Enabling 4.6 V LiNi0.6Co0.2Mn0.2O2 cathodes with excellent structural stability: combining surface LiLaO2 self-assembly and subsurface La-pillar engineering
Abstract
Although Ni-rich layered materials with the general formula LiNi1-x-yCoxMnyO2 (0 < x, y < 1, NCM) hold great promise as high-energy-density cathodes in commercial lithium-ion batteries, their practical application is greatly hampered by poor cyclability and safety. Herein, a LiNi0.6Co0.2Mn0.2O2 (NCM622) cathode modified with a surface self-assembling LiLaO2 coating and subsurface La pillars demonstrates stabilized cycling at 4.6 V. The LiLaO2-coated NCM622 benefits from the suppression of interfacial side reactions, which relieves the layer-to-rock salt phase transformation and therefore improves the capacity retention under high voltages. Moreover, the La dopant, as a pillar in the NCM622 lattice, plays a dual role in expanding the c lattice parameter to enhance the Li-ion diffusion capability, as well as suppressing Ni antisite defect formation upon cycling. Consequently, the dual-modified NCM622 cathode exhibits an initial Coulombic efficiency of over 85% and a high capacity of over 200 mAh g-1 at 0.1 C. A specific capacity of 188 mAh g-1 with a capacity retention of 76% is achieved at 1 C after 200 cycles within a voltage range of 3.0-4.6 V. These findings lay a solid foundation for the materials design and performance optimization of high-energy-density cathodes for Li-ion batteries.
Keywords
INTRODUCTION
Despite the rapid development of lithium-ion batteries (LIBs) in recent decades, their finite energy and power densities are regarded as the main barriers to their further application[1-4]. The cathode is the most critical component and is crucial in determining the working voltage, energy density and cost of a LIB[5-9]. Various promising cathode candidates with high capacity, including LiCoO2 and Ni-rich materials, have been extensively designed for LIBs[10,11]. Among the available candidates, nickel-rich layered cathode materials, namely, LiNixCoyMn1-x-yO2 (NCM, x ≥ 0.6), have demonstrated excellent market prospects owing to their advantages of high specific capacities, large discharge plateaus, low price and hypotoxicity[12-14]. Although the specific capacity of NCM materials increases significantly with the nickel content, the resulting cation mixing and interfacial side reactions lead to a rapid performance decay upon cycling. In addition, the NCM materials with more than a 70% nickel content manifest apparent safety concerns, thereby hindering their large-scale application[15-19]. The increasing cutoff voltage of NCM can also increase its specific capacity. For instance, the discharge capacity of LiNi0.6Co0.2Mn0.2O2 (NCM622) can be enhanced from 180 to 200 mAh g-1 with an increase in the cutoff voltage from 4.4 to 4.6 V. However, at
Many approaches, including elemental doping and surface coating, have been adopted to overcome the aforementioned issues and enhance the structural stability of Ni-rich cathodes. Lattice doping using a variety of extrinsic ions, such as Ti[25], Na[26], Mg[27,28], Zr[29], Nb[30], Al[31] and La[32], has been already used to plausibly improve the electrochemical performance of NCM materials. Inorganic oxides, such as MgO[33],
La is a relatively inexpensive rare-earth material that is widely used in industry. The La-O bond has an energy of 798 kJ mol-1, much higher than that for Ni-O (392 kJ mol-1), Co-O (368 kJ mol-1) and Mn-O
EXPERIMENTAL
Materials preparation. The Ni0.6Co0.2Mn0.2(OH)2 precursor was synthesized using a hydroxide coprecipitation method. The details of the coprecipitation process are displayed in the supporting information. NCM622 samples were prepared by mixing Ni0.6Co0.2Mn0.2(OH)2 precursors and Li2CO3 (Li:Ni ratio of 1:0.56). After calcined at 870 °C for 10 h, the samples were cooled to 570 °C and then sintered at
Materials characterization. The structure and morphology of the samples were evaluated by X-ray diffraction (XRD, Rigaku DMAX 2500, Tokyo, Japan) with Cu Kα radiation and scanning electron microscopy (SEM, Hitachi S-4800, Tokyo, Japan), respectively. The lattice parameters of the samples were refined by Full Prof Suite software. High-resolution transmission electron microscopy (HR-TEM, Tecnai G2 F30) was conducted to further understand the microstructure of the samples. The chemical states of the main elements of the materials were analyzed by X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi spectrometer).
Electrochemical measurements. The cell in a coin configuration (CR2016) assembled with the cathode, separator, lithium plate and electrolyte in an argon-filled glove box was used to investigate the electrochemical properties. The cathode was fabricated by coating the slurry composed of the active material, carbon black and binder at a ratio of 80:10:10 wt.% on Al foil. The electrode mass loadings of NCM622, NCM622-La and NCM622-La@LLO were 1.20, 1.18 and 1.22 mg/cm2, respectively. Li metal was used as the counter and reference electrodes. Celgard 2400 was used as the separator. The electrolyte was
RESULTS AND DISCUSSION
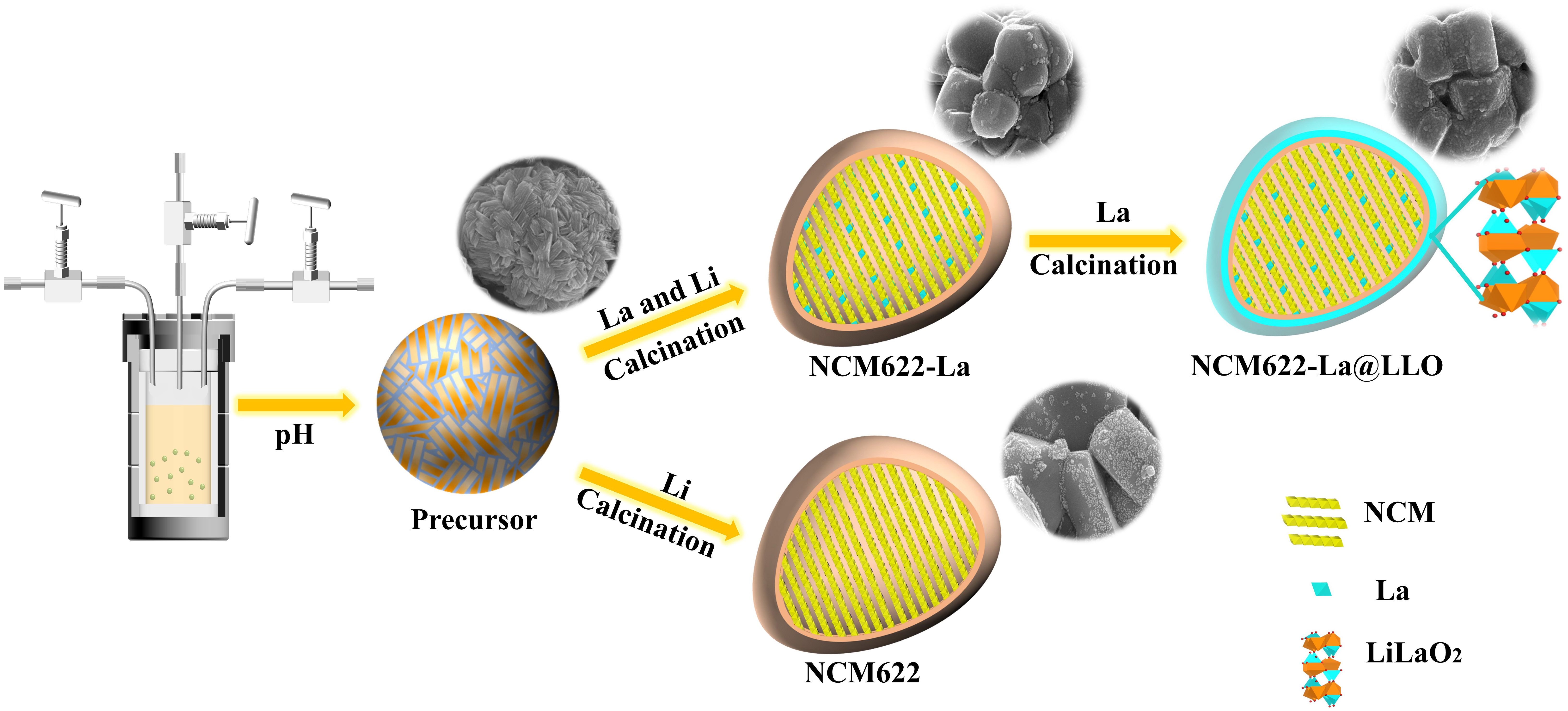
The crystal structures of NCM622, La-doped NCM622 (NCM622-La) and La-doped and LiLaO2-coated NCM622 (NCM622-La@LLO) were studied by XRD, as shown in Figure 1A-C. The diffraction peaks of the (003), (101), (104), (105), (107) and (113) planes are observed [Figure 1A] and indexed to the hexagonal 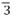 m
m
Figure 1. (A) XRD patterns of NCM622, NCM622-La and NCM622-La@LLO. (B and C) Comparison of (003) and (104) peaks for the three samples. (D-F) XPS comparison of Ni element for the three samples. (G-I) SEM morphology images of NCM622, NCM622-La and NCM622-La@LLO and (J) the corresponding energy-dispersive spectroscopy mapping results of NCM622-La@LLO. TEM images of (K and L) NCM622 and (M and N) NCM622-La@LLO.
XPS was used to evaluate the surface composition and oxidation states of the samples. As illustrated in Figure 1D-F, the peaks of Ni-2p3/2 at 855.60 and 854.53 eV represent Ni3+ and Ni2+, respectively. Apparently, compared to that in the pristine sample, the decrease in the Ni2+ content in both NCM622-La and NCM622-La@LLO is mainly due to the introduction of La3+, whose electrostatic repulsion prevents more Ni2+ from migrating to the Li site, thereby inhibiting cation mixing[54,55]. The XPS analysis comparison of La in the three samples indicates that the La particles exist in both the NCM622-La and NCM622-La@LLO electrodes [Supplementary Figure 2]. The Rietveld refinement results further confirm that the Ni-Li cation mixing decreases after the La doping and LiLaO2 coating [Supplementary Figure 3 and Supplementary Table 1].
The sizes of the NCM622-La@LLO particles are ~0.5 μm, smaller than that of the pristine NCM622 particles (~1 μm), as shown by the SEM images in Figure 1G-I. The introduction of La in the lithiation of the hydroxide precursor changes the surface energy, which changes the fusion of nanosheets during high-temperature calcination[56]. Interestingly, compared with the modified NCM622, many small particles appear on the surface of the original sample, which are residual lithium (Li2CO3/LiOH)[38]. The surface of the NCM622-La@LLO particles displays a blurred edge compared with the pristine NCM622 particles, preliminarily proving the LiLaO2 formed on the surface of NCM622. Moreover, the integral morphologies of the three samples show no significant differences, indicating that the overall morphology of the host material is free from the destruction of the doping and coating processes [Supplementary Figure 4]. The energy-dispersive spectroscopy mapping images of NCM622-La@LLO
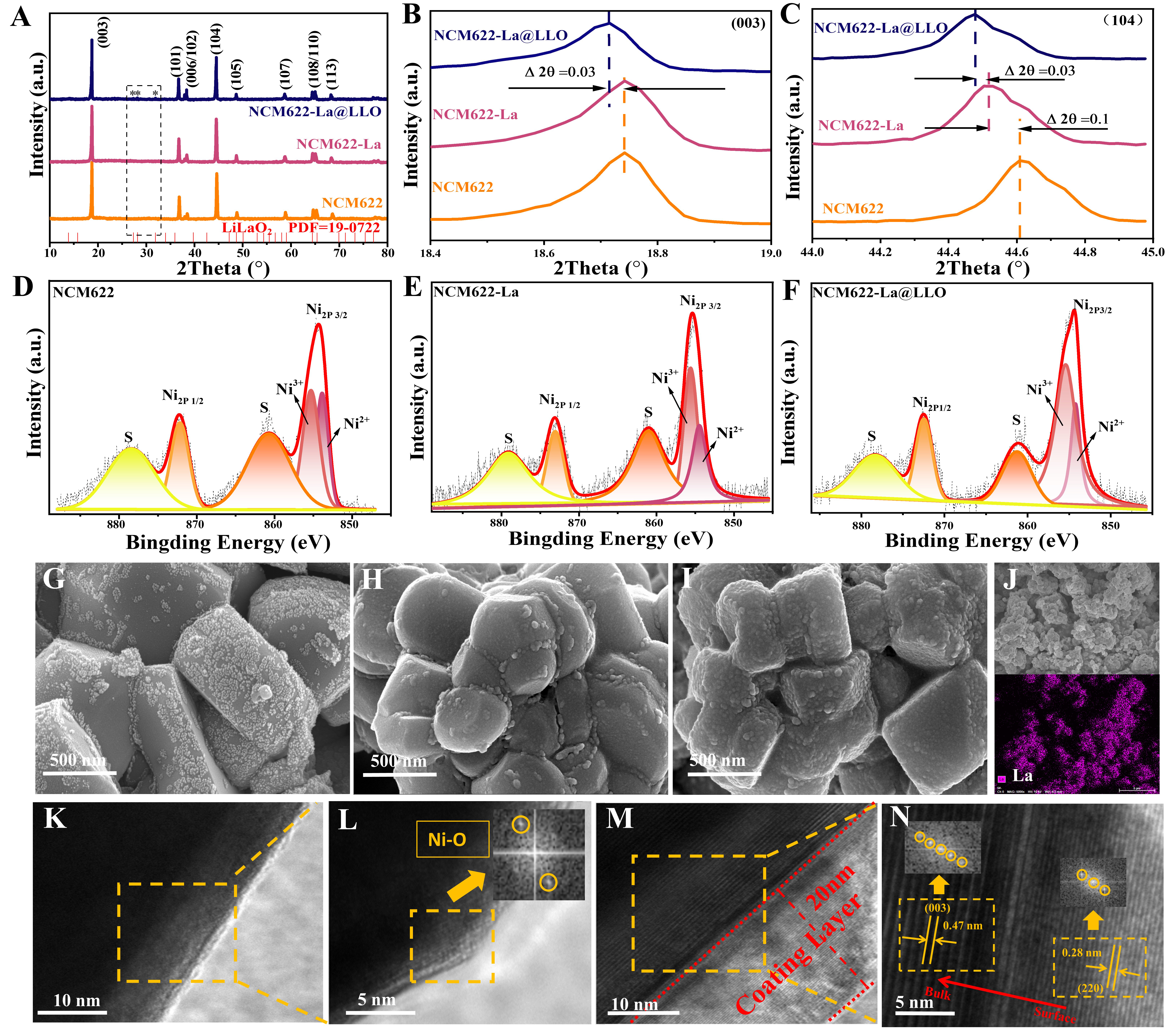
DFT calculations were used to estimate the surface structure of NCM622 before and after modification with La doping and LiLaO2 coating, as shown in Figure 2A-I. Using the same structure, the calculated density of states (DOS) of NCM622-La@LLO is much larger than that of NCM622 and NCM622-La at the Fermi level [Figure 2D-F and Supplementary Figure 6], indicating that the electronic conductivity of NCM622-La@LLO is improved[57]. Figure 2G-I and Supplementary Figures 7 and 8 exhibit the charge density differences between the three samples. An evident electron transfer phenomenon occurs among Ni, Co, La and the surrounding O atoms, and becomes clearer after La doping and LiLaO2 coating. In addition, the La-O has a high electron aggregation degree compared to the Ni-O, suggesting that La-O has a stronger chemical bond, which will be beneficial to stabilize lattice oxygen during cycling, especially at a high cutoff voltage. Stable lattice oxygen can inhibit the layer-to-spinel/rock salt phase transformation and improve the electrochemical performance.
Figure 2. (A-C) Structural diagrams, (D-F) DOS and (G-I) differential charge densities of the three samples.
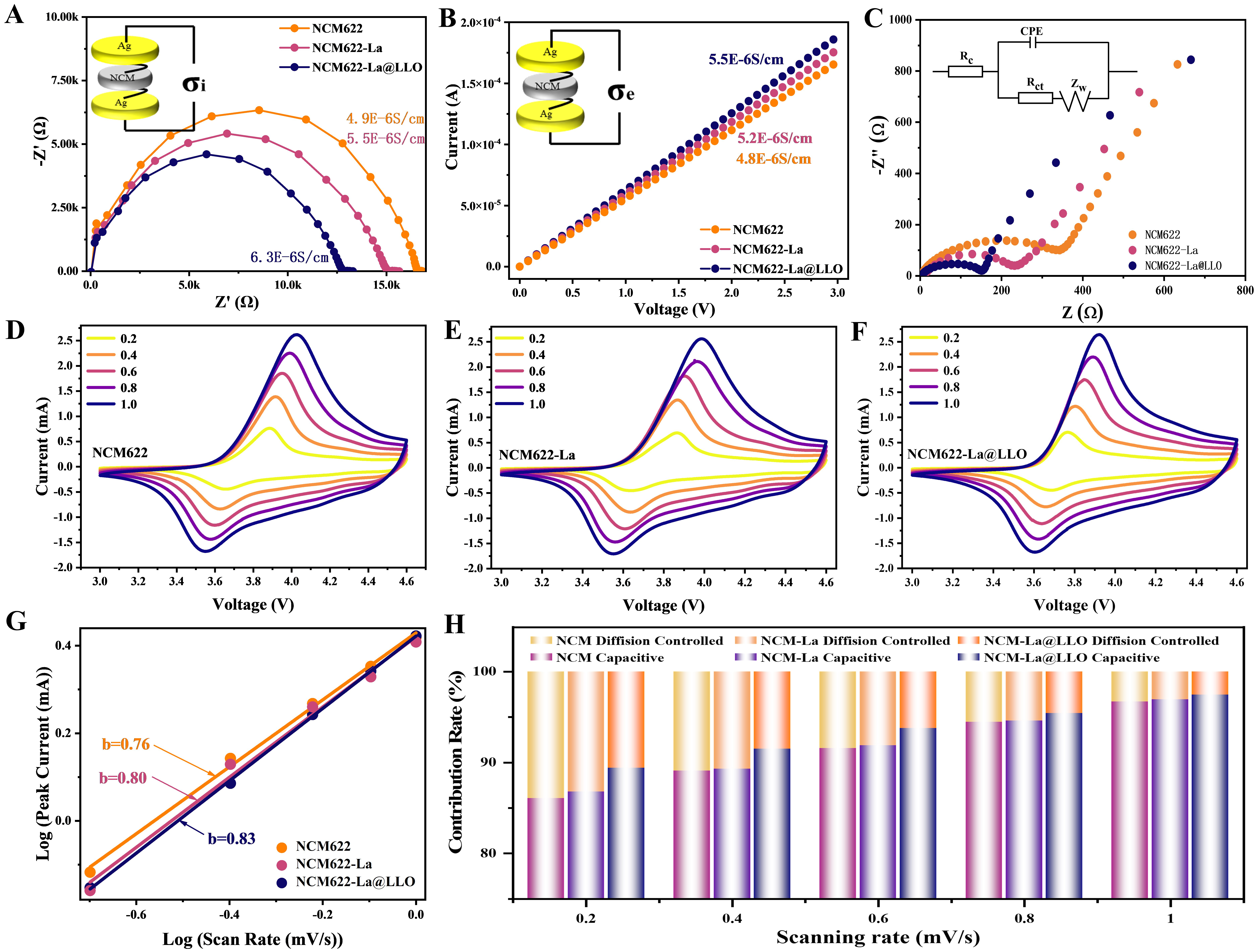
The ionic and electronic conductivities of NCM622, NCM622-La and NCM622-La@LLO measured with a blocking electrode method show that NCM622-La@LLO has the highest values [Figure 3A and B]. The Nyquist plots of the half-cells also reveal the best lithium-ion transport capability of NCM622-La@LLO [Figure 3C]. To understand the dynamic behavior of the three samples, the galvanostatic intermittent titration technique was used at a pulsed current of 20 mA/g [Supplementary Figure 9]. The lithium-ion diffusion coefficient in NCM622-La@LLO is estimated to be ~1.4 × 10-10 cm2 s-1, higher than that in NCM622 (5.1 × 10-12 cm2 s-1) and NCM622-La (4.0 × 10-11 cm2 s-1), suggesting that the La doping and LiLaO2 coating boost the lithium-ion diffusion kinetics of NCM622. CV was carried out at a scan rate of 0.1 mV s-1 within the potential range of 3.0-4.6 V [Supplementary Figure 10]. The potential difference (ΔEp) values of the three samples between the oxidation/reduction peaks are 286 mV (NCM622), 187 mV (NCM622-La) and 166 mV (NCM622-La@LLO), respectively. The minimum positional deviation of the oxidation/deoxidization peaks indicates that NCM622-La@LLO exhibits the best electrochemical activity and cycle reversibility. The CV curves of the three samples at different potential scan rates from 0.2 to
Figure 3. (A) Ionic and (B) electronic conductivity of NCM622, NCM622-La and NCM622-La@LLO samples. (C) Nyquist plots of the three electrodes. (D-F) CV curves of the three samples at different potential scanning rates. (G) Relationship between logarithmic anode peak current and logarithmic scan rates of the three electrodes. (H) Capacitance contribution calculations of the three electrodes at various scan rates.
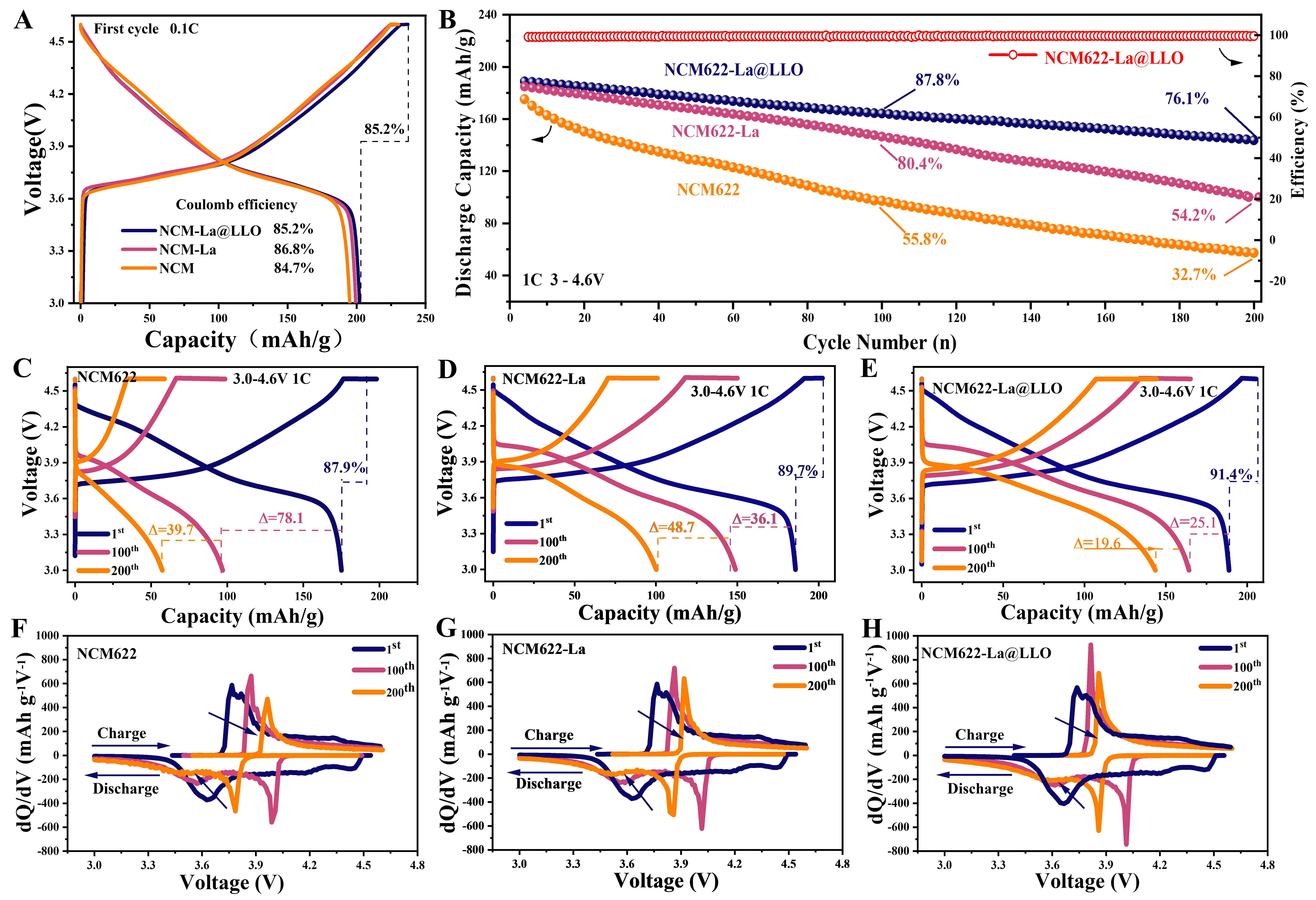
The first-cycle discharge/charge capacities of NCM622-La@LLO are 237.2/202.2 mAh g-1, with a corresponding initial Coulombic efficiency (ICE) of 85.2% [Figure 4A]. There is little difference among the ICEs compared with NCM622 (84.7%) and NCM622-La (86.8%) [Supplementary Figure 11], indicating that La doping and LiLaO2 coating have little effect on the ICE of NCM622 materials. Figure 4B further compares the electrochemical cycling performances of the three samples after various cycles at 1 C. For NCM622-La@LLO, excellent capacity retention can be achieved at 76.1% after 200 cycles. In contrast, pristine NCM622 undergoes rapid capacity decay, resulting in a lower capacity retention of only 32.7% after 200 cycles. Furthermore, NCM622-La@LLO shows a good charge and discharge plateau above 3.6 V after 200 cycles [Figure 4C-E]. Figure 4F-H provides a detailed analysis of the evolution process of the differential capacitance (dQ/dV) curves of the three samples. The dQ/dV curves display two redox peaks during cycling. However, for the NCM622 sample, the intensity of the redox peak becomes weaker than that of the NCM622-La and NCM622-La@LLO samples when the cycle number increases, indicating that La-pillared and LiLaO2-coated NCM622 exhibits a more stable structure, a stronger ability to withstand high voltages and better electrochemical performance.
Figure 4. (A) Charge and discharge curves of the first cycles of NCM, NCM-La and NCM622-La@LLO at a current density of 20 mA g-1 (0.1 C) and (B) corresponding cycling performance at 1 C in the voltage range of 3.0-4.6 V. Charge and discharge curves of different cycles and corresponding dQ/dV curves of (C and F) NCM622, (D and G) NCM622-La and (E and H) NCM622-La@LLO.
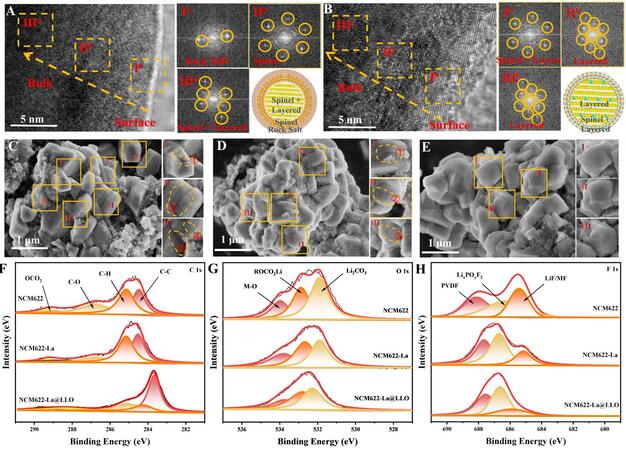
The crystal structures and surface morphologies of the samples after 200 cycles were investigated by TEM and SEM, respectively. The TEM images of NCM-La@LLO show that although no rock-salt phase is observed on the NCM-La@LLO surface, it conserves a well-ordered layered structure [Figure 5B]. In contrast, the rock-salt phase exists on the surface region of NCM622 and the spinel phase is detected from the diffraction spots, as shown in Figure 5A. The NCM622 exhibits a salt-spinel-layered structure from the surface to the inner phase, but the NCM-La@LLO exhibits a slight mixture of the layered and spinel phases on the surface. This obvious evidence indicates that La doping and LiLaO2 coating significantly inhibit the transformation of the layered phase to the rock-salt phase and maintain the structural stability of the NCM material, thereby improving the reversible specific capacity and cycle life. The surface morphologies of NCM622-La@LLO after 200 cycles are shown in Figure 5E. Compared with the NCM622 and NCM622-La particles [Figure 5C and D], no cracks exist on the surface of NCM622-La@LLO, indicating that La doping and LiLaO2 coating significantly inhibit the phase transition to maintain the structural stability of NCM at high voltages, thus improving the electrochemical performance.
Figure 5. TEM images of (A) NCM622 and (B) NCM622-La@LLO after 200 cycles, where Ia, IIa, IIIa, Ib, IIb and IIIb are the fast Fourier transform conversion graphs of the corresponding regions. Surface morphologies of (C) NCM622, (D) NCM622-La and (E) NCM622-La@LLO after 200 cycles. (F) C1s, (G) O1s and (H) F1s XPS spectra of NCM622, NCM622-La and NCM622-La@LLO after 200 cycles.
To further verify the protective effect of the coating layers on the cathode-electrolyte interface, XPS measurements on NCM622, NCM622-La and NCM622-La@LLO after 200 cycles were conducted
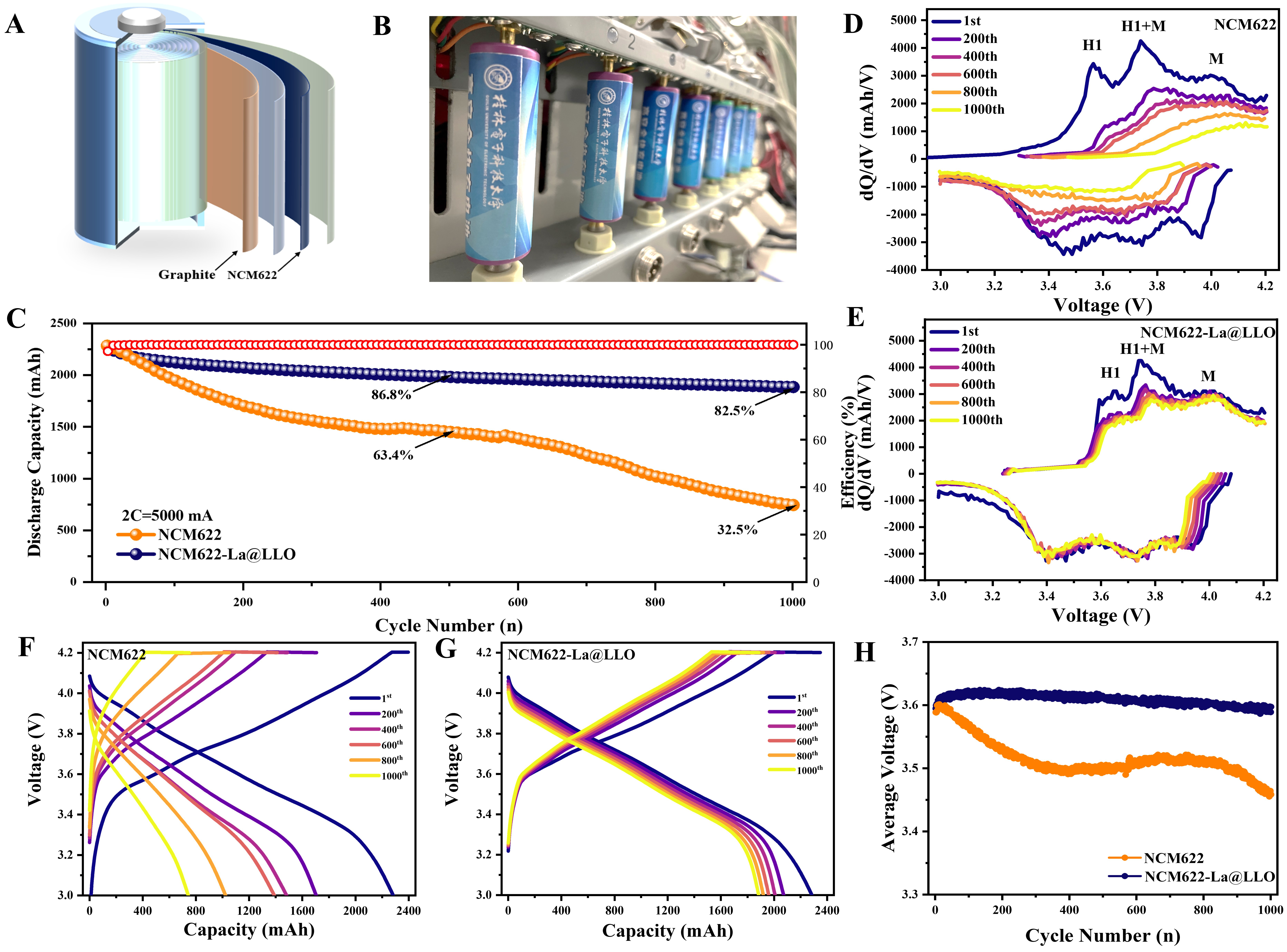
Cylindrical full batteries with a design capacity of 2500 mAh were assembled to compare the cycling stabilities of NCM622 and NCM622-La@LLO in real applications. Schematic diagrams of the cylindrical full batteries are shown in Figure 6A and B. The NCM622-La@LLO cell undergoes 1000 cycles with an excellent capacity retention of 82.5% [Figure 6C]. The overlap of the dQ/dV curves of the NCM622-La@LLO cathode demonstrates that La doping and LiLaO2 coating can maintain the structural integrity of NCM materials
Figure 6. Electrochemical characterization of cylindrical full batteries. (A) Schematic and (B) photographs of the configuration of a graphite//NCM622-La@LLO full battery. (C) Cycling performance of full batteries with both pristine NCM622 and NCM622-La@LLO as the cathode and graphite as the anode at 2 C. (D and E) dQ dV-1 curves at selected cycles. (F and G) Corresponding charge/discharge curves. (H) Comparison of voltage platform attenuation.
CONCLUSIONS
In summary, we present a practical solution to enhance the stability of NCM622 at 4.6 V via the self-assembly of a LiLaO2 coating on its surface and La pillars in its subsurface. The dual-modified NCM622-La@LLO cathode exhibits a capacity of more than 200 mAh g-1 and an initial Coulombic efficiency of 85% at a current density of 0.1 C. Even after 200 cycles, NCM622-La@LLO maintains a capacity retention of 1.7 times higher than the pristine material (76% vs. 45%) at 1 C and 4.6 V. Two factors are responsible for the superior electrochemical performance of the NCM622-La@LLO cathode. The first is that the LiLaO2 coating on the surface of NCM622 is beneficial for inhibiting the side reactions between cathodes and electrolytes and suppressing the phase transformation from the layered phase to the rock-salt phase, thereby improving the capacity retention at high voltage. The other reason is that the La atom dopants, as pillars in the NCM622 lattice, increase the c-axis distance to improve the Li+ diffusion rate, and suppress nickel taking the place of lithium. We expect this strategy could provide a direction for managing the internal structure and interfacial stability of NCM, which can be extended to the applications of other cathodes, such as
DECLARATIONS
Authors’ contributionsMethodology, formal analysis and writing manuscript: Yu Z, Cheng Y
Data analysis and technical support: Li H, An W
Data acquisition: Tong Q, Yang P, Zhao G
Supervision, writing - review and editing: Yan D, Lu X, Tian B
Availability of data and materialsThe data supporting our findings can be found in the Supplementary Information.
Financial support and sponsorshipThis study was financially supported by the Department of Science and Technology of Guangxi Province (Grant Nos.: AB21220027, AD19110090, AD19110077, and AA17204063), the Guangxi Natural Science Foundation (Grant Nos. 2020GXNSFAA159059, 2020GXNSFAA159037, and 2018GXNSFGA281001), Engineering Research Center Foundation of Electronic Information Materials and Devices (Grant No. EIMD-AA202005), and National Natural Science Foundation of China (Grant Nos. 21805055, 22075328, and 51974098).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
Supplementary MaterialsREFERENCES
1. Asadi M, Sayahpour B, Abbasi P, et al. A lithium-oxygen battery with a long cycle life in an air-like atmosphere. Nature 2018;555:502-6.
2. Zhu X, Meng F, Zhang Q, et al. LiMnO2 cathode stabilized by interfacial orbital ordering for sustainable lithium-ion batteries. Nat Sustain 2021;4:392-401.
3. Niu Y, Yu Z, Zhou Y, et al. Constructing stable Li-solid electrolyte interphase to achieve dendrites-free solid-state battery: a nano-interlayer/Li pre-reduction strategy. Nano Res 2022;15:7180-9.
4. Yu Z, Zhou L, Cheng Y, et al. Preset lithium source electrolyte boosts SiO anode performance for lithium-ion batteries. ACS Sustain Chem Eng 2022;10:10351-60.
5. Pomerantseva E, Bonaccorso F, Feng X, Cui Y, Gogotsi Y. Energy storage: the future enabled by nanomaterials. Science 2019;366:eaan8285.
6. Cheng Y, Wei K, Yu Z, et al. Ternary Si-SiO-Al composite films as high-performance anodes for lithium-ion batteries. ACS Appl Mater Interfaces 2021;13:34447-56.
7. Chi X, Li M, Di J, et al. A highly stable and flexible zeolite electrolyte solid-state Li-air battery. Nature 2021;592:551-57.
8. Ding Z, Zhang C, Xu S, et al. Stable heteroepitaxial interface of Li-rich layered oxide cathodes with enhanced lithium storage. Energy Storage Mater 2019;21:69-76.
9. Yu Z, Tian B, Li Y, et al. Lithium titanate matrix-supported nanocrystalline silicon film as an anode for lithium-ion batteries. ACS Appl Mater Interfaces 2019;11:534-40.
10. Feng X, Wu H, Gao B, Świętosławski M, He X, Zhang Q. Lithiophilic N-doped carbon bowls induced Li deposition in layered graphene film for advanced lithium metal batteries. Nano Res 2022;15:352-60.
11. Ke C, Shao R, Zhang Y, et al. Synergistic engineering of heterointerface and architecture in new-type ZnS/Sn heterostructures in situ encapsulated in nitrogen-doped carbon toward high-efficient lithium-ion storage. Adv Funct Mater 2022;32:2205635.
12. Fan X, Hu G, Zhang B, et al. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 2020;70:104450.
13. Kim H, Kim MG, Jeong HY, Nam H, Cho J. A new coating method for alleviating surface degradation of LiNi0.6Co0.2Mn0.2O2 cathode material: nanoscale surface treatment of primary particles. Nano Lett 2015;15:2111-9.
14. Hussain N, Li M, Tian B, Wang H. Co3Se4 quantum dots as an ultrastable host material for potassium-ion intercalation. Adv Mater 2021;33:e2102164.
15. Tsai P, Wen B, Wolfman M, et al. Single-particle measurements of electrochemical kinetics in NMC and NCA cathodes for Li-ion batteries. Energy Environ Sci 2018;11:860-71.
16. Kim JH, Ryu HH, Kim SJ, Yoon CS, Sun YK. Degradation mechanism of highly Ni-Rich Li[NixCoyMn1-x-y]O2 cathodes with x > 0.9. ACS Appl Mater Interfaces 2019;11:30936-42.
17. Kim A, Strauss F, Bartsch T, et al. Stabilizing effect of a hybrid surface coating on a Ni-Rich NCM cathode material in all-solid-state batteries. Chem Mater 2019;31:9664-72.
18. Heenan TMM, Wade A, Tan C, et al. Identifying the origins of microstructural defects such as cracking within Ni-Rich NMC811 cathode particles for lithium-ion batteries. Adv Energy Mater 2020;10:2002655.
19. Yu Z, Qu X, Wan T, et al. Synthesis and mechanism of high structural stability of nickel-rich cathode materials by adjusting li-excess. ACS Appl Mater Interfaces 2020;12:40393-403.
20. Fan X, Chen L, Ji X, et al. Highly fluorinated interphases enable high-voltage Li-metal batteries. Chem 2018;4:174-85.
21. Zhu Z, Liang Y, Hu H, et al. Enhanced structural and electrochemical stability of LiNi0.83Co0.11Mn0.06O2 cathodes by zirconium and aluminum co-doping for lithium-ion battery. J Power Sources 2021;498:229857.
22. Lee S, Kim M, Jeong JH, et al. Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted sol-gel method: improved thermal stability and high-voltage performance. J Power Sources 2017;360:206-14.
23. Han S, Zhang H, Fan C, Fan W, Yu L. 1,4-Dicyanobutane as a film-forming additive for high-voltage in lithium-ion batteries. Solid State Ionics 2019;337:63-9.
24. Fu J, Mu D, Wu B, et al. Enhanced electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode at high cutoff voltage by modifying electrode/electrolyte interface with lithium metasilicate. Electrochim Acta 2017;246:27-34.
25. Yang J, Huang B, Yin J, et al. Structure integrity endowed by a ti-containing surface layer towards ultrastable LiNi0.8Co0.15Al0.05O2 for all-solid-state lithium batteries. J Electrochem Soc 2016;163:A1530-4.
26. Wang Y, Sun Y, Liu S, Li G, Gao X. Na-Doped LiNi0.8Co0.15Al0.05O2 with excellent stability of both capacity and potential as cathode materials for li-ion batteries. ACS Appl Energy Mater 2018;1:3881-9.
27. Yu Z, Tong Q, Zhao G, Zhu G, Tian B, Cheng Y. Combining surface holistic Ge coating and subsurface Mg doping to enhance the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathodes. ACS Appl Mater Interfaces 2022;14:25490-500.
28. Huang Y, Zhu Y, Fu H, et al. Mg-pillared LiCoO2: towards stable cycling at 4.6 V. Angew Chem Int Ed 2021;60:4682-8.
29. Xue W, Huang M, Li Y, et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat Energy 2021;6:495-505.
30. Li J, Zhang M, Zhang D, Yan Y, Li Z. An effective doping strategy to improve the cyclic stability and rate capability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode. Chem Eng J 2020;402:126195.
31. Du F, Li X, Wu L, et al. Tailoring the Al distribution in secondary particles for optimizing the electrochemical performance of LiNi0.8Co0.1Mn0.1O2. Ceramics Inter 2021;47:12981-91.
32. Zhang M, Wang C, Zhang J, Li G, Gu L. Preparation and electrochemical characterization of La and Al Co-doped NCM811 cathode materials. ACS Omega 2021;6:16465-71.
33. Yoon W, Nam K, Jang D, et al. Structural study of the coating effect on the thermal stability of charged MgO-coated LiNi0.8Co0.2O2 cathodes investigated by in situ XRD. J Power Sources 2012;217:128-34.
34. Hu D, Du F, Cao H, et al. An effective strategy to control thickness of Al2O3 coating layer on nickel-rich cathode materials. J Electroanal Chem 2021;880:114910.
35. Woo S, Yoon CS, Amine K, Belharouak I, Sun Y. Significant improvement of electrochemical performance of AlF3-Coated LiNi0.8Co0.1Mn0.1O2 Cathode Materials. J Electrochem Soc 2007;154:A1005.
36. Song HG, Kim JY, Kim KT, Park YJ. Enhanced electrochemical properties of Li(Ni0.4Co0.3Mn0.3)O2 cathode by surface modification using Li3PO4-based materials. J Power Sources 2011;196:6847-55.
37. Cho W, Kim S, Song JH, et al. Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating. J Power Sources 2015;282:45-50.
38. Liu Y, Tang L, Wei H, et al. Enhancement on structural stability of Ni-rich cathode materials by in-situ fabricating dual-modified layer for lithium-ion batteries. Nano Energy 2019;65:104043.
39. Chen Y, Zhang Y, Chen B, Wang Z, Lu C. An approach to application for LiNi0.6Co0.2Mn0.2O2 cathode material at high cutoff voltage by TiO2 coating. J Power Sources 2014;256:20-7.
40. Ahn J, Jang EK, Yoon S, et al. Ultrathin ZrO2 on LiNi0.5Mn0.3Co0.2O2 electrode surface via atomic layer deposition for high-voltage operation in lithium-ion batteries. Appl Surf Sci 2019;484:701-9.
41. Huang X, Zhu W, Yao J, et al. Suppressing structural degradation of Ni-rich cathode materials towards improved cycling stability enabled by a Li2MnO3 coating. J Mater Chem A 2020;8:17429-41.
42. Yang H, Wu H, Ge M, et al. Simultaneously dual modification of ni-rich layered oxide cathode for high-energy lithium-ion batteries. Adv Funct Mater 2019;29:1808825.
43. Ming Y, Xiang W, Qiu L, et al. Dual elements coupling effect induced modification from the surface into the bulk lattice for ni-rich cathodes with suppressed capacity and voltage decay. ACS Appl Mater Interfaces 2020;12:8146-56.
44. Wu L, Tang X, Rong Z, et al. Studies on electrochemical reversibility of lithium tungstate coated Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode material under high cut-off voltage cycling. Appl Surf Sci 2019;484:21-32.
45. Gan Z, Lu Y, Hu G, et al. Surface modification on enhancing the high-voltage performance of LiNi0.8Co0.1Mn0.1O2 cathode materials by electrochemically active LiVPO4F hybrid. Electrochim Acta 2019;324:134807.
46. Fan Q, Lin K, Yang S, et al. Constructing effective TiO2 nano-coating for high-voltage Ni-rich cathode materials for lithium ion batteries by precise kinetic control. J Power Sources 2020;477:228745.
47. Li J, Wang J, Lu X, et al. Enhancing high-potential stability of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode with PrF3 coating. Ceram Int 2021;47:6341-51.
48. Wang R, Zhang T, Zhang Q, Zheng M, Xu K, Yan W. Enhanced electrochemical performance of La and F co-modified Ni-rich cathode. Ionics 2020;26:1165-71.
49. Yang J, Chen Y, Li Y, et al. A simple strategy to prepare the La2Li0.5Al0.5O4 modified high-performance ni-rich cathode material. Mater Chem Phys 2020;249:123135.
50. Cheng Z, Lv F, Xu N, et al. Enhanced rate performance and cycle stability of LiNi0.6Co0.2Mn0.2O2 at high cut-off voltage by Li6.1La3Al0.3Zr2O12 surface modification. Appl Surf Sci 2020;524:146556.
51. Jung C, Kim D, Eum D, et al. New insight into microstructure engineering of Ni-rich layered oxide cathode for high performance lithium ion batteries. Adv Funct Mater 2021;31:2010095.
52. Qu X, Huang H, Wan T, et al. An integrated surface coating strategy to enhance the electrochemical performance of nickel-rich layered cathodes. Nano Energy 2022;91:106665.
53. Liu Y, Zeng T, Li G, et al. The surface double-coupling on single-crystal LiNi0.8Co0.1Mn0.1O2 for inhibiting the formation of intragranular cracks and oxygen vacancies. Energy Storage Mater 2022;52:534-46.
54. Han B, Xu S, Zhao S, et al. Enhancing the structural stability of Ni-Rich layered oxide cathodes with a preformed Zr-concentrated defective nanolayer. ACS Appl Mater Interfaces 2018;10:39599-607.
55. Liu S, Chen X, Zhao J, et al. Uncovering the role of Nb modification in improving the structure stability and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode charged at higher voltage of 4.5 V. J Power Sources 2018;374:149-57.
56. Kim U, Park G, Son B, et al. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge. Nat Energy 2020;5:860-9.
57. Fan X, Ou X, Zhao W, et al. In situ inorganic conductive network formation in high-voltage single-crystal Ni-rich cathodes. Nat Commun 2021;12:5320.
58. Wei K, Zhou L, Wang S, et al. Watermelon-like texture lithium titanate and silicon composite films as anodes for lithium-ion battery with high capacity and long cycle life. J Alloys Compd 2021;885:160994.
59. Shi Y, Zhang Z, Jiang P, et al. Unlocking the potential of P3 structure for practical Sodium-ion batteries by fabricating zero strain framework for Na+ intercalation. Energy Storage Mater 2021;37:354-62.
60. Yu Z, Yu K, Wei J, Lu Q, Cheng Y, Pan Z. Improving electrode properties by sputtering Ge on SiO anode surface. Ceram Int 2022;48:26784-90.
61. Yu Z, Zhou L, Tong J, Guan T, Cheng Y. Improving electrochemical performance of thick silicon film anodes with implanted solid lithium source electrolyte. J Phys Chem Lett 2022;13:8725-32.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Yu Z, Tong Q, Cheng Y, Yang P, Zhao G, Li H, An W, Yan D, Lu X, Tian B. Enabling 4.6 V LiNi0.6Co0.2Mn0.2O2 cathodes with excellent structural stability: combining surface LiLaO2 self-assembly and subsurface La-pillar engineering. Energy Mater 2022;2:200037. http://dx.doi.org/10.20517/energymater.2022.42
AMA Style
Yu Z, Tong Q, Cheng Y, Yang P, Zhao G, Li H, An W, Yan D, Lu X, Tian B. Enabling 4.6 V LiNi0.6Co0.2Mn0.2O2 cathodes with excellent structural stability: combining surface LiLaO2 self-assembly and subsurface La-pillar engineering. Energy Materials. 2022; 2(5): 200037. http://dx.doi.org/10.20517/energymater.2022.42
Chicago/Turabian Style
Yu, Zhaozhe, Qilin Tong, Yan Cheng, Ping Yang, Guiquan Zhao, Huacheng Li, Weifeng An, Dongliang Yan, Xia Lu, Bingbing Tian. 2022. "Enabling 4.6 V LiNi0.6Co0.2Mn0.2O2 cathodes with excellent structural stability: combining surface LiLaO2 self-assembly and subsurface La-pillar engineering" Energy Materials. 2, no.5: 200037. http://dx.doi.org/10.20517/energymater.2022.42
ACS Style
Yu, Z.; Tong Q.; Cheng Y.; Yang P.; Zhao G.; Li H.; An W.; Yan D.; Lu X.; Tian B. Enabling 4.6 V LiNi0.6Co0.2Mn0.2O2 cathodes with excellent structural stability: combining surface LiLaO2 self-assembly and subsurface La-pillar engineering. Energy Mater. 2022, 2, 200037. http://dx.doi.org/10.20517/energymater.2022.42
About This Article
Copyright
Data & Comments
Data
 Cite This Article 23 clicks
Cite This Article 23 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.