiple small bulbs, showing good practical application prospects (insert of Figure 4F).
CONCLUSIONS
In summary, a NiCo2O4@MnO2/CNTs-Ni foam self-supported electrode was successfully designed and synthesized by a facial hydrothermal self-assembly process. The as-synthesized NiCo2O4@MnO2/CNTs-Ni foam self-supported electrode has a unique tremella-like 3D hierarchical structure with an abundant heterojunction and evolves rich Mn3+ active sites. The unique hierarchical structure effectively promotes electrolyte penetration, reactant (O2 and OH-) diffusion, and product emission. In addition, the abundant Mn3+ active sites activated by bimetallic ions effectively improve the oxygen catalytic intrinsic performance. Furthermore, the steric hindrance effect and providing self-assembly site mechanism of the Ni substrate were studied. The introduction of Ni foam can significantly avoid the agglomeration of metal oxides and induce the evolution of the nanoparticles on the surface of MnO2 into two-dimensional nanosheets, which realize the rapid formation of the abundant three-phase reaction interfaces. As expected, the NiCo2O4@MnO2/CNTs-Ni foam demonstrates a promising ORR and OER performance with high catalytic activity (total overpotential of 0.67 V) and the rapid intrinsic kinetics (a small Tafel slope of 105 mV dec-1). In addition, the ZABs with the NiCo2O4@MnO2/CNTs-Ni foam cathode also show a high peak power density of 226 mW cm-2, a large specific capacity of 814 mAh g-1 and a stable charge-discharge cycles with low voltage gap of 0.72 V for 165 h at 5 mA cm-2. These findings provide inspiration for the design of novel performance-oriented Mn-based self-supported electrodes for wider applications in metal-air batteries.
DECLARATIONS
Authors’ contributionsMethodology, formal analysis, investigation, writing manuscript: Yin Z, He R
Validation, resources, formal analysis: Chen J, Wang Y
Methodology, visualization, investigation: Ye X, Huang H, Xue H
Project administration, conceptualization, funding acquisition, supervision, writing manuscript: Xu N,
The data supporting our work can be found in the supplementary information.
Financial support and sponsorshipThis work was financially supported by the "Scientific and Technical Innovation Action Plan" Hong Kong, Macao and Taiwan Science & Technology Cooperation Project of Shanghai Science and Technology Committee (19160760600), the National Natural Science Foundation of China (21972017) and Shanghai Sailing Program (22YF1400700).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
Supplementary MaterialsREFERENCES
1. Bae J, Shin D, Jeong H, et al. Facet-dependent Mn doping on shaped Co3O4 crystals for catalytic oxidation. ACS Catal 2021;11:11066-74.
2. Chen C, Su H, Lu L, et al. Interfacing spinel NiCo2O4 and NiCo alloy derived N-doped carbon nanotubes for enhanced oxygen electrocatalysis. Chem Eng J 2021;408:127814.
3. Liu Y, Deng H, Lu Z, Zhong X, Zhu Y. The study of MnO2 with different crystalline structures for U(VI) elimination from aqueous solution. J Mol Liquids 2021;335:116296.
4. Xiao X, Hu X, Liang Y, et al. Anchoring NiCo2O4 nanowhiskers in biomass-derived porous carbon as superior oxygen electrocatalyst for rechargeable Zn-air battery. J Power Sources 2020;476:228684.
5. Li H, Ma L, Han C, et al. Advanced rechargeable zinc-based batteries: recent progress and future perspectives. Nano Energy 2019;62:550-87.
6. Pan J, Xu YY, Yang H, Dong Z, Liu H, Xia BY. Advanced architectures and relatives of air electrodes in Zn-air batteries. Adv Sci (Weinh) 2018;5:1700691.
7. Yin M, Miao H, Chen B, et al. Self-supported metal sulfide electrode for flexible quasi-solid-state zinc-air batteries. J Alloys Compd 2021;878:160434.
8. Cheng H, Chen JM, Li QJ, et al. A modified molecular framework derived highly efficient Mn-Co-carbon cathode for a flexible Zn-air battery. Chem Commun (Camb) 2017;53:11596-9.
9. Yang Z, Zheng C, Wei Z, et al. Multi-dimensional correlation of layered Li-rich Mn-based cathode materials. Energy Mater 2022;2:200006.
10. Xu N, Liu J, Qiao J, Huang H, Zhou X. Interweaving between MnO2 nanowires/nanorods and carbon nanotubes as robust multifunctional electrode for both liquid and flexible electrochemical energy devices. J Power Sources 2020;455:227992.
11. Xu N, Nie Q, Luo L, et al. Controllable hortensia-like MnO2 synergized with carbon nanotubes as an efficient electrocatalyst for long-term metal-air batteries. ACS Appl Mater Interfaces 2019;11:578-87.
12. Lee S, Nam G, Sun J, et al. Enhanced intrinsic catalytic activity of λ-MnO2 by electrochemical tuning and oxygen vacancy generation. Angew Chem Int Ed 2016;55:8599-604.
13. Xu N, Zhang Y, Wang M, et al. High-performing rechargeable/flexible zinc-air batteries by coordinated hierarchical Bi-metallic electrocatalyst and heterostructure anion exchange membrane. Nano Energy 2019;65:104021.
14. Xu N, Zhang Y, Wang Y, et al. Hierarchical bifunctional catalysts with tailored catalytic activity for high-energy rechargeable Zn-air batteries. Appl Energy 2020;279:115876.
15. Singh A, Ojha AK. Designing vertically aligned porous NiCo2O4@MnMoO4 Core@Shell nanostructures for high-performance asymmetric supercapacitors. J Colloid Interface Sci 2020;580:720-9.
16. Li A, Kong S, Guo C, et al. Enhancing the stability of cobalt spinel oxide towards sustainable oxygen evolution in acid. Nat Catal 2022;5:109-18.
17. Xu N, Cai Y, Peng L, et al. Superior stability of a bifunctional oxygen electrode for primary, rechargeable and flexible Zn-air batteries. Nanoscale 2018;10:13626-37.
18. Wang XT, Ouyang T, Wang L, Zhong JH, Ma T, Liu ZQ. Redox-Inert Fe3+ ions in octahedral sites of Co-Fe spinel oxides with enhanced oxygen catalytic activity for rechargeable zinc-air batteries. Angew Chem Int Ed 2019;58:13291-6.
19. Gangadharan PK, Bhange SN, Kabeer N, Illathvalappil R, Kurungot S. NiCo2O4 nanoarray on CNT sponge: a bifunctional oxygen electrode material for rechargeable Zn–air batteries. Nanoscale Adv 2019;1:3243-51.
20. Kumar R. NiCo2O4 nano-/microstructures as high-performance biosensors: a review. Nanomicro Lett 2020;12:122.
21. Yang C, Gao N, Wang X, et al. Phosphate boosting stable efficient seawater splitting on porous NiFe (oxy)hydroxide@NiMoO4 Core-Shell micropillar electrode. Energy Mater 2021;1:100015.
22. Ma TY, Dai S, Qiao SZ. Self-supported electrocatalysts for advanced energy conversion processes. Mater Today 2016;19:265-73.
23. Ye L, Hong Y, Liao M, et al. Recent advances in flexible fiber-shaped metal-air batteries. Energy Stor Mater 2020;28:364-74.
24. Pei Z, Yuan Z, Wang C, et al. A flexible rechargeable zinc-air battery with excellent low-temperature adaptability. Angew Chem Int Ed 2020;59:4793-9.
25. Wang B, Chen Y, Wang X, et al. A microwave-assisted bubble bursting strategy to grow Co8FeS8/CoS heterostructure on rearranged carbon nanotubes as efficient electrocatalyst for oxygen evolution reaction. J Power Sources 2020;449:227561.
26. Xu N, Zhang Y, Zhang T, Liu Y, Qiao J. Efficient quantum dots anchored nanocomposite for highly active ORR/OER electrocatalyst of advanced metal-air batteries. Nano Energy 2019;57:176-85.
27. Xu N, Liu Y, Zhang X, et al. Self-assembly formation of Bi-functional Co3O4/MnO2-CNTs hybrid catalysts for achieving both high energy/power density and cyclic ability of rechargeable zinc-air battery. Sci Rep 2016;6:33590.
28. Xu N, Wilson JA, Wang Y, et al. Flexible self-supported bi-metal electrode as a highly stable carbon- and binder-free cathode for large-scale solid-state zinc-air batteries. Appl Catal B-Environ 2020;272:118953.
29. Wang A, Hu Y, Wang H, et al. Activating inverse spinel NiCo2O4 embedded in N-doped carbon nanofibers via Fe substitution for bifunctional oxygen electrocatalysis. Mater Today Phys 2021;17:100353.
30. Ma R, Lin G, Ju Q, et al. Edge-sited Fe-N4 atomic species improve oxygen reduction activity via boosting O2 dissociation. Appl Catal B: Environ 2020;265:118593.
31. Singh T, Das C, Bothra N, et al. MOF Derived Co3O4@Co/NCNT nanocomposite for electrochemical hydrogen evolution, flexible zinc-air batteries, and overall water splitting. Inorg Chem 2020;59:3160-70.
32. Wang Z, Ang J, Liu J, et al. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl Catal B: Environ 2020;263:118344.
33. Xie W, Li J, Song Y, Li S, Li J, Shao M. Hierarchical carbon microtube@nanotube core-shell structure for high-performance oxygen electrocatalysis and Zn-air battery. Nanomicro Lett 2020;12:97.
34. Zhang X, Han X, Jiang Z, et al. Atomically dispersed hierarchically ordered porous Fe-N-C electrocatalyst for high performance electrocatalytic oxygen reduction in Zn-air battery. Nano Energy 2020;71:104547.
35. Zheng X, Cao X, Sun Z, et al. Indiscrete metal/metal-N-C synergic active sites for efficient and durable oxygen electrocatalysis toward advanced Zn-air batteries. Appl Catal B: Environ 2020;272:118967.
36. Wang Q, Xue Y, Sun S, Yan S, Miao H, Liu Z. Facile synthesis of ternary spinel Co-Mn-Ni nanorods as efficient bi-functional oxygen catalysts for rechargeable zinc-air batteries. J Power Sources 2019;435:226761.
37. Deng Y, Jiang Y, Luo D, et al. Hierarchical porous double-shelled electrocatalyst with tailored lattice alkalinity toward bifunctional oxygen reactions for metal-air batteries. ACS Energy Lett 2017;2:2706-12.
38. Su HY, Gorlin Y, Man IC, et al. Identifying active surface phases for metal oxide electrocatalysts: a study of manganese oxide bi-functional catalysts for oxygen reduction and water oxidation catalysis. Phys Chem Chem Phys 2012;14:14010-22.
39. Chen Z, Yu A, Ahmed R, Wang H, Li H, Chen Z. Manganese dioxide nanotube and nitrogen-doped carbon nanotube based composite bifunctional catalyst for rechargeable zinc-air battery. Electrochim Acta 2012;69:295-300.
40. Han C, Zhang T, Li J, Li B, Lin Z. Enabling flexible solid-state Zn batteries via tailoring sulfur deficiency in bimetallic sulfide nanotube arrays. Nano Energy 2020;77:105165.
41. Han J, Meng X, Lu L, Wang ZL, Sun C. Triboelectric nanogenerators powered electrodepositing tri-functional electrocatalysts for water splitting and rechargeable zinc-air battery: a case of Pt nanoclusters on NiFe-LDH nanosheets. Nano Energy 2020;72:104669.
42. Wang Y, Li Z, Zhang P, et al. Flexible carbon nanofiber film with diatomic Fe-Co sites for efficient oxygen reduction and evolution reactions in wearable zinc-air batteries. Nano Energy 2021;87:106147.
43. Wang Z, Huang J, Wang L, et al. Cation-tuning induced d-band center modulation on Co-based spinel oxide for oxygen reduction/evolution reaction. Angew Chem Int Ed 2022;61:e202114696.
44. Yan L, Xu Z, Hu W, Ning J, Zhong Y, Hu Y. Formation of sandwiched leaf-like CNTs-Co/ZnCo2O4@NC-CNTs nanohybrids for high-power-density rechargeable Zn-air batteries. Nano Energy 2021;82:105710.
45. Zhang Z, Sun H, Li J, et al. S-doped CoMn2O4 with more high valence metallic cations and oxygen defects for zinc-air batteries. J Power Sources 2021;491:229584.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Yin Z, He R, Xue H, Chen J, Wang Y, Ye X, Xu N, Qiao J, Huang H. A bimetallic-activated MnO2 self-assembly electrode with a dual heterojunction structure for high-performance rechargeable zinc-air batteries. Energy Mater 2022;2:200021. http://dx.doi.org/10.20517/energymater.2022.17
AMA Style
Yin Z, He R, Xue H, Chen J, Wang Y, Ye X, Xu N, Qiao J, Huang H. A bimetallic-activated MnO2 self-assembly electrode with a dual heterojunction structure for high-performance rechargeable zinc-air batteries. Energy Materials. 2022; 2(3): 200021. http://dx.doi.org/10.20517/energymater.2022.17
Chicago/Turabian Style
Yin, Zhengyu, Rui He, Huaibin Xue, Jingjian Chen, Yue Wang, Xiaoxiao Ye, Nengneng Xu, Jinli Qiao, Haitao Huang. 2022. "A bimetallic-activated MnO2 self-assembly electrode with a dual heterojunction structure for high-performance rechargeable zinc-air batteries" Energy Materials. 2, no.3: 200021. http://dx.doi.org/10.20517/energymater.2022.17
ACS Style
Yin, Z.; He R.; Xue H.; Chen J.; Wang Y.; Ye X.; Xu N.; Qiao J.; Huang H. A bimetallic-activated MnO2 self-assembly electrode with a dual heterojunction structure for high-performance rechargeable zinc-air batteries. Energy Mater. 2022, 2, 200021. http://dx.doi.org/10.20517/energymater.2022.17
About This Article
Copyright
Data & Comments
Data
 Cite This Article 15 clicks
Cite This Article 15 clicks















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.