Anodization fabrication techniques and energy-related applications for nanostructured anodic films on transition metals
Abstract
Nanostructured anodic films on transition metals prepared using the electrochemical anodization method have recently attracted particular attention owing to their extraordinary properties and potential use in a variety of applications. Herein, we provide a thorough review of the anodization fabrication of anodic films with different nanostructures, including nanopores, nanotubes, nanoflowers, nanoneedles and nanowires on transition metals, focusing on the growth processes of nanostructured anodic films on three representative transition metals, namely, iron, copper and zinc. Specific consideration is given to the anodization behavior and formed film nanostructures of these transition metals. We conclude that electrolyte composition plays a key role in influencing the final morphologies of anodic films. Fluoride-containing solutions represent universal electrolytes for forming nanostructured anodic films on transition metals. The main applications of the resulting nanostructured anodic films, especially in energy-related fields, such as photoelectrochemical water splitting and supercapacitors, are also presented and discussed. Finally, we indicate the main challenges associated with the fabrication of anodic films with highly ordered nanostructures and the potential future directions of this field are indicated.
Keywords
INTRODUCTION
Transition metal oxides, especially nanostructured oxides, are well known as a fascinating class of materials because of their rich and unique physical, chemical and mechanical properties. Among the various methods used for the fabrication of nanostructured transition metal oxides, the electrochemical anodization technique has gained increasing attention due to its simplicity, reproducibility and low-cost processing. To date, the most successful efforts to produce nanostructured anodic films have been for the anodization of aluminum (Al). Porous anodic aluminum oxide (AAO) films can be grown on Al by anodizing Al metal in acidic electrolytes (e.g., sulfuric (H2SO4), oxalic and phosphoric (H3PO4) acids)[1]. These porous AAO films consist of a thin barrier oxide layer over the Al surface and an overlying porous oxide layer, where each cylindrical nanopore and its surrounding oxide region form a hexagonal cell orientated perpendicular to the Al surface. These highly ordered AAO films with a honeycomb-like cell arrangement can be achieved under specific anodization conditions[2-5]. Consequently, by taking advantage of their highly ordered nanopores, porous AAO films have been widely utilized as templates for the fabrication of functional nanostructures.
Following the success of the anodization route in making highly ordered AAO films, this anodization method for the production of nanostructures has been applied to other metals, including transition metals. In 2001, the Grimes group first reported the formation of uniform TiO2 nanotube arrays by the anodization of titanium (Ti) in a dilute aqueous hydrofluoric acid (HF) electrolyte[6]. However, the obtained nanotube arrays exhibited relatively poor ordering of the nanotube arrangement and the maximum nanotube lengths were limited to ~500 nm. Later, the Schmuki group demonstrated the growth of longer and ordered TiO2 nanotube arrays during the anodization of Ti in fluoride-containing ethylene glycol (EG) electrolytes[7-9]. Since then, there have been considerable advances in the anodization of Ti. The morphological control of anodic TiO2 nanotubes has become of significant interest and a large number of studies in this field have been published[10]. Anodization is currently a well-established technique for the fabrication of TiO2 nanotube arrays.
Motivated by the success of anodization in the development of TiO2 nanotubes, a variety of other transition metals have also been explored for the fabrication of nanostructured anodic oxides. Fluoride-based organic electrolytes were found to provide a very versatile approach to form nanostructured anodic films on other transition metal surfaces. For instance, a highly ordered nanoporous vanadium oxide film with a thickness of 13 μm and a pore diameter of ~15 nm was obtained by the anodization of vanadium foil at 120 V for 2 h in an EG electrolyte containing 0.2 mol L-1 HF and 300 ppm Ti. A nanotubular structure was formed by increasing the anodization time to 24 h[11]. Furthermore, self-ordered nanoporous vanadium oxide films can be grown in EG-containing aqueous NH4BF4 and NaBF4 solutions[11-13]. Similarly, other transition metal oxides (e.g., cobalt[14,15], molybdenum[16-19] and tungsten[20]) with ordered nanopores and other morphologies were obtained by anodization in fluoride-based electrolytes.
Over the past two decades, there has been growing interest in the anodization of various transition metals. Anodic films on a variety of transition metals with different nanostructures have been developed, leading to the discovery of many exciting properties and applications for transition metal oxides. The main goal of this review is to fully understand the state-of-the-art regarding nanostructured anodic films on transition metals by outlining the anodization behavior of several transition metals. Since a number of excellent reviews on anodic TiO2 nanotubes have been published[10,21-23], the transition metals discussed here do not include Ti. Considering the tremendous amount of research involving the electrochemical anodization of transition metals, it is not possible to review all examples in the literature. Therefore, we select iron (Fe), copper (Cu) and zinc (Zn) as representative transition metals, because they are three of the most widely studied transition metals in this field in addition to Ti. This review article provides a comprehensive outlook of these three transition metals. Particular attention is given to their anodization behavior and resultant nanostructures. The main applications of the corresponding nanostructured anodic films on transition metals, especially in energy-related fields, such as photoelectrochemical (PEC) water splitting and supercapacitors, are presented and discussed. Finally, the potential future directions and challenges for the anodization of transition metals are considered.
IRON
Benefitting from the success of the electrochemical anodization route for making ordered TiO2 nanotube arrays[21], the Grimes group was the first to report a successful attempt to synthesize nanostructured iron oxides by anodization in 2006[24]. They obtained iron(III) oxide with nanoporous structures by the anodization of Fe foil in glycerol electrolytes containing HF, NH4F and HNO3. Since then, there has been a surge of interest regarding the fabrication of nanostructured iron oxides by anodization.
Nanoporous and nanotubular arrays
To avoid local acidification at the oxide-electrolyte interface, the Grimes group chose glycerol as the electrolytic medium[24]. They prepared nanoporous anodic films on Fe foils by the potentiostatic anodization of Fe in glycerol containing HF, NH4F and HNO3 at 10 °C. Unlike the anodization currents observed for the anodization of Al[1] or Ti[10], the anodization current for Fe decreased monotonically with time during anodization, whereas the typical current-time behavior for the anodization of Al or Ti is characterized by an increase in the current amplitude after an initial rapid current drop. The Grimes group explored the influence of anodization bath temperature, electrolyte composition and applied voltage on the pore size of the as-anodized nanoporous structures. For example, nanoporous films with a barrier layer thickness of ~800 nm could be formed by anodization at 90 V for 2 h at 10 °C. Nevertheless, the as-anodized nanoporous films obtained in the electrolyte containing HF, NH4F and HNO3 in glycerol had relatively disordered porous structures with a typical pore depth of only ~500 nm. Interestingly, the authors obtained a highly ordered nanoporous structure by anodization at a constant voltage of 40 V at 10 °C when using
Following the pioneering work of Grimes and coworkers, high-aspect-ratio and relatively ordered nanoporous anodic films were later developed. The Schmuki group utilized NH4F/EG-based electrolytes similar to those used in the fabrication of TiO2 nanotube arrays to prepare relatively ordered nanoporous anodic films on Fe at 20 °C[27]. The resulting porous layer had a significantly increased thickness (up to
Schmuki and coworkers observed an interesting temperature effect, where anodization at higher temperatures could induce a morphological change from the porous layer to nanotube arrays under otherwise identical conditions[27]. Later, LaTempa et al. reported the formation of anodic oxide nanotube arrays by the potentiostatic anodization of Fe in NH4F/EG-based electrolytes at higher temperatures[32]. The authors were able to produce iron oxide nanotube arrays using EG electrolytes containing
Consequently, the formation of nanotubes was believed to be associated with the extended anodization times in addition to the electrolyte temperature. In reality, the stirring of the electrolyte[28,36], the applied magnetic field[36] and the rotation speed of the Fe electrode[37,38] during anodization can also influence the nanostructures of anodic films on a Fe substrate. Of course, the purity of the Fe substrate also plays an important role in determining the formation of iron oxide nanostructures. Lee et al. investigated the anodization behavior of two Fe substrates with purities of 99.5% and 99.99% at room temperature, at 60 °C and under ultrasonication-assisted conditions, respectively[39]. They found that there was a notable difference in the thickness of the obtained anodic films for the two Fe substrates regardless of which anodization conditions were used. Although there was no significant difference in the top morphologies for both substrates, the anodic films grown on the 99.99% purity Fe substrate were typically twice as thick as those grown on the 99.5% purity substrate for the same anodization time[39].
Some fabrication techniques available for anodic TiO2 nanotubes and porous AAO can also be used in the electrochemical anodization of Fe. For example, Fe anodization experiments were carried out using ultrasonic waves[39-42]. Nanotubular array films on Fe foils could be grown by ultrasound-assisted anodization due to the introduction of additional energy[39-41]. To prepare porous anodic iron oxide films with more ordered nanopores, the so-called “two-step anodization” process was also utilized in the anodization of Fe[43-47]. In this process, an anodic film grown on the Fe foil by the first anodization process was removed to obtain a Fe surface textured with arrays of concave features. After the second anodization, a self-ordered porous anodic film was formed, apparently because these concave features guide the growth of nanopores. In addition to the usual potentiostatic and galvanostatic anodization, sinusoidally modulated current waveforms were also employed in the anodization of Fe[48]. Interestingly, periodically structured porous anodic films were obtained by applying a sine-wave current or a voltage pulse to the Fe electrode[49]. By changing the periodicity of the sine-wave current, the structural periodicity of the obtained anodic films and the film color could be tuned[48]. The reported anodization conditions for the fabrication of nanoporous or nanotubular iron oxide arrays are listed in Table 1, along with their morphological parameters.
Anodization conditions and morphological parameters of samples anodized in various electrolyte systems
| Solvent | Electrolyte (wt.%) | Water content | Anodization voltage/current | Anodization duration | Bath emperature (°C) | Pore/Tube diameter (nm) | Nano structure | Degree of ordering | Pore/Tube length (µm) | Refs. |
| Glycerol | 0.5 wt.% NH4F + 1wt.% HF + 0.2 wt.% HNO3 | 0 | 40-90 V | 2 h | 10 | 50-250 | nanopore | poor | 0.3-0.6 | [24] |
| EG | 0.3 wt.% NH4F | 0 | 40 V | 15 min | 10 | 30 | nanopore | good | 2.5 | [24] |
| EG | 0.1 M NH4F | 1 M | 10-100 V | 1 h | 20 | 40-110 | nanopore | good | 1-13 | [27] |
| EG | 0.1-0.4 wt.% NH4F | 1.5-3.5 vol.% | 30-100 V | 2.5 h | 10-20 | 92 | nanopore | fair | 10 | [28] |
| EG | 0.1 M NH4F | 0.1-1.5 M | 5 mA cm-2 | 900 s | 20 | 8-50 | nanopore | poor | 0.7 | [29] |
| EG | 0.1 M NH4F | 0.3-3.5 M | 1-10 mA cm-2 | 0.5-2 h | 10-30 | 20-125 | nanopore | fair | 7 | [30] |
| EG | 0.2-0.5 wt.% NH4F | 2%-4% | 30-60 V | 30-180 s | 60-75 | 35-65 | nanotube | good | 4 | [32] |
| EG | 0.1 M NH4F | 3 vol.% | 50 V | 15 min | RT | 54-100 | nanotube | fair | 1.5 | [33,34] |
| EG | 0.5 wt.% NH4F | 3 vol.% | 50 V | 13 min | using ultrasonic waves | 50-60 | nanotube | fair | 3.77 | [40] |
| EG | 0.5 wt.% NH4F | 3 vol.% | - | - | using ultrasonic waves | 40 | nanotube | fair | 2 | [41] |
| EG | 0.3 wt.% NH4F | 2 vol.% | 50 V (two-step) | 5 min | RT | 40 | nanopore | fair | 2 | [43] |
| EG | 0.1 M NH4F | 1 M | 40 V (two-step) | 1 h | 20 | 60-100 | nanopore | good | 5 | [45,46] |
| EG | 0.1 M NH4F | 3 vol.% | 50 V (two-step) | 15 min | 30 | 50 | nanopore | fair | 0.6 | [47] |
| EG | 0.1 M NH4F | 1.5 vol.% | sine-wave current | 405-645 s | 60 | 30-120 | nanopores with structural periodicities | good | 3 | [48] |
| EG | 0.5 wt.% NH4F | 3 wt.% | 50 V | 5 min | 60 | 120 | nanotube | good | 3.6 | [50] |
| EG | 0.5 wt.% NH4F | 3 vol.% | 50 V | 5 min | 60 | 100 | nanotube | good | 3 | [51] |
| EG | 0.1 M NH4F | 1 M | 40 V | 1 h | 25 | 50-70 | nanotube | fair | - | [52] |
| EG | 0.35 wt.% NH4F | 2.7 wt.% | 50 V | 15 min | RT | 30 | nanopore | fair | 1.8 | [53] |
| EG | 0.5 wt.% NH4F | 1.3 M | 60 V | 90-150 s | - | 47-51 | nanopore | fair | 1.4-1.8 | [54] |
| EG | 0.8 wt.% NH4F | 2 M | 60 V | 90-150 s | - | 62-79 | nanotube | fair | 2.2-2.7 | [54] |
| EG | 0.35 wt.% NH4F | 3 vol.% | 50 V | 10-20 min | 20-50 | 50 | nanotube | fair | - | [55] |
| EG | 0.2 M NH4F | 3 vol.% | 50 V | 5 min | 20 | - | nanopore | fair | 1 | [56] |
| EG | 0.7 wt.% NH4F | 2 vol.% | 40 V | 15 min | - | 40 | nanopore | fair | - | [57] |
| EG | 0.1 M NH4F | 3 vol.% | 50 V | 15 min | 25 (electrode rotation) | 75 | nanotube | fair | 0.8-0.95 | [58] |
| EG | 0.2 M NH4F | 2 M | 50 V | 5 min | 23 (stirring) | 40 | nanopore | fair | 1.5 | [59] |
| EG | 0.2 M NH4F | 0.5 M | 20-60 V | 1 h | 20 (not stirring) | 60-89 | nanopore | fair | 1.98-3.61 | [60] |
Formation mechanism
For the potentiostatic anodization of Fe, the current-time transients during anodization typically display three different stages, as shown in Figure 1A[50], which are very similar to those observed for the anodization of Al or Ti[1,10,32,34,40,45,47,50,52]. Therefore, it is presumed that the nanostructured anodic films on Fe were formed in a manner analogous to the growth of porous AAO or TiO2 nanotube arrays. Following the suggested mechanism for the formation of anodic TiO2 nanotubes, some authors proposed the possible formation mechanism of iron oxide nanopores or nanotubes formed by anodization in NH4F/EG-based electrolytes[47,50,59]. It is generally believed that the formation of iron oxide nanopores or nanotubes involves two electrochemical/chemical processes, namely, the field-assisted oxidation of Fe metal either to form iron oxides or Fe ions dissolved in the electrolyte and the chemical dissolution of iron oxides owing to etching by fluoride ions. A delicate equilibrium between the formation and dissolution of iron oxide films results in nanopore or nanotube formation.
Figure 1. (A) Current density transient during the anodization of Fe foil at 50 V in EG + 0.5 wt.% NH4F + 3.0 wt.% water for 300 s at
The current-time behavior shown in Figure 1A can be understood in terms of the above-described model. In NH4F/EG-based electrolytes containing water, an initial oxide layer is first formed on the Fe substrate at the metal-electrolyte interface according to Equation 1 when an anodization voltage is applied. This process corresponds to a rapid decrease in current density, as shown in Figure 1A at the initial stage (stage I), as a result of the fast formation of the insulating iron oxide layer. Subsequently, localized chemical etching of the iron oxide can take place to form some disordered small pits in the oxide layer due to the formation of soluble fluoro complexes, according to Equation 2. In this stage (stage II, Figure 1A), a slight rise in current occurs since these small pits increase the surface area of the Fe anode. At the bottom of the small pits, the electric field strength is higher than at other locations due to the relatively thin barrier layer, thereby giving rise to the emergence and/or growth of pores. As anodization proceeds, the pores tend to share the anodization current equally, leading to the formation of an ordered nanoporous film. In this stage (stage III, Figure 1A), the anodization current stays almost constant, implying the establishment of an equilibrium between the formation and dissolution of iron oxides. With a further increase in the anodization time, transformation of the ordered nanoporous structures into nanotube arrays eventually occurs, largely due to the selective dissolution of the Fe fluoride-rich layer at the cell boundaries. Xie et al. investigated systematically the effects of anodization conditions on the morphology and growth behavior of anodic iron oxide films and presented the morphological evolution of anodic films during anodization, as depicted in Figure 1B-G[50].
2Fe + 3H2O → Fe2O3 (anodic) + 3H2↑ (cathodic) (1)
Fe2O3 + 12F- + 6H+ → 2[FeF6]3- + 3H2O (2)
Recently, some researchers[59,61] have argued that the formation of nanoporous anodic films on Fe can also be explained in terms of the electronic current and oxygen bubble model suggested by our group in the case of porous AAO or anodic TiO2 nanotubes[62-66]. This model emphasizes that the formation of nanopores is closely related to oxygen gas generation during anodization. The anodization currents (consisting of ionic and electronic currents) are not all consumed to form the oxide. During anodization, the ionic currents originating from the migration of oxygen/metal ions lead to oxide growth, while the electronic currents give rise to the evolution of oxygen gas at the bottoms of the pores. Furthermore, the generation of bubbles all over the Fe anode surface could be observed clearly during the Fe anodization. It was suggested that nanopores in the anodic films on Fe were generated by the flow of the material in the barrier layer from the pore bases towards the pore walls around the gas bubble under the pressure of bubbles and growth stresses.
Other nanostructures
In addition to the most commonly found nanoporous and nanotubular structures, the anodization of Fe in the NH4F-H2O-EG electrolyte system can also result in anodic films with other morphologies[67,68].
Figure 2. SEM images of iron oxide nanostructures prepared by anodization of Fe foil at 50 V for 1 h using EG electrolytes of various compositions: (A) 0.125 wt.% NH4F and 1.0 vol.% H2O; (B) 0.250 wt.% NH4F and 2.0 vol.% H2O; (C) 0.500 wt.% NH4F and 3.0 vol.% H2O; (D) 1.000 wt.% NH4F and 4.0 vol.% H2O[67]. Reproduced from Ref.[67] with permission from Wiley.
Several investigations have sought to generate other nanostructured anodic films on Fe substrates in fluoride-free electrolyte systems[69-71]. Sagu et al. employed a 10 mol L-1 aqueous NaOH solution as the electrolyte for the anodization of steel[69]. The surface layer of the anodized steel was confirmed to be Fe2O3 with a highly porous sponge-like structure. Recently, our group[70,71] carried out the anodization of Fe using an aqueous oxalic acid solution. Interestingly, the anodic film grown on Fe exhibited a rod-like morphology, with the rods having relatively regular hexahedral shapes with feature sizes of 250-700 nm. FTIR and XRD analyzes showed that the as-anodized films essentially consisted of amorphous ferrous oxalate[70].
Finally, it is worth emphasizing that, unlike anodic TiO2 nanotubes, the nanostructures (nanopores and nanotubes) of as-anodized iron oxide films generally undergo a significant change after thermal annealing due to an amorphous to crystalline transition[31,36,38,46,50,53,55,56,68,72]. To inhibit the morphological changes of the anodic iron oxide films as a result of the agglomeration of the hematite particles during thermal annealing, Jun et al. specifically devised a 10 nm-thick alumina layer deposited on the surface of nanopores as a shielding layer to maintain the vertically ordered nanoporous structure of the hematite[44].
Applications
Iron oxides, like FeO, Fe2O3 and Fe3O4, have attracted intense research interest for various applications, including photocatalysis[49], PEC water splitting[38,39,44,67], lithium-ion batteries[45,46], supercapacitors[51] and biomaterials[54,73], because of their unique electrochemical/PEC properties, crystallographic polymorphism, earth abundance, eco-friendliness and low cost. Here, we focus on their applications in the PEC fields and electrochemical energy storage devices.
PEC applications
Since α-Fe2O3 (hematite) has a band gap of 2.0-2.2 eV, which allows the harvesting of nearly 40% of the solar spectrum with a maximum theoretical photoconversion efficiency of 12.9%[74,75], it has increasingly gained attention as a promising material for PEC applications. In fact, investigations of nanoporous iron(III) oxide synthesized by anodization, first reported by the Grimes group, have been mainly focused on PEC applications[24]. They found that annealed nanoporous Fe2O3 films exhibited a net photocurrent density of 0.51 mA cm-2 at 0.6 V vs. Ag/AgCl in an electrolyte containing 0.5 mol L-1 H2O2 and 1 mol L-1 NaOH under AM 1.5 simulated solar illumination. Since then, many studies have featured the PEC properties of nanostructured iron oxide films fabricated by anodization[31-35,37-40,42-44,49,52,53,56-58,67,72-75]. The Misra group demonstrated that a photoanode with a two-layer oxide structure comprising a top layer with a nanodendrite morphology and a bottom layer with a nanoporous morphology, obtained by the two-step anodization of Fe, exhibited noticeably improved photoactivity compared to that of a single layer nanotubular oxide[33,34]. Moreover, the authors produced ordered and ultrathin Fe2O3 nanotube arrays on Fe foil by an ultrasonic wave-assisted anodization process[40]. These ultrathin Fe2O3 nanotube arrays with a wall thickness of 5-7 nm and a length of 3-4 μm showed a photocurrent density of 1.41 mA cm-2 at 0.5 V vs. Ag/AgCl with a maximum solar-to-hydrogen conversion efficiency of 0.84% under illumination with AM 1.5 solar light.
As mentioned above, the fabrication of vertically aligned iron oxide nanoflakes on the surface of a Fe foam using electrochemical anodization was reported by Kang et al. in 2017[67]. The anodized Fe foam photoanode was observed to have a photocurrent density exceeding 5 mA cm-2 before reaching the dark current onset, which is the maximum value reported to date for PEC water splitting based on iron oxides. They attributed this excellent performance to the large surface area and low electrical resistance of the Fe foams[67]. More recently, Xue et al. reported the in-situ construction of a Fe2O3/Fe3O4 heterojunction by a facile two-step annealing process[76]. The as-anodized Fe2O3 nanotubes were annealed first at 400 °C in air and then at
It has been well-documented that the nanoscale morphology of iron oxides plays a crucial role in determining their PEC properties. For instance, Jun et al. fabricated highly ordered honeycomb hematite films by employing two-step anodization and a subsequent alumina shielding treatment before annealing[44]. The highly ordered nanoporous hematite films showed significantly higher photocurrent values compared to those with lower degrees of ordering. Mushove et al. found that the incident photon-to-current efficiency at 350 nm for the wave-like hematite nanotube arrays was about three times greater than that of single-layer nanotube arrays and about 12 times higher than that of multilayer nanotube arrays[49]. Furthermore, the PEC water splitting performance of the anodized iron oxides was found to be influenced by many factors, such as the purity of the Fe substrate[39], the annealing temperature[53], the anodization time[58], the rotation speed of the Fe substrate and electrolyte temperature during anodization[37,38] and the annealing atmosphere[56,72]. In addition to their very promising applications in PEC water splitting, anodic iron oxide nanotube arrays have also shown good photocatalytic performance in the degradation of methylene blue under visible light[42,52] and excellent oxygen reduction reaction catalytic activity[55]. In addition, Joseph et al. reported the synthesis of metal-doped iron oxide nanoporous structures achieved by an additional simple electrochemical process after Fe anodization[57,77]. The metal-doped iron oxide nanostructures exhibited enhanced photocatalytic activity.
Electrochemical energy storage devices
The wide variety of valence states and crystal structures of iron oxides makes it possible to use them as electrochemical energy storage devices, either as battery electrodes or supercapacitors[78,79]. The application of anodic Fe2O3 nanotubes in supercapacitors was first reported by Xie et al. in 2011[51]. They demonstrated that the resultant α-Fe2O3 nanotube arrays had a high specific capacitance of 138 F·g-1 at 1.3 A·g-1 and good rate capability (91 F·g-1 at 12.8 A·g-1). However, the cycling stability was found to be relatively poor, with a capacitance retention of only 88.9% after 500 cycles. More recently, Li et al. prepared a porous Fe3O4 film on Fe foil by anodization, followed by the electrodeposition of polyaniline onto the film by the electropolymerization of aniline[80]. The obtained Fe3O4/polyaniline hybrid electrode showed a specific capacitance of 47.11 mF·cm-2 at 1.3 mA·cm-2 and good cycling stability with a capacitance retention of more than 90% after 1500 cycles. Pervez et al. utilized Fe2O3 nanotube layers grown by anodization as a Li-ion battery anode[45,46]. They performed a comparative study of the electrochemical performance of crystalline and amorphous anodic iron oxide nanotube layers. The results indicated that the crystalline nanotubes exhibit greatly enhanced electrochemical properties, with a high specific capacity of 2.775 mAh cm-2 at
COPPER
According to the Pourbaix diagram of Cu, its passivity at room temperature occurs in alkaline environments, meaning that alkaline electrolytes, such as NaOH, are typically used during the anodization of Cu. The investigation of the anodic behavior of Cu in alkaline solutions can be traced back to the 1940s[81]. The early studies focused on identifying the compositions of anodic films on Cu. The main products of Cu anodization in alkaline media were cuprous oxide Cu2O, cupric oxide CuO, cupric hydroxide Cu(OH)2 and water-soluble coordination anions like Cu(OH)42-[81,84]. Later, the fabrication of nanostructured copper oxides/hydroxides attracted significant attention due to the advantageous properties of nanomaterials. The most straightforward route to nanostructures is template synthesis. For example,
Nanoneedles, nanowires and nanotubes
As early as 1976, Shoesmith et al. thoroughly investigated the anodic oxidation of Cu in a LiOH solution using potentiostatic, galvanostatic and voltammetric sweep techniques[87]. The main anodization product was demonstrated to be Cu(OH)2, with the formation of CuO and Cu2O phases also possible in terms of the anodization conditions. The authors also discussed the nucleation and growth mechanism of Cu(OH)2 films. Furthermore, the acicular morphology of the resultant films was first observed in their pioneering work[87]. In 2005, the Shi group utilized KOH-based electrolytes to form nanostructured anodic films on Cu under galvanostatic conditions[88]. As shown in Figure 3, the typical anodic products exhibit a pine needle-like morphology, which was confirmed to be mainly Cu(OH)2 with traces of Cu2O. It is noteworthy that unlike the amorphous nature of the majority of anodic films on metals, the as-anodized products formed on Cu are crystalline. Figure 3 shows the morphological evolution of anodic films on Cu during anodization. After anodization for 1500 s, Cu(OH)2 nanoneedles oriented roughly perpendicular to the Cu substrate covered the whole Cu surface [Figure 3D]. These nanoneedles had an average length of 15 μm with a typical diameter of 500-550 nm at their roots and 100-120 nm at their sharp tips. As the electrolyte temperature decreased, the number density of nanoneedles clearly increased, whereas their average length decreased correspondingly. This could be attributed to the easier nucleation of Cu(OH)2 crystals at lower temperatures. Under otherwise identical conditions, scroll-like Cu(OH)2 nanotubes were formed on Cu foil when the KOH concentration was increased to over 2.5 mol L-1. Moreover, the current density also played an important role in governing the resulting anodic products. At a low current density of 0.5 mA cm-2, black nanosheets or nanoflowers of CuO, together with a certain amount of Cu2O, were generated. When the current density was increased to over 1 mA cm-2, Cu(OH)2 rather than CuO nanoneedles was obtained. The increase in current density facilitated the formation of Cu(OH)2 and prevented the generation of the side product Cu2O. The optimized anodization conditions for fabricating Cu(OH)2 nanoneedles or nanotubes are listed in Table 2. In addition, Cu(OH)2 nanoneedles can be readily converted to CuO nanoneedles by annealing at 200 °C for 3 h under a nitrogen atmosphere[88].
Products formed by anodization of Cu at 1.5 mA cm-2 in media with different concentrations of KOH (CKOH) and at different temperatures (T)[88]. Reproduced from Ref.[88] with permission from the American Chemical Society
| T (°C) | CKOH, 2.0 mol L-1 | CKOH, 2.5 mol L-1 | CKOH, 3.0 mol L-1 | CKOH, 3.5 mol L-1 |
| 2 ± 2 | Cu(OH)2 nanoneedles | Cu(OH)2 nanoneedles | Cu(OH)2 nanoneedles | Cu(OH)2 nanoneedles |
| 15 ± 2 | Cu(OH)2 nanoneedles | Cu(OH)2 nanoneedles | Cu(OH)2 nanotubes | Cu(OH)2 nanotubes |
| 28 ± 2 | Cu(OH)2 nanoneedles | Cu(OH)2 nanotubes | Cu(OH)2 nanotubes | CuO nanoparticles |
The formation of nanoneedles by the anodization of Cu in KOH or NaOH electrolytes was further confirmed in subsequent studies[89-103]. Allam and Grimes found that the reaction of Cu foil in an aqueous KOH electrolyte was pH dependent[89]. No film was formed on the Cu surface when the reaction was performed in KOH solutions with pH ≤ 10. Zhang and Wang explored the anodization of electrodeposited Cu films in a 3 mol L-1 solution NaOH at different current densities[90]. They found that the anodic films prepared at a lower current density were Cu2O with polyhedral structures, while those prepared at a higher current density were Cu(OH)2 with a randomly packed nanowire (or nanoneedle) structure. This was in reasonable agreement with the experimental findings of the Shi group[88]. Moreover, different annealing environments and temperatures were found to result in the formation of different CuO and/or Cu2O nanostructures when the Cu(OH)2 nanoneedles were annealed[92,104]. For instance, when anodized Cu foam was heated under a nitrogen atmosphere at 150 °C for 3 h and at 200 °C for 3 h, CuO nanowires were formed on the Cu foam. In contrast, when the anodized sample was heated in air at 200 °C for 15 min, CuO nanosheets were generated on the Cu foam[91]. When the Cu foam was anodized at
Nanoporous and leaf-like nanoarchitectures
Inspired by the fact that the anodization of metals like Ti in fluoride-containing media can lead to the formation of nanoporous/nanotubular structures, the Grimes group studied the anodization of Cu foil in halide-containing solutions in 2011 in an attempt to obtain nanoporous copper oxide films[89]. They investigated the combined effect of KOH and NH4Cl on Cu anodization and found that no porous structure could be obtained using NH4Cl-based electrolytes. This result was also verified in a more recent study[108]. The Grimes group[89] discovered that a nanoporous structure appeared on the entire Cu surface after the Cu foil was anodized at 6 V for 300 s in an aqueous electrolyte containing 0.15 mol L-1 KOH and 0.1 mol L-1 NH4F. When the KOH concentration was increased to 0.2 mol L-1 while maintaining all other conditions, anodic products with porous microspheroids were obtained. However, when Cu thin films of ~450 nm thickness were anodized in the same aqueous electrolytes containing 0.1 mol L-1 NH4F under similar anodization conditions, the anodic products still showed nanowire structures[109].
The Grimes group also showed that changing the electrolyte solvent from water to EG still did not produce the nanoporous/nanotubular structures like those found after Ti anodization[89]. Nevertheless, the formation of leaf-like nanoarchitectures with a thickness of ~160 nm was observed upon the anodization at 30 V for 300 s of Cu foil in an EG electrolyte containing 0.15 mol L-1 KOH, 0.1 mol L-1 NH4F and 3% H2O. Increasing the KOH concentration to 0.2 mol L-1 led to the growth of thicker oxide films of ~500 nm with the same leaf-like morphology[89]. However, Jerez et al. obtained nanoporous mixed Cu2O/CuO films when the Cu anodization was conducted at a relatively lower temperature (5 °C) in similar EG electrolytes[110]. Furthermore, the current-time transients during potentiostatic anodization were similar to those observed for Ti anodization. Wang et al. found that the calcination temperature played a key role in determining the final morphology of the anodization products[111]. Nanoporous Cu2O films were formed by anodization in an EG electrolyte containing 0.75 wt.% KOH, 0.20 wt.% NaF and 3.0 wt.% H2O. The formation of a dense array of CuO nanowires embedded within the surface of the Cu2O layer was observed after the anodized films were annealed at 400 °C for 60 min.
Shu et al. also prepared leaf-like copper oxide nanosheets in the NaOH-NaCl-polyethylene glycol (PEG) aqueous electrolyte system[112,113]. They found that higher anodization temperatures were required for generating copper oxide nanosheets in NaOH-NaCl-PEG electrolytes. No oxide film was observed on the Cu surface when the anodization temperatures were lower than 50 °C[112]. Furthermore, they found that the addition of ammonium molybdate (AM) in the NaOH-NaCl-PEG solutions inhibited the Cu → Cu+ oxidation reaction, resulting in the formation of single-phase CuO nanosheet films. Typically, in an aqueous solution containing 1 mol L-1 NaOH, 2.5 mol L-1 NaCl, 1 g L-1 PEG and 20 g L-1 AM, single-phase CuO nanosheets were obtained directly on Cu foam when it was anodized at a constant current of 10 mA cm-2 at 65 °C. These grown nanosheets were ~2 μm in size with a thickness of ~30-50 nm[113].
Other nanostructures and anodization products
Since the aqueous solutions of carbonate salts of alkali metals are weakly alkaline, they have also been used as electrolytes for Cu anodization in addition to the commonly used NaOH or KOH solutions.
Applications
Copper oxides (CuO and Cu2O) have attracted significant attention for a wide variety of applications due to their relatively low cost, nontoxicity, abundance and high chemical stability. As indicated above, numerous research efforts have focused on tuning the nanostructure and function of copper oxide-based materials by anodization, which has led to the discovery of many fascinating properties and applications. In addition to the PEC and supercapacitor applications commonly encountered, copper oxide-based materials have found applications in sensors[43,109,119,127,128], pH-controllable water permeation[93], superhydrophobic anticorrosion coatings[94,95,129], oil/water separation[122] and surface-enhanced Raman scattering substrates[108].
PEC applications
In contrast to iron oxides, copper oxides (CuO and Cu2O) are p-type semiconductors and are thus suitable for use as photocathode materials. Compared to CuO, Cu2O is a more attractive photocatalyst for PEC hydrogen production with a direct bandgap of 2 eV and a corresponding theoretical photocurrent of
Similarly, a Cu2O-CuO composite film on a Cu substrate, in the form of CuO nanowires embedded in a dense layer of Cu2O, was obtained by Wang et al.[111]. This composite film was able to generate
Supercapacitors
Over the past decade, copper oxides have experienced a surge in interest for their application in supercapacitors owing to the rich variety of their electrochemical responses and nanostructures[133]. Because copper oxides/hydroxides are grown in situ on Cu substrates by the anodization route, unlike powder-type active materials, they can be directly employed as electrodes in supercapacitors without using any binder. Moreover, nanostructured copper oxides/hydroxides with various morphologies can also be obtained by anodization, as described above. Consequently, copper oxides/hydroxides prepared by anodization have been studied extensively for use in supercapacitors[91,96,98-101,103,105,113,125,134].
Generally, nanostructured CuO, Cu2O or Cu(OH)2 films (e.g., nanowires and nanosheets) obtained by the anodization technique have produced cyclic voltammograms with ill-defined or broad redox peaks in alkaline solutions, although their capacitances were believed to originate from Faradaic redox reactions[91,98,103,105]. Figure 4 displays the typical cyclic voltammograms of CuO nanowires, nanosheets and nanoflowers in a 6.0 mol L-1 KOH solution. It has been proposed that the following redox reactions of these copper oxides/hydroxides in alkaline solution may be involved in the transition between Cu(I) and Cu(II) species:
Figure 4. Cyclic voltammograms of (a) CuO nanowires, (b) CuO nanosheets and (c) CuO nanoflowers recorded in a 6.0 mol L-1 KOH solution at a scan rate of 20 mV s-1. The inset shows the cyclic voltammograms of the Cu foam substrate and the CuO nanowires on the Cu foam electrode with the same geometrical area of 1 cm2[91]. Reproduced from Ref.[91] with permission from Elsevier.
½Cu2O + OH- ↔ CuO + ½H2O + e- (3)
½Cu2O + ½H2O + OH- ↔ Cu(OH)2 + e- (4)
CuOH + OH- ↔ CuO + H2O + e- (5)
CuOH + OH- ↔ Cu(OH)2 + e- (6)
The broad redox peaks in their cyclic voltammograms, as illustrated in Figure 4, could be attributed to the overlap of the redox processes expressed in Equations 3-6[91]. These nanostructured copper oxides/hydroxides in alkaline solutions can exhibit relatively high specific capacitances and good cycling stability. For example, leaf-like CuO-Cu2O nanosheets on Cu foam showed a high capacitance of
As mentioned above, Lu et al. successfully fabricated graphene-like CuO nanofilms on Cu foam by in-situ anodization[125]. As shown in Figure 5A-C, the fabricated electrode was comprised of interconnected CuO nanofilms with open mesopores. Furthermore, the individual CuO nanofilms with a thickness of ~2 nm had a graphene-like flexible and folded structure [Figure 5D]. Unlike the aforementioned copper oxides/hydroxides, the CuO nanofilms displayed well-defined redox peaks [Figure 5E and F], corresponding to the transition between Cu(I) and Cu(II) species (Equation 3). The dramatically different electrochemical response was primarily attributed to the ultrathin graphene-like structure with short pathways for ion transport. Moreover, the CuO nanofilms exhibited a much higher specific capacitance
Figure 5. Morphologies of CuO nanofilms: (A-C) SEM images taken at different magnifications; (D) TEM image. Cyclic voltammograms of (E) different electrodes at 10 mV s-1 and (F) CuO nanofilm electrode at various scan rates. The inset of (A) shows an SEM image of the bare Cu foam[125]. Reproduced from Ref.[125] with permission from the American Chemical Society.
ZINC
Because alkaline batteries with Zn electrodes are manufactured commercially and used on a large scale, the anodization behavior of Zn in alkaline solutions has been widely investigated as early as the 1950s[135-137]. Early work primarily focused on the understanding of the anodic ZnO formation processes and the related electrochemical reactions in alkaline solutions. In contrast, relatively little information on the microscopic morphologies of the anodic films has been published[138-143]. Over the past two decades, there has been increasing interest in the anodic fabrication of nanostructured ZnO with various morphologies owing to its many promising applications. For Zn anodization, it is noteworthy that even small changes in electrolyte composition or anodization voltage may lead to dramatic changes in both the morphology and structure of the as-anodized films.
Nanoneedles, nanorods, nanowires and nanotubes
Similar to the case of Cu(OH)2 nanoneedles discussed above, the first report on the anodization preparation of ZnO nanoneedles was also published by the Shi group in 2006[144]. They demonstrated that highly oriented ZnO nanoneedle arrays could be prepared by the anodization of Zn foil in a saturated zincate [Zn(OH)42-] solution at room temperature. The electrolyte used was a freshly prepared saturated aqueous zincate solution, which was prepared by adding 10 mL of 1.0 mol L-1 Zn(NO3)2 into 10 mL of 4.0 mol L-1 KOH with vigorous stirring and then removing the precipitate by centrifugation. Crystalline ZnO nanoneedle arrays were obtained by anodization in a freshly prepared 0.5 mol L-1 zincate aqueous solution at 0.3 mA cm-2 and 1.5 C cm-2. As shown in Figure 6, these ZnO nanoneedles were aligned perpendicular to the Zn substrate surface and coated on the substrate uniformly and compactly, typically having a length of 550-650 nm with a tip diameter of 10-15 nm and a root diameter of 50-60 nm. They found that the concentration of zincate may play a key role in the formation of anodic ZnO nanoneedles. The nanoneedles were not formed in electrolytes with low zincate concentrations. The anodization of Zn in a pure aqueous KOH solution (2.0 M) only led to the dissolution of Zn in the solution[144]. Subsequent studies also demonstrated that ZnO nanoneedles could be formed by anodization in a concentrated NaOH solution
Several years later, Hu and coworkers reported the rapid synthesis of ultralong ZnO nanowire films by anodization in a slightly basic solution[148]. They found that high-aspect-ratio nanowire arrays of tens of microns in length and 30-100 nm in diameter can be formed on Zn foil rapidly by anodizing in an aqueous 5-50 mM KHCO3 solution at room temperature. The as-anodized nanowires were demonstrated to be highly crystalline materials that may have the composition Zn(OH)n(CO3)m and could be readily converted to ZnO nanowires by annealing at 250 °C for 1 h in air. Figure 7 shows the morphological evolution during anodization of Zn at 10 V. In the initial stages of anodization, the Zn foil was dissolved to form irregular, discrete pits over the surface of the metal [Figure 7a]. Furthermore, nanoflower-like products appeared inside the pits [Figure 7A]. As the anodization time increased, the morphology of the anodic films was transformed gradually from discrete nanoflower-like structures to a uniform film of aligned nanowires [Figure 7]. Numerous follow-up studies further revealed that the nanowire length and diameter and density of the nanoflower-like structures were directly proportional to the anodization time[149-154]. In addition, nanowires can be grown at a rather high rate (~1.3 μm min-1)[149-154] in an aqueous KHCO3 solution.
In addition to the aqueous KHCO3 solution, other bicarbonate electrolytes, including NaHCO3 and
In work by Katwal et al., it was observed that nanotubes appeared at the edges (< 5% of the area) of the Zn foil, although the foil was covered predominantly by nanowires [Figure 8A], while the Zn anodization was performed first at 10 V in a 6.8 mM NaHCO3 solution, followed by a second anodization at 20 V in a
Figure 8. SEM images of a zinc oxide nanotube-nanowire mixed structure fabricated in an aqueous electrolyte containing (A) only NaHCO3, in which < 5% of the substrate area was covered with nanotubes, and (B and C) both Na2CO3 and NaHCO3. (D) TEM image showing a nanotube and a nanowire[166]. Reproduced from Ref.[166] with permission from the American Chemical Society.
Nanoporous films
Several reports have mentioned that, in oxalic acid electrolytes, nanoporous ZnO thin films were typically formed by the electrochemical anodization of Zn[173,174]. Interestingly, ZnO porous structures on Zn metal were also obtained by Zn anodization just using deionized/distilled water as an electrolyte at room temperature[175,176]. As the deionized water itself was slightly acidic (pH 5.8), the Zn anodization could be carried out in water without the need for the addition of any extra electrolyte[175]. The morphologies of the ZnO thin films were found to be highly dependent on the anodization voltages[176].
As described above, Zn anodization in a concentrated NaOH solution led to the formation of ZnO nanoneedles[145]. In contrast, it was also reported that nanoporous anodic ZnO films could be produced in dilute NaOH solutions (< 1 M)[147,177-179]. For example, a porous anodic film with a thickness of ~10 μm was obtained by anodization in a 0.1 mol L-1 NaOH solution at 20 V for 90 min, which consisted of crystalline ZnO with a straight cellular structure similar to that of AAO[177]. It was suggested that the addition of EG to the NaOH solution could improve the film properties and current efficiency of anodization[177]. When a
Other nanostructures and anodization products
So far, it has been shown that the compositions of the electrolytes used in anodization play a particularly influential role in determining the nanostructures of anodic ZnO films. Apart from the commonly used NaOH/KOH and bicarbonate electrolytes, other electrolytes, such as silicate-based solutions[181], H2SO4 solutions[182], methanol solutions containing HF and water[183], mixed solutions of (NH4)2SO4 and NH4Cl[184], H3PO4/NH4H2PO4 solutions[185] and mixed solutions of (NH4)2SO4 and NaOH[186], could also be employed to make nanostructured anodic films by Zn anodization. In 2008, Kim and Choi utilized H2SO4 solutions in ethanol instead of in water to fabricate ZnO films by anodization[182]. Unlike the formation of hexagonal etched arrays or the stair-like morphology of ZnO obtained in aqueous H2SO4 solutions, anodization in a H2SO4 solution in ethanol led to the formation of self-assembled ZnO stripes consisting of 800 nm polygonal flakes, which may be attributed to the water-selective dissolution of ZnO and the hydrodynamic flow of water by convection[182]. In 0.1 wt.% HF solutions in a mixture of methanol and water, ZnO nanoflowers composed of hundreds of sheet-like nanopetals were found to be grown on a Zn substrate after a potentiostatic anodization of 15 min[183]. In contrast, in a mixed solution of 0.2 mol L-1 (NH4)2SO4 and
Applications
Zinc oxide (ZnO) is a well-known piezoelectric material and also an n-type semiconductor with a wide direct bandgap (~3.37 eV). It offers many unique and promising properties, such as a large exciton binding energy (60 meV), high electron mobility, good photocatalytic activity, excellent thermal and chemical stability, large piezoelectric constant and easily modified electric conductivity[188]. Therefore, nanostructured ZnO materials have been studied for many potential applications, including photoluminescence[142,146,189], gas sensors[173,190,191], biological applications[166,167,192], photodetectors[151], control of surface wettability[152,183,193], photocatalysts[141,177,186,194-198], dye-sensitized solar cells[149] and PEC water splitting[168-171,199,200].
Unsurprisingly, the majority of research efforts have focused on the PEC applications of ZnO due to its efficient PEC response. For example, the Schmuki group fabricated hierarchically structured self-organized ZnO nanotubes by Zn anodization in a sulfide-based aqueous electrolyte containing a small amount of
Very recently, as mentioned earlier, Batista-Grau et al. fabricated different nanostructured ZnO electrodes by anodization in NaHCO3 and NaHCO3 solutions containing ethanol or glycerol under hydrodynamic conditions[168,169]. Their experimental results established that the obtained ZnO nanostructures presented high photocurrent density response during water splitting measurements in a sacrificial polysulfide electrolyte. In particular, the samples anodized at 5000 rpm in NaHCO3 solutions showed a ~159% improvement in the photocurrent density response compared to those anodized under stagnant conditions[168]. It was observed that when 10% v/v of ethanol or 25% v/v of glycerol was added to the aqueous NaHCO3 electrolyte, the photocurrent density response for the electrodes obtained in the organic solvent containing an electrolyte under stagnant conditions was greatly increased. The photocurrent density response of the ZnO electrode anodized in a NaHCO3 electrolyte containing 10% v/v ethanol (0.34 mA cm-2) was twice that of the sample anodized under the same conditions in an aqueous NaHCO3 electrolyte
CONCLUSION AND OUTLOOK
This review provides a comprehensive survey of the current research progress in the field of anodization of transition metals, with specific emphasis on the growth process of nanostructured anodic films on Fe, Cu and Zn metals and their corresponding applications. Typically, as-anodized films on Fe are essentially amorphous, whereas the anodic films prepared by Cu and Zn anodization are relatively crystalline. Anodic films with various nanostructures, such as nanopores, nanotubes, nanoflowers, nanoneedles and nanowires, have been successfully produced in different electrolytes. We conclude that the anodization conditions, including anodization voltage, anodization duration, electrolyte composition, the organic solvent, anodization bath temperature and water content, have profound effects on the morphologies of anodic films. Fluoride-containing solutions were found to be a universal type of electrolyte for the formation of nanostructured anodic films on transition metal surfaces. Moreover, in some electrolytes containing special anionic species, including iodide, phosphate and sulfide, the as-anodized products were metal iodides (or phosphates or sulfides) instead of metal oxides/hydroxides. The anodized films on transition metals have found widespread applications in PEC water splitting, photocatalysis, lithium-ion batteries, supercapacitors and biomaterials[201,202].
At present, anodization has become a widely used technique for preparing metal oxides with various nanostructures. In addition to transition metals, some non-transition metals, like tin, have also been anodized to fabricate nanomaterials with various unique properties[203-208]. However, compared with the highly ordered AAO, the anodized films on transition metals, such as Fe, Cu and Zn, showed fewer perfect nanostructures with relatively low degrees of ordering. More recently, Yanagishita and coworkers showed the formation of anodic porous oxides with ordered hole array structures by employing a pretexturing process for Cu, Zn and Ni[209]. However, the fabricated ordered nanohole arrays of Cu2O, ZnO and NiO had limited thickness (less than 500 nm). The controllable fabrication of anodic films with highly ordered nanostructures on transition metals is still a major challenge. Although some formation mechanisms for nanostructured anodic films, especially nanoporous and nanotubular films, have been suggested, the nature of the nanostructure formation process is not very well understood and is still debated. In particular, the relationship between electrolyte systems and the morphologies of anodic films deserves further study. The understanding of formation mechanisms and continuous improvements in anodization techniques are crucial for the future of anodic films on transition metals. In short, the anodization field still holds many challenges and opportunities for researchers.
DECLARATIONS
Authors’ contributions
Preparing the manuscript draft, writing-review, editing, funding acquisition: Jiang L
Writing-review: Li P, Wang S, van Ree T
Collecting literature: Liu R
Funding acquisition, supervision: Zhu X, Song Y
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the project from National Natural Science Foundation of China (Grant Nos. 51777097, 51577093).
Conflicts of interest
All authors declares that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2022.
REFERENCES
1. Lee W, Park SJ. Porous anodic aluminum oxide: anodization and templated synthesis of functional nanostructures. Chem Rev 2014;114:7487-556.
2. Masuda H, Fukuda K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995;268:1466-8.
3. Nielsch K, Choi JS, Schwirn K, Wehrspohn RB, Gösele U. Self-ordering regimes of porous alumina: The 10% porosity rule. Nano Lett 2002;2:677-80.
4. Lee W, Schwirn K, Steinhart M, Pippel E, Scholz R, Gösele U. Structural engineering of nanoporous anodic aluminium oxide by pulse anodization of aluminium. Nat Nanotech 2008;3:234-9.
5. Lee K, Tang Y, Ouyang M. Self-ordered, controlled structure nanoporous membranes using constant current anodization. Nano Lett 2008;8:4624-9.
6. Gong D, Grimes CA, Varghese OK, et al. Titanium oxide nanotube arrays prepared by anodic oxidation. J Mater Res 2001;16:3331-4.
7. Macak JM, Tsuchiya H, Taveira L, Aldabergerova S, Schmuki P. Smooth anodic TiO2 nanotubes. Angew Chem Int Ed 2005;44:7463-5.
8. Macak JM, Schmuki P. Anodic growth of self-organized anodic TiO2 nanotubes in viscous electrolytes. Electrochim Acta 2006;52:1258-64.
9. Albu SP, Ghicov A, Macak JM, Hahn R, Schmuki P. Self-organized, free-standing TiO2 nanotube membrane for flow-through photocatalytic applications. Nano Lett 2007;7:1286-9.
10. Lee K, Mazare A, Schmuki P. One-dimensional titanium dioxide nanomaterials: nanotubes. Chem Rev 2014;114:9385-454.
11. Yang Y, Albu SP, Kim D, Schmuki P. Enabling the anodic growth of highly ordered V2O5 nanoporous/nanotubular structures. Angew Chem Int Ed 2011;50:9071-5.
12. Yang Y, Lee K, Zobel M, et al. Formation of highly ordered VO2 nanotubular/nanoporous layers and their supercooling effect in phase transitions. Adv Mater 2012;24:1571-5.
13. Lee H, Kumbhar VS, Lee J, Choi Y, Lee K. Highly reversible crystal transformation of anodized porous V2O5 nanostructures for wide potential window high-performance supercapacitors. Electrochim Acta 2020;334:135618.
14. Lee CY, Lee K, Schmuki P. Anodic formation of self-organized cobalt oxide nanoporous layers. Angew Chem Int Ed 2013;52:2077-81.
15. Li Y, Wei B, Yu Z, et al. Bifunctional porous cobalt phosphide foam for high-current-density alkaline water electrolysis with 4000 h long stability. ACS Sustainable Chem Eng 2020;8:10193-200.
16. Yang Y, Fei H, Ruan G, Xiang C, M J. Tour. Edge-oriented MoS2 nanoporous films as flexible electrodes for hydrogen evolution reactions and supercapacitor devices. Adv Mater 2014;26:8163-8.
17. Jin B, Zhou X, Huang L, Licklederer M, Yang M, Schmuki P. Aligned MoOx/MoS2 core-shell nanotubular structures with a high density of reactive sites based on self-ordered anodic molybdenum oxide nanotubes. Angew Chem Int Ed 2016;55:12252-56.
18. Szkoda M, Trzciński K, Siuzdak K, Lisowska-Oleksiak A. Photocatalytical properties of maze-like MoO3 microstructures prepared by anodization of Mo plate. Electrochim Acta 2017;228:139-45.
19. Jin B, Hejazi S, Chu H, et al. MoP-protected Mo oxide nanotube arrays for long-term stable supercapacitors. Appl Mater Today 2019;17:227-35.
20. Yanagishita T, Masuda T, Kondo T, Masuda H. Highly ordered anodic porous oxides of transition metals fabricated by anodization combined with a pretexturing process. Electrochem Commun 2021;123:106916.
21. Mor GK, Varghese OK, Paulose M, Shankar K, Grimes CA. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol Energy Mater Sol Cells 2006;90:2011-75.
22. Regonini D, Bowen CR, Jaroenworaluck A, Stevens R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mat Sci Eng R 2013;74:377-406.
23. Riboni F, Nguyen NT, So S, Schmuki P. Aligned metal oxide nanotube arrays: key-aspects of anodic TiO2 nanotube formation and properties. Nanoscale Horiz 2016;1:445-66.
24. Prakasam HE, Varghese OK, Paulose M, Mor GK, Grimes CA. Synthesis and photoelectrochemical properties of nanopores iron (III) oxide by potentiostatic anodization. Nanotechnology 2006;17:4285-91.
25. Song Y, Jiang L, Qi W, Lu C, Zhu X, Jia H. High-field anodization of aluminum in concentrated acid solutions and at higher temperatures. J Electroanal Chem 2012;673:24-31.
26. Yang J, Huang H, Lin Q, et al. Morphology defects guided pore initiation during the formation of porous anodic alumina. ACS Appl Mater Interfaces 2014;6:2285-91.
27. Albu SP, Ghicov A, Schmuki P. High aspect ratio, self-ordered iron oxide nanopores formed by anodization of Fe in ethylene glycol/NH4F electrolytes. Phys Status Solidi RRL 2009;3:64-6.
28. Jagminas A, Mažeika K, Bernotas N, Klimas V, Selskis A, Baltrūnas D. Compositional and structural characterization of nanoporous films produced by iron anodizing in ethylene glycol solution. Appl Surf Sci 2011;257:3893-7.
29. Habazaki H, Konno Y, Aoki Y, Skeldon P, Thompson GE. Galvanostatic growth of nanoporous anodic films on iron in ammonium fluoride-ethylene glycol electrolytes with different water contents. J Phys Chem C 2010;114:18853-9.
30. Konno Y, Tsuji E, Skeldon P, Thompson GE, Habazaki H. Factors influencing the growth behaviour of nanoporous anodic films on iron under galvanostatic anodizing. J Solid State Electrochem 2012;16:3887-96.
31. Santamaria M, Terracina S, Konno Y, Habazaki H, Di Quarto F. Physicochemical characterization and photoelectrochemical analysis of iron oxide films. J Solid State Electrochem 2013;17:3005-14.
32. LaTempa TJ, Feng X, Paulose M, Grimes CA. Temperature-dependent growth of self-assembled hematite (α-Fe2O3) nanotube arrays: rapid electrochemical synthesis and photoelectrochemical properties. J Phys Chem C 2009;113:16293-8.
33. Rangaraju RR, Panday A, Raja KS, Misra M. Nanostructured anodic iron oxide film as photoanode for water oxidation. J Phys D Appl Phys 2009;42:135303.
34. Rangaraju RR, Raja KS, Panday A, Misra M. An investigation on room temperature synthesis of vertically oriented arrays of iron oxide nanotubes by anodization of iron. Electrochim Acta 2010;55:785-93.
35. Wu J, Liu L, Liu S, et al. High responsivity photoconductors based on iron pyrite nanowires using sulfurization of anodized iron oxide nanotubes. Nano Lett 2014;14:6002-9.
36. Pawlik A, Hnida K, Socha RP, Wiercigroch E, Małek K, Sulka GD. Effects of anodizing conditions and annealing temperature on the morphology and crystalline structure of anodic oxide layers grown on iron. Appl Surf Sci 2017;426:1084-93.
37. Lucas-Granados B, Sánchez-Tovar R, Fernández-Domene RM, García-Antón J. Controlled hydrodynamic conditions on the formation of iron oxide nanostructures synthesized by electrochemical anodization: effect of the electrode rotation speed. Appl Surf Sci 2017;392:503-13.
38. Lucas-Granados B, Sánchez-Tovar R, Fernández-Domene RM, García-Antón J. Influence of electrolyte temperature on the synthesis of iron oxide nanostructures by electrochemical anodization for water splitting. Int J Hydrogen Energ 2018;43:7923-37.
39. Lee CY, Wang L, Kado Y, Killian MS, Schmuki P. Anodic nanotubular/porous hematite photoanode for solar water splitting: substantial effect of iron substrate purity. ChemSusChem 2014;7:934-40.
40. Mohapatra SK, John SE, Banerjee S, Misra M. Water photooxidation by smooth and ultrathin α-Fe2O3 nanotube arrays. Chem Mater 2009;21:3048-55.
41. Yu SH, Shin J, Kim JJ, Lee KJ, Sung YE. Vertically aligned iron oxide nanotube arrays and porous magnetite nanostructures as three-dimensional electrodes for lithium ion microbatteries. RSC Adv 2012;2:12177-81.
42. Zhang Z, Hossain MF, Takahashi T. Fabrication of shape-controlled α-Fe2O3 nanostructures by sonoelectrochemical anodization for visible light photocatalytic application. Mater Lett 2010;64:435-8.
43. Zhang Z, Hossain MF, Takahashi T. Self-assembled hematite α-Fe2O3 nanotube arrays for photoelectrocatalytic degradation of azo dye under simulated solar light irradiation. Appl Catal B Environ 2010;95:423-9.
44. Jun H, Im B, Kim JY, et al. Photoelectrochemical water splitting over ordered honeycomb hematite electrodes stabilized by alumina shielding. Energy Environ Sci 2012;5:6375-82.
45. Pervez SA, Kim D, Farooq U, et al. Crystalline iron oxide nanotube arrays with high aspect ratio as binder free anode for Li-ion batteries. Phys Status Solidi A 2014;211:1-6.
46. Pervez SA, Kim D, Farooq U, et al. Comparative electrochemical analysis of crystalline and amorphous anodized iron oxide nanotube layers as negative electrode for LIB. ACS Appl Mater Interfaces 2014;6:11219-24.
47. He J, Mao M, Lu Y, Jiang W, Liang B. Superhydrophobic anodized Fe surface modified with fluoroalkylsilane for application in LiBr-water absorption refrigeration process. Ind Eng Chem Res 2017;56:495-504.
48. Cheng H, Zheng L, Tsang CK, et al. Electrochemical fabrication and optical properties of periodically structured porous Fe2O3 films. Electrochem Commun 2012;2:178-81.
49. Mushove T, Breault TM, Thompson LT. Synthesis and characterization of hematite nanotube arrays for photocatalysis. Ind Eng Chem Res 2015;54:4285-92.
50. Xie K, Guo M, Huang H, Liu Y. Fabrication of iron oxide nanotube arrays by electrochemical anodization. Corros Sci 2014;88:66-75.
51. Xie K, Li J, Lai Y, et al. Highly ordered iron oxide nanotube arrays as electrodes for electrochemical energy storage. Electrochem Commun 2011;13:657-60.
52. Momeni MM, Ghayeb Y, Mohammadi F. Fe2O3 nanotube films prepared by anodisation as visible light photocatalytic. Surf Eng 2015;31:452-7.
53. Rozana M, Razak KA, Yew CK, Lockman Z. Annealing temperature-dependent crystallinity and photocurrent response of anodic nanoporous iron oxide film. J Mater Res 2016;31:1681-90.
54. Yang Y, Zhou J, Detsch R, et al. Biodegradable nanostructures: degradation process and biocompatibility of iron oxide nanostructured arrays. Mat Sci Eng C 2018;85:203-13.
55. Xue Y, Jin W, Du H, Wang S, Zheng S, Zhang Y. Tuning a-Fe2O3 nanotube arrays for the oxygen reduction reaction in alkaline media. RSC Adv 2016;6:41878-84.
56. Makimizu Y, Nguyen NT, Tucek J, et al. Activation of α-Fe2O3 for photoelectrochemical water splitting strongly enhanced by low temperature annealing in low oxygen containing ambient. Chem Eur J 2020;26:2685-92.
57. Joseph JA, Nair SB, John KA, et al. Aluminium doping in iron oxide nanoporous structures to tailor material properties for photocatalytic applications. J Appl Electrochem 2020;50:81-92.
58. Lucas-Granados B, Sánchez-Tovar R, Fernández-Domene RM, Estívalis-Martínez JM, García-Antón J. How does anodization time affect morphological and photocatalytic properties of iron oxide nanostructures? J Mater Sci Technol 2020;38:159-69.
59. Martín-González M, Martinez-Moro R, Aguirre MH, Flores E, Caballero-Calero O. Unravelling nanoporous anodic iron oxide formation. Electrochim Acta 2020;330:135241.
60. Syrek K, Kemona S, Czopor J, Zaraska L, Sulka GD. Photoelectrochemical properties of anodic iron oxide layers. J Electroanal Chem 2022;909:116143.
61. Cao J, Gu Q, Gao N, et al. Designing micro-nano structure of anodized iron oxide films by metallographic adjustment on T8 steel. Ceram Int 2021;47:32954-62.
62. Zhu X, Liu L, Song Y, et al. Oxygen bubble mould effect: serrated nanopore formation and porous alumina growth. Monatsh Chem 2008;139:999-1003.
63. Zhu XF, Song Y, Liu L, et al. Electronic currents and the formation of nanopores in porous anodic alumina. Nanotechnology 2009;20:475303.
64. Zhong XM, Yu DL, Zhang SY, et al. Fabrication and formation mechanism of triple-layered TiO2 nanotubes. J Electrochem Soc 2013;160:125-9.
65. Yu DL, Song Y, Zhu XF, Yang RQ. Han AJ. Morphological evolution of TiO2 nanotube arrays with lotus-root-shaped nanostructure. Appl Surf Sci 2013;276:711-6.
66. Li C, Ni Y, Gong J, Song Y, Gong T, Zhu X. A review: research progress on the formation mechanism of porous anodic oxides. Nanoscale Adv 2022;4:322-33.
67. Kang JS, Noh Y, Kim J, et al. Iron oxide photoelectrode with multidimensional architecture for highly efficient photoelectrochemical water splitting. Angew Chem Int Ed 2017;56:6583-8.
68. Ali G, Park YJ, Hussain A, Cho SO. A novel route to the formation of 3D nanoflower-like hierarchical iron oxide nanostructure. Nanotechnology 2019;30:095601.
69. Sagu JS, Wijayantha KGU, Bohm M, Bohm S, Rout TK. Anodized steel electrodes for supercapacitors. ACS Appl Mater Interfaces 2016;8:6277-85.
70. Wang Q, Liu Q, Ni Y, Yang Y, Zhu X, Song Y. N-Doped FeS2 achieved by thermal annealing of anodized Fe in ammonia and sulfur atmosphere: applications for supercapacitors. J Electrochem Soc 2021;168:080522.
71. Wang H, Chen B, Zhang S, et al. Preparation and supercapacitive properties of 3D flower-like iron metaphosphates based on anodization of iron. Thin Solid Films 2022;742:139045.
72. Makimizu Y, Yoo J, Poornajar M, et al. Effects of low oxygen annealing on the photoelectrochemical water splitting properties of α-Fe2O3. J Mater Chem A 2020;8:1315-25.
73. Li Y, Cheng YF. Photocatalytic anti-bioadhesion and bacterial deactivation on nanostructured iron oxide films. J Mater Chem B 2018;6:1458-69.
74. Murphy AB, Barnes PRF, Randeniya LK, et al. Efficiency of solar water splitting using semiconductor electrodes. Int J Hydrogen Energy 2006;31:1999-2017.
75. Alexander BD, Kulesza PJ, Rutkowska I, Augustynski J. Metal oxide photoanodes for solar hydrogen production. J Mater Chem 2008;18:2298-303.
76. Xue J, Zhang N, Shen Q, et al. In-situ construction of photoanode with Fe2O3/Fe3O4 heterojunction nanotube array to facilitate charge separation for efficient water splitting. J Alloy Compd 2022;918:165787.
77. Joseph JA, Nair SB, John SS, Remillard SK, Shaji S, Philip RR. Zinc-doped iron oxide nanostructures for enhanced photocatalytic and antimicrobial applications. J Appl Electrochem 2021;51:521-38.
78. Yu SH, Lee SH, Lee DJ, Sung YE, Hyeon T. Conversion reaction-based oxide nanomaterials for lithium ion battery anodes. Small 2016;12:2146-72.
79. Zeng Y, Yu M, Meng Y, Fang P, Lu X, Tong Y. Iron-based supercapacitor electrodes: advances and challenges. Adv Energy Mater 2016;6:1601053.
80. Li R, Gao N, Wang C, Ding G, Wang Y, Ma H. A facile strategy to in situ synthesize metal oxide/conductive polymer hybrid electrodes for supercapacitors. Soft Matter 2022;18:2517-21.
81. Hickling A, Taylor D. The anodic behaviour of metals. part V-copper. Trans Faraday Soc 1948;44:262-8.
82. Halliday JS. The anodic behaviour of copper in caustic soda solutions. Trans Faraday Soc 1954;50:171-8.
83. Ashworth Y, Fairhurst D. The anodic formation of Cu2O in alkaline solutions. J Electrochem Soc 1977;124:506.
84. Marchiano SL, Elsner CI, Arvia AJ. The anodic formation oxide films on copper and cathodic reduction of cuprous in sodium hydroxide solutions. J Appl Electrochem 1980;10:365-77.
85. Daltin AL, Addad A, Chopart JP. Potentiostatic deposition and characterization of cuprous oxide films and nanowires. J Cryst Growth 2005;282:414-20.
86. Lee YH, Leu IC, Wu MT, Yen JH, Fung KZ. Fabrication of Cu/Cu2O composite nanowire arrays on Si via AAO template-mediated electrodeposition. J Alloy Compd 2007;427:213-8.
87. Shoesmith DW, Rummery TE, Owen D, Lee W. Anodic oxidation of copper in alkaline solutions I. nucleation and growth of cupric hydroxide films. J Electrochem Soc 1976;123:790-9.
88. Wu X, Bai H, Zhang J, Chen F, Shi G. Copper hydroxide nanoneedle and nanotube arrays fabricated by anodization of copper. J Phys Chem B 2005;109:22836-42.
89. Allam NK, Grimes CA. Electrochemical fabrication of complex copper oxide nanoarchitectures via copper anodization in aqueous and non-aqueous electrolytes. Mater Lett 2011;65:1949-55.
90. Zhang Z, Wang P. Highly stable copper oxide composite as an effective photocathode for water splitting via a facile electrochemical synthesis strategy. J Mater Chem 2012;22:2456-64.
91. Li Y, Chang S, Liu X, et al. Nanostructured CuO directly grown on copper foam and their supercapacitance performance. Electrochim Acta 2012;85:393-8.
92. Hyam RS, Lee J, Cho E, Khim J, Lee H. Synthesis of copper hydroxide and oxide nanostructures via anodization technique for efficient photocatalytic application. J Nanosci Nanotechnol 2012;12:8396-400.
93. Cheng Z, Du M, Fu K, Zhang N, Sun K. pH-controllable water permeation through a nanostructured copper mesh film. ACS Appl Mater Interfaces 2012;4:5826-32.
94. Jiang W, He J, Xiao F, Yuan S, Lu H, Liang B. Preparation and antiscaling application of superhydrophobic anodized CuO nanowire surfaces. Ind Eng Chem Res 2015;54:6874-83.
95. Xiao F, Yuan S, Liang B, Li G, Pehkonen SO, Zhang TJ. Superhydrophobic CuO nanoneedle-covered copper surfaces for anticorrosion. J Mater Chem A 2015;3:4374-88.
96. Zhao J, Shu X, Wang Y, et al. Construction of CuO/Cu2O@CoO core shell nanowire arrays for high-performance supercapacitors. Surf Coat Tech 2016;299:15-21.
97. Stepniowski WJ, Stojadinović S, Vasilić R, et al. Morphology and photoluminescence of nanostructured oxides grown by copper passivation in aqueous potassium hydroxide solution. Mater Lett 2017;198:89-92.
98. Wan J, Pang A, He D, Liu J, Suo H, Zhao C. A high-performance supercapacitor electrode based on three-dimensional poly-rowed copper hydroxide nanorods on copper foam. J Mater Sci Mater Electron 2018;29:2660-7.
99. Du X, Xia C, Li Q, Wang X, Yang T, Yin F. Facile fabrication of CuxO composite nanoarray on nanoporous copper as supercapacitor electrode. Mater Lett 2018;233:170-3.
100. Wang B, Cao B, Wang C, Zhang Y, Yao H, Wang Y. The optical and electrical performance of CuO synthesized by anodic oxidation based on copper foam. Materials 2020;13:5411.
101. Zhang R, Chen C, Yu H, et al. All-solid-state wire-shaped asymmetric supercapacitor based on binder-free CuO nanowires on copper wire and PPy on carbon fiber electrodes. J Electroanal Chem 2021;893:115323.
102. Song G, Liu S, Xia C, Song L, Yang T, Li Q. Synthesis and application of Cu(OH)2 nanowires on nanoporous copper prepared by dealloying Ti50Cu50 and Ti25Zr25Cu50 amorphous alloys. Mater Charact 2021:178;111258.
103. Gong S, Liu X, Yue X, et al. Needle-like Cu(OH)2 in situ grown on nanoporous copper ribbon via anodizing route for supercapacitors. Mater Chem Phys 2022;283:126046.
104. Ghadge TS, Lokhande BJ. Post annealing temperature-dependent morphological and electrochemical properties of copper hydroxide thin film electrodes obtained by anodization of copper. J Mater Sci 2016;51:9879-88.
105. He D, Wang G, Liu G, Suo H, Zhao C. Construction of leaf-like CuO-Cu2O nanocomposites on copper foam for high-performance supercapacitors. Dalton Tran 2017;46:3318-24.
106. Anantharaj S, Sugime H, Noda S. Ultrafast growth of a Cu(OH)2-CuO nanoneedle array on Cu foil for methanol oxidation electrocatalysis. ACS Appl Mater Interfaces 2020;12:27327-38.
107. Anantharaj S, Sugime H, Yamaoka S, Noda S. Pushing the limits of rapid anodic growth of CuO/Cu(OH)2 nanoneedles on cu for the methanol oxidation reaction: Anodization pH Is the game changer. ACS Appl Energy Mater 2021;4:899-912.
108. Wang C, Cao J, Gao Z, Ji S, Ma H, Wang Y. Synthesizing robust cuprous oxide film with adjustable morphologies as surface-enhanced raman scattering substrate by copper anodization. Mater Chem Phys 2021;264:124470.
109. Şişman O, Kılınç N, Öztürk ZZ. Structural, electrical and H2 sensing properties of copper oxide nanowires on glass substrate by anodization. Sensor Actuat B 2016;236:1118-25.
110. Jerez DPO, Teijelo ML, Cervantes WR, et al. Nanostructuring of anodic copper oxides in fluoride-containing ethylene glycol media. J Electroanal Chem ;807:181-6.
111. Wang P, Ng YH, Amal R. Embedment of anodized p-type Cu2O thin films with CuO nanowires for improvement in photoelectrochemical stability. Nanoscale 2013;5:2952-8.
112. Shu X, Zheng H, Xu G, et al. The anodization synthesis of copper oxide nanosheet arrays and their photoelectrochemical properties. Appl Surf Sci 2017;412:505-16.
113. Shu X, Wang Y, Cui J, et al. Supercapacitive performance of single phase CuO nanosheet arrays with ultra-long cycling stability. J Alloy Compd 2018;753:731-9.
114. Stępniowski WJ, Paliwoda D, Chen Z, Landskron K, Misiołek WZ. Hard anodization of copper in potassium carbonate aqueous solution. Mater Lett 2019;252:182-5.
115. Stępniowski WJ, Misiołek WZ. The influence of electrolyte usage on the growth of nanostructured anodic films on copper in sodium carbonate aqueous solution. J Electroanal Chem 2020;857:113491.
116. Abd-Elnaiem AM, Abdel-Rahim MA, Abdel-Latief AY, Mohamed AAR, Mojsilović K, Stępniowski WJ. Fabrication, characterization and photocatalytic activity of copper oxide nanowires formed by anodization of copper foams. Materials 2021;14:5030.
117. Stępniowski WJ, Paliwoda D, Abrahami ST, et al. Nanorods grown by copper anodizing in sodium carbonate. J Electroanal Chem 2020;857:113628.
118. Stępniowski WJ, Wang KK, Chandrasekar S, Paliwoda D, Nowak-Stępniowska A, Misiołek WZ. The impact of ethylenediaminetetraacetic acid (EDTA) additive on anodization of copper in KHCO3 - hindering Cu2+ re-deposition by EDTA influences morphology and composition of the nanostructures. J Electroanal Chem 2020;871:114245.
119. Babu TGS, Ramachandran T. Development of highly sensitive non-enzymatic sensor for the selective determination of glucose and fabrication of a working model. Electrochim Acta 2010;55:1612-8.
120. Huang HY, Chien DJ, Huang GG, Chen PY. Electrochemical preparation of photoelectrochemically active CuI thin films from room temperature ionic liquid. Electrochim Acta 2012;65:204-9.
121. Vishwanath RS, Kandaiah S. Electrochemical preparation of crystalline γ-CuI thin films through potential-controlled anodization of copper and its photoelectrochemical investigations. J Solid State Electr 2016;20:2093-102.
122. Zhou C, Cheng J, Hou K, Zhu Z, Zheng Y. Preparation of CuWO4@Cu2O film on copper mesh by anodization for oil/water separation and aqueous pollutant degradation. Chem Eng J 2017;307:803-11.
123. Hu H, Wang X, Gong L, Yu X, Yang X, Zhao J. Preparation of leaflike copper phosphate films by anodic oxidation and their catalytic oxidation performance. Catal Commun 2017;95:46-9.
124. Antonopoulos IA, Karantonis A. Electrochemistry of copper in methanolic solutions: anodic oxidation and fabrication of hydrophobic surfaces. Electrochim Acta 2017;240:195-202.
125. Lu Y, Liu X, Qiu K, et al. Facile synthesis of graphene-like copper oxide nanofilms with enhanced electrochemical and photocatalytic properties in energy and environmental applications. ACS Appl Mater Interfaces 2015;7:9682-90.
126. Liu S, Wang J, Pei X, et al. The reversible wetting transition between superhydrophilicity and superhydrophobicity of tremella-like CuxO@CuxS nanosheets prepared by one-step anodization and the application of on-demand oil/water separation. J Alloy Compd 2021;889:161793.
127. Meng M, Li R, Zuo L, Luo X, Zhang T. Fabrication of hierarchical porous metallic glasses decorated with Cu nanoparticles as integrated electrodes for high-performance non-enzymatic glucose sensing. Scripta Mater 2021;199:113884.
128. Sisman O, Kilinc N, Akkus UO, et al. Hybrid liquid crystalline zinc phthalocyanine@Cu2O nanowires for NO2 sensor application. Sens Actuators B Chem 2021;345:130431.
129. Yamamoto R, Kowalski D, Zhu R, et al. Fabrication of superhydrophobic copper metal nanowire surfaces with high thermal conductivity. Appl Surf Sci 2021;537:147854.
130. Paracchino A, Laporte V, Sivula K, Grätzel M, Thimsen E. Highly active oxide photocathode for photoelectrochemical water reduction. Nature Mater 2011;10:456-61.
131. Joya KS, de Groot HJM. Controlled surface-assembly of nanoscale leaf-type Cu-oxide electrocatalyst for high activity water oxidation. ACS Catal 2016;6:1768-71.
132. Zhang Z, Zhong C, Liu L, Teng X, Wu Y, Hu W. Electrochemically prepared cuprous oxide film for photo-catalytic oxygen evolution from water oxidation under visible light. Sol Energy Mater Sol Cells 2015;132:275-81.
133. Majumdar D, Ghosh S. Recent advancements of copper oxide based nanomaterials for supercapacitor applications. J Energy Storage 2021;34:101995.
134. Chen J, Xu J, Zhou S, Zhao N, Wong CP. Facile and scalable fabrication of three-dimensional Cu(OH)2 nanoporous nanorods for solid-state supercapacitors. J Mater Chem A 2015;3:17385-91.
135. Huber K. Anodic formation of coatings on magnesium, zinc, and cadmium. J Electrochem Soc 1953; 100:376-82.
136. Dirkse TP. Electrolytic oxidation of zinc in alkaline solutions. J Electrochem Soc 1955; 102:497-501.
137. Fry H, Whitaker M. The anodic oxidation of zinc and a method of altering the characteristics of the anodic films. J Electrochem Soc 1959;106:606-11.
138. Bockris JO, Nagy Z, Damjanovic A. On the deposition and dissolution of zinc in alkaline solutions. J Electrochem Soc 1972;119:285-95.
139. Szpak S, Gabriel CJ. The Zn-KOH system: the solution-precipitation path for anodic ZnO formation. J Electrochem Soc 1979;126:1914-23.
140. El Ela AH, Bahay ME, El-Raghy SM. Anodic oxidation and self-polarization of zinc metal. J Mater Sci 1981;16:2726-36.
141. Liu MB, Cook GM, Yao NP. Passivation of zinc anodes in KOH electrolytes J Electrochem Soc 1981;128:1663-8.
142. Nanto H, Minami T, Takata S. Intense white photoluminescence in ZnO thin film formed by anodization. J Mater Sci 1983;18:2721-6.
143. Yamaguchi Y, Yamazaki M, Yoshihara S, Shirakashi T. Photocatalytic ZnO films prepared by anodizing. J Electroanal Chem 1998;442:1-3.
144. Wu X, Lu G, Li C, Shi G. Room-temperature fabrication of highly oriented ZnO nanoneedle arrays by anodization of zinc foil. Nanotechnology 2006;17:4936-40.
145. Sreekantan S, Gee LR, Lockman Z. Room temperature anodic deposition and shape control of one-dimensional nanostructured zinc oxide. J Alloy Compd 2009;476:513-18.
146. Huang GS, Wu XL, Cheng YC, Shen JC, Huang AP, Chu PK. Fabrication and characterization of anodic ZnO nanoparticles. Appl Phys A 2007;86:463-7.
147. Masuda R, Kowalski D, Kitano S, Aoki Y, Nozawa T, Habazaki H. Characterization of dark-colored nanoporous anodic films on zinc. Coatings 2020;10:1014.
148. Hu Z, Chen Q, Li Z, Yu Y, Peng LM. Large-scale and rapid synthesis of ultralong ZnO nanowire films via anodization. J Phys Chem C 2010;114:881-9.
149. Kim YT, Park J, Kim S, Park DW, Choi J. Fabrication of hierarchical ZnO nanostructures for dye-sensitized solar cells. Electrochim Acta 2012;78:417-21.
150. Park J, Kim K, Choi J. Formation of ZnO nanowires during short durations of potentiostatic and galvanostatic anodization. Curr Appl Phys 2013;13:1370-5.
151. Samir N, Eissa DS, Allam NK. Self-assembled growth of vertically aligned ZnO nanorods for light sensing applications. Mater Lett 2014;137:45-8.
152. Yavaş A, Güler S, Erol M. Growth of ZnO nanoflowers: effects of anodization time and substrate roughness on structural, morphological, and wetting properties. J Aust Ceram Soc 2020;56:995-1003.
153. Tantray AM, Shah MA. Photo electrochemical ability of dense and aligned ZnO nanowire arrays fabricated through electrochemical anodization. Chem Phys Lett 2020;747:137346.
154. Tantray AM, Mir JF, Mir MA, Rather J, Shah MA. Random oriented ZnO nanorods fabricated through anodization of zinc in KHCO3 electrolyte. ECS J Solid State Sci 2021;10:081003.
155. Miles DO, Cameron PJ, Mattia D. Hierarchical 3D ZnO nanowire structures via fast anodization of zinc. J Mater Chem A 2015;3:17569-77.
156. Mah CF, Beh KP, Yam FK, Hassan Z. Rapid formation and evolution of anodized-Zn nanostructures in NaHCO3 solution. ESC J Solid State Sci Technol 2016;5:M105-12.
157. Zaraska L, Mika K, Syrek K, Sulka GD. Formation of ZnO nanowires during anodic oxidation of zinc in bicarbonate electrolytes. J Electroanal Chem 2017;801:511-20.
158. Zaraska L, Mika K, Hnida KE, et al. High aspect-ratio semiconducting ZnO nanowires formed by anodic oxidation of Zn foil and thermal treatment. Mater Sci Eng B 2017;226:94-8.
159. Zamora-Peredo L, Ceballos-Valle A, Báez-Rodríguez A, Hernández-Torres J, García- González L, Orozco-Cruz R. Raman spectroscopy of ZnO nanowires obtained by electrochemical anodization: effect of thermal treatment, voltage and anodizing time. ECS Trans 2019;94:329-37.
160. Tantray AM, Shah MA. Photo electrochemical stability response of ZnO nanoflowers fabricated through single step electrochemical anodization. Chem Pap 2021;75:1739-47.
161. Lee W, Ji R, Gösele U, Nielsch K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat Mater 2006;5:741-7.
162. Chu SZ, Wada K, Inoue S, Isogai M, Yasumori A. Fabrication of ideally ordered nanoporous alumina films and integrated alumina nanotubule arrays by high-field anodization. Adv Mater 2005;17:2115-9.
163. Huang W, Yu M, Cao S, Wu L, Shen X, Song Y. Fabrication of highly ordered porous anodic alumina films in 0.75 M oxalic acid solution without using nanoimprinting. Mater Res Bull 2019;111:24-33.
164. Kim SJ, Lee J, Choi J. Understanding of anodization of zinc in an electrolyte containing fluoride ions. Electrochim Acta 2008;53:7941-45.
165. Tello A, Boulett A, Sánchez J, et al. An unexplored strategy for synthesis of ZnO nanowire films by electrochemical anodization using an organic-based electrolyte. Morphological and optical properties characterization. Chem Phys Lett 2021;778:138825.
166. Katwal G, Paulose M, Rusakova IA, Martinez JE, Varghese OK. Rapid growth of zinc oxide nanotube−nanowire hybrid architectures and their use in breast cancer-related volatile organics detection. Nano Lett 2016;16:3014-21.
167. Dong H, Zhou J, Virtanen S. Fabrication of ZnO nanotube layer on Zn and evaluation of corrosion behavior and bioactivity in view of biodegradable applications. Appl Surf Sci 2019;494:259-65.
168. Batista-Grau P, Sánchez-Tovar R, Fernández-Domene RM, García-Antón J. Formation of ZnO nanowires by anodization under hydrodynamic conditions for photoelectrochemical water splitting. Surf Coat Tech 2020;381:125197.
169. Batista-Grau P, Fernández-Domene RM, Sánchez-Tovar R, García-Antón J. Control on the morphology and photoelectrocatalytic properties of ZnO nanostructures by simple anodization varying electrolyte composition. J Electroanal Chem 2021;880:114933.
170. Shrestha NK, Lee K, Hahn R, Schmuki P. Anodic growth of hierarchically structured nanotubular ZnO architectures on zinc surfaces using a sulfide based electrolyte. Electrochem Commun 2013;34:9-13.
171. Sanz-Marco A, Sánchez-Tovar R, Bajo MM, Fernández-Domene RM, García-Antón J. Cathodoluminescence characterization of ZnO/ZnS nanostructures anodized under hydrodynamic conditions. Electrochim Acta 2018;269:553-9.
172. Aydın EB. Electrochemical synthesize and characterization of ZnO/ZnS nanostructures for hydrogen production. Int J Energy Res 2020;44:1-16.
173. Basu PK, Bhattacharyya P, Saha N, Saha H, Basu S. The superior performance of the electrochemically grown ZnO thin films as methane sensor. Sensor Actuat B 2008;133:357-63.
174. Basu PK, Bontempi E, Maji S, Saha H, Basu S. Variation of optical band gap in anodically grown nanocrystalline ZnO thin films at room temperature-effect of electrolyte concentrations. J Mater Sci Mater Electron 2009;20:1203-7.
175. Shetty A, Nanda KK. Synthesis of zinc oxide porous structures by anodization with water as an electrolyte. Appl Phys A 2012;109:151-7.
176. Voon CH, Tukiman N. T, Lim BY, et al. Synthesis of zinc oxide thin film by anodizing. In 2014 IEEE International Conference on Semiconductor Electronics, 2014. p. 420-3.
177. Ono S, Kobayashi Y, Asoh H. Self-organized and high aspect ratio nanoporous zinc oxide prepared by anodization. ECS Trans 2008;13:183-9.
178. Dong J, Liu Z, Dong J, et al. Self-organized ZnO nanorods prepared by anodization of zinc in NaOH electrolyte. RSC Adv 2016;6:72968-74.
179. Mika K, Socha RP, Nyga P, et al. Electrochemical synthesis and characterization of dark nanoporous zinc oxide films. Electrochim Acta 2019;305:349-59.
180. Shrestha NK, Hahn R, Lee K, Tighineanu A, Schmuki P. Electrochemically assisted self-assembling of ZnF2-ZnO nanospheres: formation of hierarchical thin porous films. ECS Electrochem Lett 2014;3:E1-E3.
181. Diomidis N, Celis JP. Effect of hydrodynamics on zinc anodizing in silicate-based electrolytes. Surf Coat Technol 2005;195:307-13.
182. Kim SJ, Choi J. Self-assembled arrays of ZnO stripes by anodization. Electrochem Commun 2008;10:175-9.
183. He S, Zheng M, Yao L, et al. Preparation and properties of ZnO nanostructures by electrochemical anodization method. Appl Surf Sci 2010;256:2557-62.
184. Zhao J, Wang X, Liu J, Meng Y, Xu X, Tang C. Controllable growth of zinc oxide nanosheets and sunflower structures by anodization method. Mater Chem Phys 2011;126:555-9.
185. Zhao J, Wang X, Sun Y, Liu J, Tang C. Preparation and formation mechanism of microporous spheric zinc phosphate. J Solid State Electrochem 2011;15:1861-5.
186. Farrukh MA, Thong CK, Adnan R, Kamarulzaman MA. Preparation and characterization of zinc oxide nanoflakes using anodization method and their photodegradation activity on methylene blue. Russ J Phys Chem A 2012;86:2041-8.
187. Heo B, Kim YT, Choi J. Electrochemical synthesis of zinc ricinoleate and its application in ammonia adsorption. J Environ Chem Eng 2021;9:105083.
188. Pearton SJ, Norton DP, Ip K, Heo YW, Steiner T. Recent progress in processing and properties of ZnO. Prog Mater Sci 2005;50:293-340.
189. Chang SS, Yoon SO, Park HJ, Sakai A. Luminescence properties of anodically etched porous Zn. Appl Surf Sci 2000;158:330-4.
190. Yang J, Liu G, Lu J, Qiu Y, Yang S. Electrochemical route to the synthesis of ultrathin ZnO nanorod/nanobelt arrays on zinc substrate. Appl Phys Lett 2007;90:103109.
191. Galstyan V, Comini E, Baratto C, Faglia G, Sberveglieri G. Nanostructured ZnO chemical gas sensors. Ceram Int 2015;41:14239-44.
192. Gilani S, Ghorbanpour M, Jadid AP. Antibacterial activity of ZnO films prepared by anodizing. J Nanostruct Chem 2016;6:183-9.
193. Ramirez-Canon A, Miles DO, Cameron PJ, Mattia D. Zinc oxide nanostructured films produced via anodization: a rational design approach. RSC Adv 2013;3:25323-30.
194. Ramirez-Canon A, Medina-Llamas M, Vezzoli M, Mattia D. Multiscale design of ZnO nanostructured photocatalysts. Phys Chem Chem Phys 2018;20:6648-56.
195. Taylor CM, Ramirez-Canon A, Wenk J, Mattia D. Enhancing the photo-corrosion resistance of ZnO nanowire photocatalysts. J Hazard Mater 2019;378:120799.
196. Ozdemir ET, Kartal U, Dikici T, Erol M, Yurddaskal M. A comparative study on structural, morphological and photocatalytic properties of anodically grown ZnO nanowires under varying parameters. J Mater Sci Mater Electron 2021;32:27398-408.
197. Batista-Grau P, Fernández-Domene RM, Sánchez-Tovar R, Blasco-Tamarit E, Solsona B, García-Antón J. Indirect charge transfer of holes via surface states in ZnO nanowires for photoelectrocatalytic applications. Ceram Int 2022;48:21856-67.
198. Zhu E, Li F, Zhao Q, et al. Preparation, parameter optimization of anodized ZnO films and their photocatalytic performance in organic dye degradation in wastewater. Appl Phys A 2022;128:697.
199. Huang MC, Wang TH, Wu BJ, Lin JC, Wu CC. Anodized ZnO nanostructures for photoelectrochemical water splitting. Appl Surf Sci 2016;360:442-50.
200. Mika K, Syrek K, Uchacz T, Sulka GD, Zaraska L. Dark nanostructured ZnO films formed by anodic oxidation as photoanodes in photoelectrochemical water splitting. Electrochim Acta 2022;414:140176.
201. Chang H, Wu YR, Han X, Yi TF. Recent developments in advanced anode materials for lithium-ion batteries. Energy Mater 2021;1:100003.
202. Chen X, Liu J, Yuan T, et al. Recent advances in earth-abundant first-row transition metal (Fe, Co and Ni)-based electrocatalysts for the oxygen evolution reaction. Energy Mater 2022;2:200028.
203. Feng S, Tang Y, Xiao T. Anodization, precursor route to flowerlike patterns composed of nanoporous tin oxide nanostrips on tin substrate. J Phys Chem C 2009;113:4809-13.
204. Lee JW, Park SJ, Choi WS, Shin HC. Well-defined meso- to macro-porous film of tin oxides formed by an anodization process. Electrochim Acta 2011;56:5919-25.
205. Wang M, Yang H, Liu Y. Current oscillations during potentiostatic anodization of tin in alkaline electrolytes. Electrochim Acta 2011;56:7051-7.
206. Bian H, Tian Y, Lee C, Yuen MF, Zhang W, Li YY. Mesoporous SnO2 nanostructures of ultrahigh surface areas by novel anodization. ACS Appl Mater Interfaces 2016;8:28862-71.
207. Li P, Niu D, He M, et al. Growth model of the tin anodizing process and the capacitive performance of porous tin oxides. J Phys Chem C 2020;124:3050-58.
208. Gao N, Cao J, Wang C, et al. Study on the crystallinity and oxidation states of nanoporous anodized tin oxide films regulated by annealing treatment for supercapacitor application. Langmuir 2022;38:164-73.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Jiang L, Li P, Wang S, Liu R, Zhu X, Song Y, van Ree T. Anodization fabrication techniques and energy-related applications for nanostructured anodic films on transition metals. Energy Mater 2022;2:200038. http://dx.doi.org/10.20517/energymater.2022.52
AMA Style
Jiang L, Li P, Wang S, Liu R, Zhu X, Song Y, van Ree T. Anodization fabrication techniques and energy-related applications for nanostructured anodic films on transition metals. Energy Materials. 2022; 2(6): 200038. http://dx.doi.org/10.20517/energymater.2022.52
Chicago/Turabian Style
Jiang, Longfei, Pengze Li, Shiyi Wang, Rui Liu, Xufei Zhu, Ye Song, Teunis van Ree. 2022. "Anodization fabrication techniques and energy-related applications for nanostructured anodic films on transition metals" Energy Materials. 2, no.6: 200038. http://dx.doi.org/10.20517/energymater.2022.52
ACS Style
Jiang, L.; Li P.; Wang S.; Liu R.; Zhu X.; Song Y.; van Ree T. Anodization fabrication techniques and energy-related applications for nanostructured anodic films on transition metals. Energy Mater. 2022, 2, 200038. http://dx.doi.org/10.20517/energymater.2022.52
About This Article
Copyright
Data & Comments
Data
 Cite This Article 20 clicks
Cite This Article 20 clicks



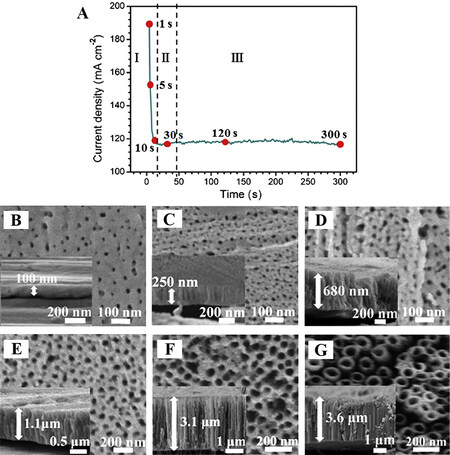
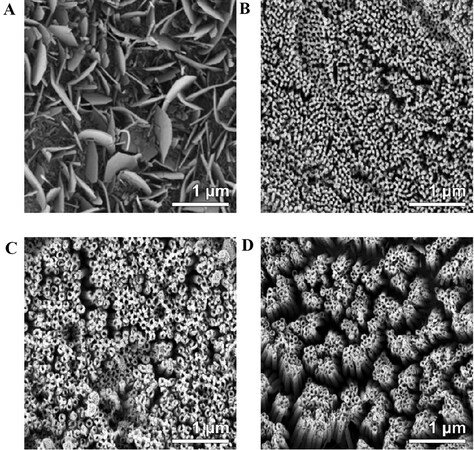
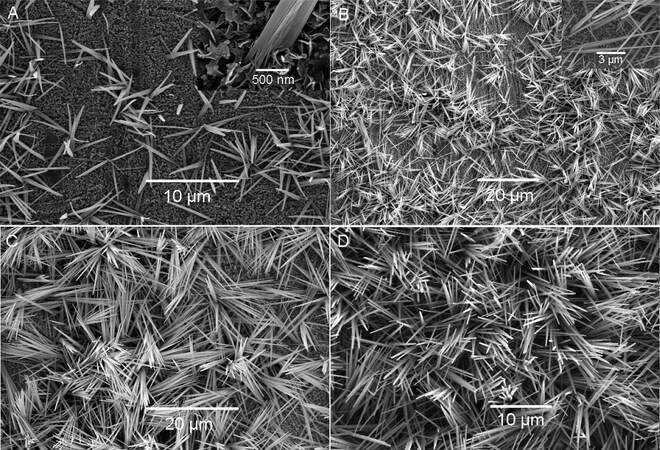
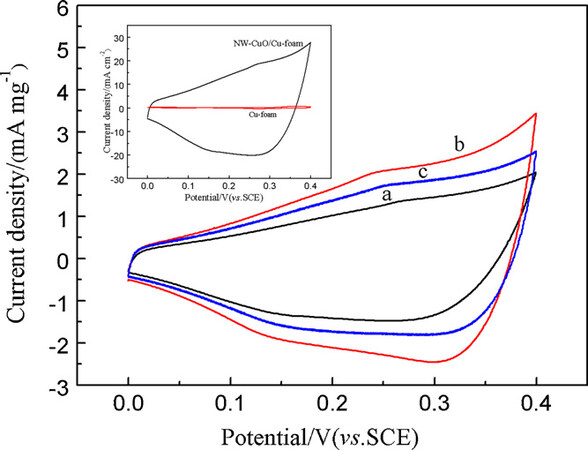
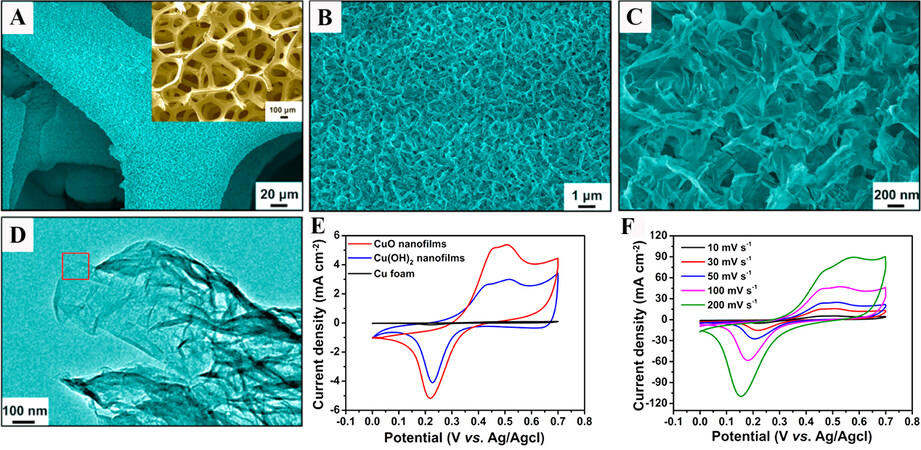
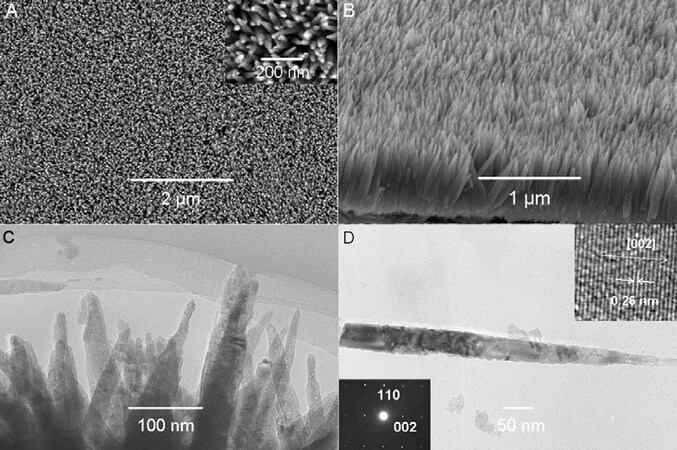
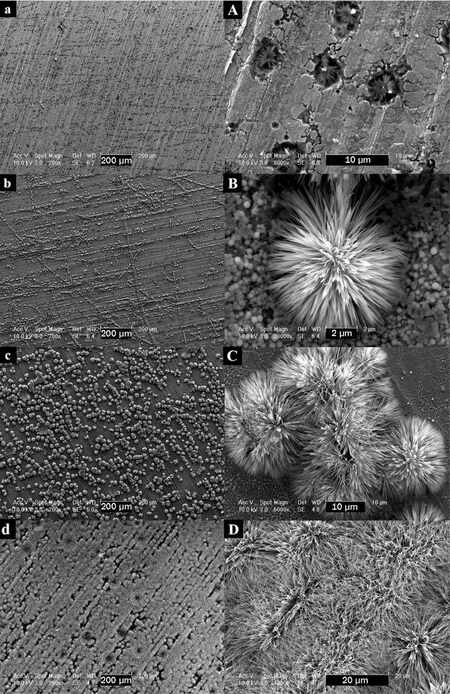
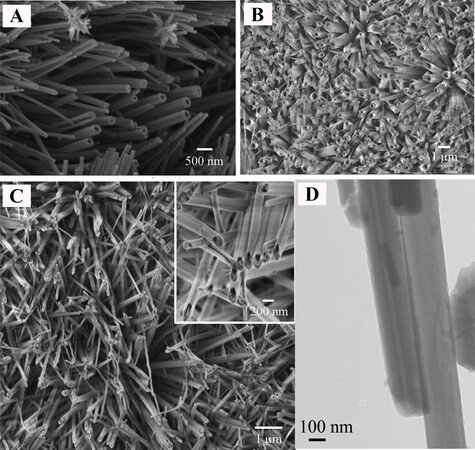











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.