Metal nitride heterostructures capsulated in carbon nanospheres to accommodate lithium metal for constructing a stable composite anode
Abstract
Although various hosts have been proposed to accommodate the Lithium (Li) metal to solve the uneven Li deposition and infinite volume change, the pulverization of the host or lithiophilic modification layer easily leads to structural damage and the poor cycling stability of the composite anode. Herein, we design a host of metal nitrides (Mo2N and WN heterostructures) nanoparticles capsulated in the hollow carbon nanospheres, which can accommodate Li metal to form a stable composite anode. The lithiophilic Mo2N guides uniform infusion and reduces the nucleation barriers of Li metal during electrochemical process. Note that the rigid WN matrix is uniformly composited with Mo2N, which can suppress the pulverization of Mo2N during the repeat Li plating/stripping, ensuring the stability of regulated deposition during long cycling. High mechanical strength, uniform surface potential distribution and good electrolyte wettability of the Li metal-based composite anode guarantee the rapid Li plating/stripping kinetics. Thus, the obtained composite anode can stably cycle 1400 h at
Keywords
INTRODUCTION
The energy density of conventional lithium (Li)-ion batteries (LIBs) based on the graphite anode is hard to meet the requirements of the rapid development of automobiles and portable electronic devices[1-4]. Li metal is the most promising anode for the next generation of high-energy batteries due to its ultrahigh capacity (3860 mA h g-1) and low reduction potential (-3.04 V vs. the standard hydrogen electrode)[5-8]. However, the critical problems, including uncontrolled Li dendrite formation[9-12], infinite volume expansion[13,14] and severe interfacial side reactions[15-20], cause safety issues and poor stability of lithium metal batteries (LMBs). Thus far, numerous strategies have been proposed to solve the above problems, such as artificial interface construction[21-23], host structure design[24-26] and electrolyte modification engineering[27-29]. Unfortunately, most of the above works are conducted under low-capacity loadings or with flooded electrolytes, far from the harsh practical conditions[9,30].
Compared with the pure Li metal anode, the Li metal-based composite anode is more promising for applications because of its better structural stability and capability for mass production[31-33]. Various materials have been used as hosts to accommodate the Li metal, including carbon-based hosts and three-dimensional metal foams[24,33]. However, these hosts have poor interfacial compatibility with lithium[34], and thus, many lithiophilic alloys such as metal nitrides and lithium compounds are used to modify the host surface to guide the uniform plating or infusion in the hosts. Nevertheless, these lithiophilic materials usually suffer the inevitable pulverization and agglomeration during long cycling because of the reaction with Li[35,36]. Therefore, the structural integrity of lithiophilic components and host materials is important to prepare a stable Li-metal-based composite anode and realize the uniform Li plating and stripping.
Herein, we design and prepare a host of metal nitrides (Mo2N and WN heterostructures) capsulated in the hollow carbon nanospheres (denoted WN/Mo2N@HCN). A composite Li metal anode was prepared by die pressing and heating the Li metal powder and WN/Mo2N@HCN host together
RESULTS AND DISCUSSION
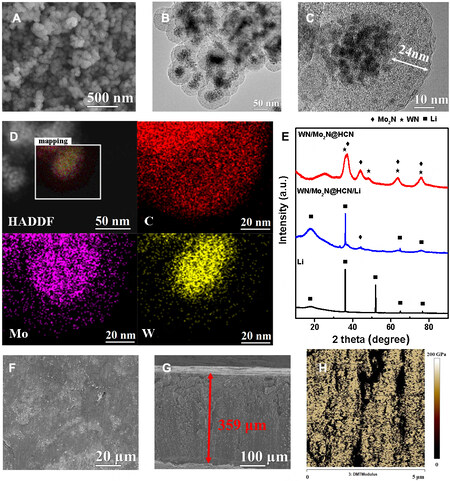
The WN/Mo2N@HCN host was obtained through micelle-interfacial copolymerization in the presence of soluble metal salts, followed by carbonization and nitridation[37-39]. The copolymerization of organic monomers triggers the condensation of metal salts at micelle interfaces, which achieves one-pot integration of nanoparticle preparation, encapsulation and shell formation. The WN/Mo2N@HCN host shows a well-defined spherical morphology and core-shell structure [Figure 1A and B]. In addition, this process also ensures the uniform growth of WN and Mo2N together to form a heterostructure-like structure due to the similar properties of the used salt precursors and simultaneous nitriding. The cavity thickness in the HCN is about ~24 nm suggested by the high-resolution TEM image [Figure 1C]. The specific surface area calculated by the Brunauer-Emmett-Teller (BET) method and the pore volume of WN/Mo2N@HCN are 206 m2 g-1 and 0.35 cm3 g-1, respectively, which are much lower than those of HCN (569 m2 g-1 and 0.54 cm3 g-1). The decrease in BET specific surface area and the disappearance of hysteresis loop suggest that the interior void of carbon is filled with the alloy nanoparticles after the incorporation of WN/Mo2N heterostructures
Figure 1. Structural characterization of WN/Mo2N@HCN and the obtained Li metal composite anode. (A) SEM image, (B and C) TEM images and (D) stacked HAADF and element mappings of WN/Mo2N@HCN; (E) XRD patterns, (F and G) surface and cross-sectional SEM images and (H) AFM image with Young’s modulus mapping of the composite anode.
The Li metal composite anode (WN/Mo2N@HCN/Li) was prepared by die pressing and heating the Li metal powder and WN/Mo2N@HCN host together with different ratios (Unless specified, the mass ratio between the WN/Mo2N@HCN and Li is 1:3), in which the molten Li uniformly infused into the host and formed compact stack with high bulk density [Supplementary Table 3]. After this process, the diffraction peaks of WN/Mo2N@HCN were weakened. The morphologies of the composite anode are shown in Figure 1F and G and Supplementary Figure 3A and B, which show a similar thickness (around 360 μm) to that of the prepared Li metal foil anode by Li metal powder. The surface modulus of the composite anode and lithium metal foil was characterized by an atomic force microscope (AFM). As shown in Figure 1H, the average Young’s modulus of the composite anode reaches 86.0 GPa, whereas that of Li metal foil is only
The reaction between Li metal powder and WN/Mo2N@HCN was investigated by X-ray photoelectron spectroscopy (XPS). The N 1s XPS peaks at 398.4 and 395.4 eV in the spectra of the composite anode
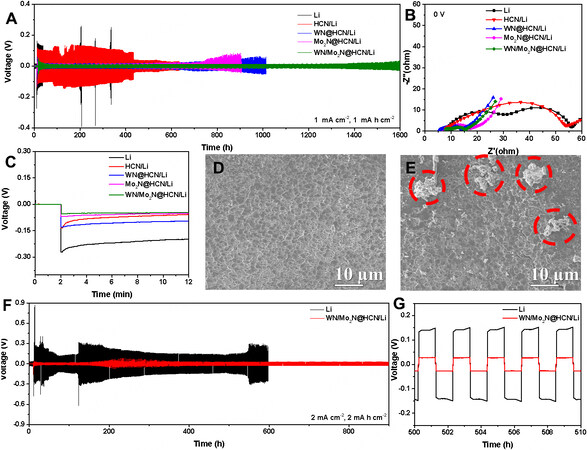
In order to investigate the electrochemical stability of the composite anode, the galvanostatic stripping/plating voltage profiles of symmetric cells with ether-based electrolyte were tested, as shown in Figure 2A. The composite anode exhibits stable cycling performance for more than 1400 h with an ultralow voltage hysteresis (~16 mV) without distinct voltage fluctuation at 1 mA cm-2 and 1 mA h cm-2. In contrast, the composite anodes of WN@HCN/Li and Mo2N@HCN/Li show enlarged overpotential after 600 h and 700 h, which should be ascribed to the inferior lithiophility of WN and the pulverization of Mo2N in long cycling. Note that the HCN/Li and Li metal foil anode show distinct voltage fluctuation in short cycling due to the nonuniform Li deposition, which induces uncontrollable dendrite growth. The comparison of semicircles at the high-frequency region of Nyquist plots [Figure 2B] also shows that the composite anode has the smallest electrochemical resistance due to improved ion diffusion and interfacial reaction kinetics, which is benefited from the introduction of WN/Mo2N@HCN. It is shown that Mo2N@HCN/Li also has a similar initial overpotential with the composite anode during the Li plating [Figure 2C], which is much smaller than those of other anodes, indicating the function of Mo2N in lowering the nucleation barrier[49]. Mo2N can serve as the preferred nucleation site for Li deposition, which can effectively reduce nucleation barriers, where Li can be deposited on Mo in an epitaxial manner with the lattice strain accommodated at the interface, leading to stronger Li-Mo bond than the Li-Li bond[41]. When the mass ratio of WN/Mo2N@HCN and lithium powder was adjusted to 1:2 and 1:4, the cycling performance became worse than that with the ratio of 1:3 [Supplementary Figure 7]. No cracks and dead Li can be observed on the composite anode surface after 200 h stripping/plating [Figure 2D and E]. Even at a high current density of 2 mA cm-2 and a high capacity of 2 mA h cm-2, the cells with the composite anode still maintain excellent cycling stability for 900 cycles [Figure 2F and G].
Figure 2. Electrochemical performance of the different anodes in symmetrical cells. (A) long-term plating/stripping profiles of composite anodes with the different hosts in symmetrical cells at 1 mA cm-2 and 1 mA h cm-2 and (B) the corresponding Nyquist plots; (C) overpotential profiles during the first Li plating cycle; SEM images of (D) WN/Mo2N@HCN/Li and (E) pure Li metal anode after 200 h cycles; (F) long-term plating/stripping profiles of WN/Mo2N@HCN/Li and pure Li metal anode in symmetrical cells at
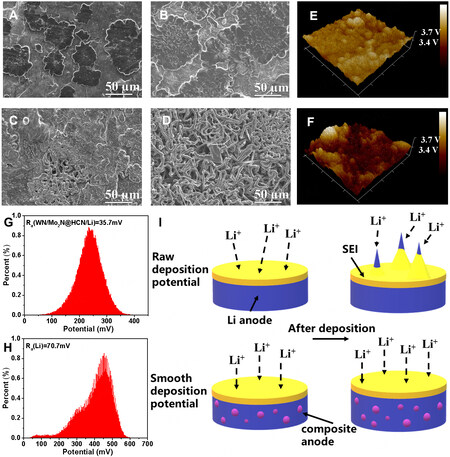
The morphology of the composite and pure Li metal anodes was characterized to reveal the different Li stripping/plating behavior at the current density of 1 mA cm-2. With the deposition capacity of
Figure 3. Lithium deposition behavior of different anodes. Lithium deposition morphology at 1 mA cm-2 for (A) 1 h and (B) 3 h for the composite anode, (C) 1 h and (D) 3 h for pure Li metal anode; surface potential mappings of (E) the composite anode and (F) pure Li metal anode through Kelvin probe force microscopy (KPFM) and corresponding (G and H) statistical distribution; (I) schematic illustration of Li deposition process on these two anodes.
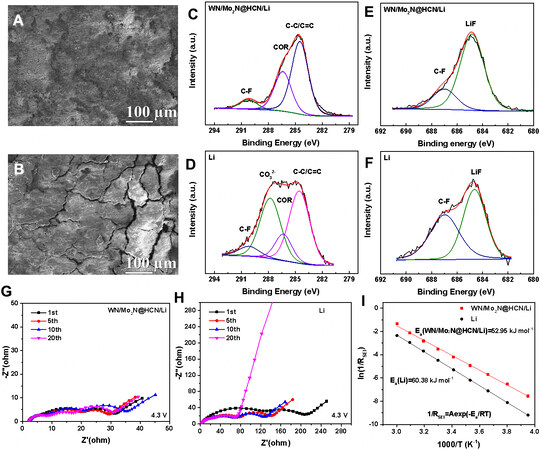
The smooth and flat surface of the composite anode was shown after 100 cycles in the full cell, but the surface of Li metal foil anode showed many cracks [Figure 4A and B]. The above results clearly prove that the lithiophilic metal nitride nanoparticles effectively guide homogeneous Li stripping/plating and the carbon shells well buffer volume changes to release the stress[22], which strengthens the structural stability of the composite anode during cycling. The compositions of the SEI formed on the composite and Li metal foil anodes in the carbonate electrolyte were analyzed by XPS [Figure 4C-F]. In the C 1s spectrum, the peaks at 284.4, 286.4, 288.1 and 290.2 eV correspond to the C-C/C=C, COR, CO32- and C-F species, respectively[50]. The disappearance of Li2CO3 peak in the composite anode indicates that the side reaction was suppressed significantly benefited from improved interfacial kinetics. The peaks at 687.6 and 684.7 eV in the F 1s spectrum are assigned to C-F and LiF[51]. The results show more LiF contained in the SEI formed on the composite anode surface due to the existence of lithium nitride in the composite anode, which can regulate lithium deposition behavior by boosting rapid ion transport. Such differences are also suggested by the electrochemical impedance spectroscopy [Figure 4G and H]. The interfacial impedances of Li metal foil anode not only fluctuated wildly at different cycles in the Nyquist plots, but also were larger than that of the composite anode. The apparent activation energy of Li-ions migration across SEI film on the interface was also calculated by the electrochemical impedance spectroscopy measurements under different temperatures[52]. As shown in Figure 4I, the ESEI of the composite anode (52.95 kJ mol-1) is obviously lower than that of Li metal foil anode (60.38 kJ mol-1), suggesting the improved dynamics of Li-ion migration through SEI for the uniform Li deposition[53].
Figure 4. Structural stability and SEI composition of different anodes. SEM images of surface topography for (A) composite anode and (B) pure Li metal anode after cycling; high-resolution XPS analysis of (C and D) C 1s and (E and F) F 1s spectra for the composite anode and pure Li metal anode after cycling; (G and H) Nyquist plots of NCM811 full cells with the above two anodes after different cycles;
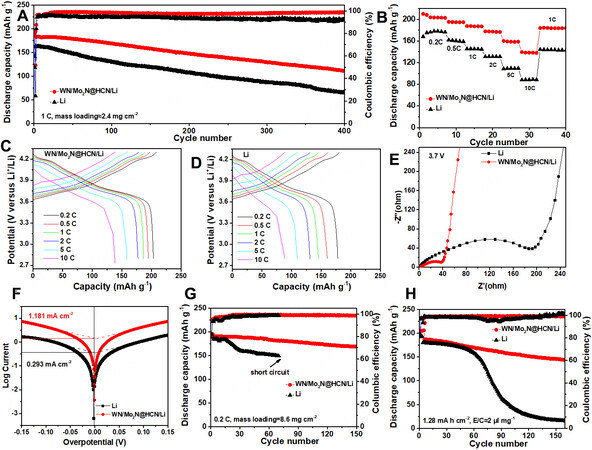
The full battery was assembled with NCM811 cathode to show the practicability of the composite anode. The full cell using the composite anode delivers a higher initial specific capacity of 182.4 mA h g-1 and superior cycling performance with a capacity retention of 61.5% after 400 cycles, which are obviously higher than that of other composite anodes due to the synergistic effect of Mo2N and WN heterostructures
Figure 5. Electrochemical performance of NCM811 full cells with different anodes. (A) Cycling performance, (B) rate performance,
The rate performances of the above two full cells were also tested from 0.2 C to 10 C, as shown in
CONCLUSIONS
In summary, a host of metal nitrides (Mo2N and WN heterostructure) capsulated in the hollow carbon nanospheres was developed, which can accommodate Li metal to form a composite anode with excellent stability. The lithiophilic Mo2N guides the uniform infusion and the electrochemical plating of Li in HCN. At the same time, WN, which is well composited with Mo2N, acts as a rigid matrix to conquer the pulverization of Mo2N during the Li plating/stripping, enhancing the structural stability of the host and regulated deposition during long cycling. The obtained composite anode thus shows excellent electrochemical performance and stability. The symmetric battery can work stably for more than 1400 h at
DECLARATIONS
Authors’ contributionsContributed equally to this work: Ding B, An X
Conceived the concept and directed the research: He YB, Lv W
Carried out the synthesis and characterization: Ding B, An X, Yu J
Gave advice to the research: Lv W, Kang F
Wrote the manuscript: Ding B, He YB
Availability of data and materialsSupplementary Information associated with this article can be found in the online version.
Financial support and sponsorshipThis work was supported by National Natural Science Foundation of China (No. U2001220 and 51972190), the National Key Research and Development Program of China (2021YFF0500600), Shenzhen All-Solid-State Lithium Battery Electrolyte Engineering Research Center (XMHT20200203006), and Shenzhen Technical Plan Project (Nos. RCJC20200714114436091).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
Supplementary MaterialsREFERENCES
1. Grey CP, Tarascon JM. Sustainability and in situ monitoring in battery development. Nat Mater 2016;16:45-56.
2. Larcher D, Tarascon JM. Towards greener and more sustainable batteries for electrical energy storage. Nat Chem 2015;7:19-29.
3. Scrosati B, Hassoun J, Sun Y. Lithium-ion batteries. A look into the future. Energy Environ Sci 2011;4:3287.
4. Tarascon JM, Armand M. Issues and challenges facing rechargeable lithium batteries. Nature 2001;414:359-67.
5. Albertus P, Babinec S, Litzelman S, Newman A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat Energy 2018;3:16-21.
6. Cheng XB, Zhang R, Zhao CZ, Zhang Q. Toward safe lithium metal anode in rechargeable batteries: a review. Chem Rev 2017;117:10403-73.
7. Lin D, Liu Y, Cui Y. Reviving the lithium metal anode for high-energy batteries. Nat Nanotechnol 2017;12:194-206.
8. Liu DH, Bai Z, Li M, et al. Developing high safety Li-metal anodes for future high-energy Li-metal batteries: strategies and perspectives. Chem Soc Rev 2020;49:5407-45.
9. Chen XR, Zhao BC, Yan C, Zhang Q. Review on Li deposition in working batteries: from nucleation to early growth. Adv Mater 2021;33:e2004128.
10. Lu Y, Tu Z, Archer LA. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat Mater 2014;13:961-9.
11. Zhang L, Yang T, Du C, et al. Lithium whisker growth and stress generation in an in situ atomic force microscope-environmental transmission electron microscope set-up. Nat Nanotechnol 2020;15:94-8.
12. Zou P, Sui Y, Zhan H, et al. Polymorph evolution mechanisms and regulation strategies of lithium metal anode under multiphysical fields. Chem Rev 2021;121:5986-6056.
14. Lu J, Chen Z, Pan F, Cui Y, Amine K. High-performance anode materials for rechargeable lithium-ion batteries. Electrochem Energ Rev 2018;1:35-53.
15. Cao X, Ren X, Zou L, et al. Monolithic solid-electrolyte interphases formed in fluorinated orthoformate-based electrolytes minimize Li depletion and pulverization. Nat Energy 2019;4:796-805.
16. Li Y, Li Y, Pei A, et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 2017;358:506-10.
17. Liu T, Lin L, Bi X, et al. In situ quantification of interphasial chemistry in Li-ion battery. Nat Nanotechnol 2019;14:50-6.
18. Wu H, Jia H, Wang C, Zhang J, Xu W. Recent progress in understanding solid electrolyte interphase on lithium metal anodes. Adv Energy Mater 2021;11:2003092.
19. Yan C, Xu R, Xiao Y, et al. Toward critical electrode/electrolyte interfaces in rechargeable batteries. Adv Funct Mater 2020;30:1909887.
20. Zhou Y, Su M, Yu X, et al. Real-time mass spectrometric characterization of the solid-electrolyte interphase of a lithium-ion battery. Nat Nanotechnol 2020;15:224-30.
21. Yu Z, Cui Y, Bao Z. Design principles of artificial solid electrolyte interphases for lithium-metal anodes. Cell Rep Phys Sci 2020;1:100119.
22. Zheng G, Lee SW, Liang Z, et al. Interconnected hollow carbon nanospheres for stable lithium metal anodes. Nat Nanotechnol 2014;9:618-23.
23. Gao Y, Yan Z, Gray JL, et al. Polymer-inorganic solid-electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat Mater 2019;18:384-9.
24. Yang CP, Yin YX, Zhang SF, Li NW, Guo YG. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat Commun 2015;6:8058.
25. Chen H, Yang Y, Boyle DT, et al. Free-standing ultrathin lithium metal-graphene oxide host foils with controllable thickness for lithium batteries. Nat Energy 2021;6:790-8.
26. Wan M, Kang S, Wang L, et al. Mechanical rolling formation of interpenetrated lithium metal/lithium tin alloy foil for ultrahigh-rate battery anode. Nat Commun 2020;11:829.
27. Jie Y, Ren X, Cao R, Cai W, Jiao S. Advanced liquid electrolytes for rechargeable Li metal batteries. Adv Funct Mater 2020;30:1910777.
28. Jin C, Liu T, Sheng O, et al. Rejuvenating dead lithium supply in lithium metal anodes by iodine redox. Nat Energy 2021;6:378-87.
29. Yang K, Chen L, Ma J, et al. Stable interface chemistry and multiple ion transport of composite electrolyte contribute to ultra-long cycling solid-state LiNi0.8Co0.1Mn0.1O2/lithium metal batteries. Angew Chem Int Ed 2021;60:24668-75.
30. Li G. Regulating mass transport behavior for high-performance lithium metal batteries and fast-charging lithium-ion batteries. Adv Energy Mater 2021;11:2002891.
31. Zhou F, Li Z, Lu YY, et al. Diatomite derived hierarchical hybrid anode for high performance all-solid-state lithium metal batteries. Nat Commun 2019;10:2482.
32. Krauskopf T, Mogwitz B, Rosenbach C, Zeier WG, Janek J. Diffusion limitation of lithium metal and Li-Mg alloy anodes on LLZO type solid electrolytes as a function of temperature and pressure. Adv Energy Mater 2019;9:1902568.
33. Zhang R, Chen X, Shen X, et al. Coralloid carbon fiber-based composite lithium anode for robust lithium metal batteries. Joule 2018;2:764-77.
34. Duan J, Zheng Y, Luo W, et al. Is graphite lithiophobic or lithiophilic? Natl Sci Rev 2020;7:1208-17.
35. Lu D, Shao Y, Lozano T, et al. Failure mechanism for fast-charged lithium metal batteries with liquid electrolytes. Adv Energy Mater 2015;5:1400993.
36. Zhan Y, Shi P, Ma X, et al. Failure mechanism of lithiophilic sites in composite lithium metal anode under practical conditions. Adv Energy Mater 2022;12:2103291.
37. Xu F, Ding B, Qiu Y, et al. Generalized domino-driven synthesis of hollow hybrid carbon spheres with ultrafine metal nitrides/oxides. Matter 2020;3:246-60.
38. Xu F, Qu C, Lu Q, et al. Atomic Sn-enabled high-utilization, large-capacity, and long-life Na anode. Sci Adv 2022;8:eabm7489.
39. Xu F, Tang Z, Huang S, et al. Facile synthesis of ultrahigh-surface-area hollow carbon nanospheres for enhanced adsorption and energy storage. Nat Commun 2015;6:7221.
40. Zhao Q, Hao X, Su S, et al. Expanded-graphite embedded in lithium metal as dendrite-free anode of lithium metal batteries. J Mater Chem A 2019;7:15871-9.
41. Luo L, Li J, Yaghoobnejad Asl H, Manthiram A. A 3D lithiophilicMo2N-modified carbon nanofiber architecture for dendrite-free lithium-metal anodes in a full cell. Adv Mater 2019;31:e1904537.
42. Wang S, Yu X, Lin Z, et al. Synthesis, crystal structure, and elastic properties of novel tungsten nitrides. Chem Mater 2012;24:3023-8.
43. Zhang W, Fan L, Tong Z, et al. Stable Li-metal deposition via a 3D nanodiamond matrix with ultrahigh young's modulus. Small Methods 2019;3:1900325.
44. Yu J, Shi K, Zhang S, et al. A lithium nucleation-diffusion-growth mechanism to govern the horizontal deposition of lithium metal anode. Sci China Mater 2021;64:2409-20.
45. Xu F, Ding B, Qiu Y, et al. Hollow carbon nanospheres with developed porous structure and retained n doping for facilitated electrochemical energy storage. Langmuir 2019;35:12889-97.
46. Shi P, Li T, Zhang R, et al. Lithiophilic LiC6 layers on carbon hosts enabling stable Li metal anode in working batteries. Adv Mater 2019;31:e1807131.
47. Zhu J, Cai D, Li J, et al. In-situ generated Li3N/Li-Al alloy in reduced graphene oxide framework optimizing ultra-thin lithium metal electrode for solid-state batteries. Energy Stor Mater 2022;49:546-54.
48. Ye W, Pei F, Lan X, et al. Stable nano-encapsulation of lithium through seed-free selective deposition for high-performance Li battery anodes. Adv Energy Mater 2020;10:1902956.
49. Yan K, Lu Z, Lee H, et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat Energy 2016;1:16010.
50. Rustomji CS, Yang Y, Kim TK, et al. Liquefied gas electrolytes for electrochemical energy storage devices. Science 2017;356:eaal4263.
51. Huang Z, Ren J, Zhang W, et al. Protecting the Li-metal anode in a Li-O2 battery by using boric acid as an sei-forming additive. Adv Mater 2018;30:e1803270.
52. Zhang W, Wu Q, Huang J, et al. Colossal granular lithium deposits enabled by the grain-coarsening effect for high-efficiency lithium metal full batteries. Adv Mater 2020;32:e2001740.
53. Li S, Wang XS, Han B, et al. Ultrathin and high-modulus LiBO2 layer highly elevates the interfacial dynamics and stability of lithium anode under wide temperature range. Small 2022;18:e2106427.
54. Wang Y, Liu F, Fan G, et al. Electroless formation of a fluorinated Li/Na hybrid interphase for robust lithium anodes. J Am Chem Soc 2021;143:2829-37.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Ding B, An X, Yu J, Lv W, Kang F, He YB. Metal nitride heterostructures capsulated in carbon nanospheres to accommodate lithium metal for constructing a stable composite anode. Energy Mater 2022;2:200039. http://dx.doi.org/10.20517/energymater.2022.53
AMA Style
Ding B, An X, Yu J, Lv W, Kang F, He YB. Metal nitride heterostructures capsulated in carbon nanospheres to accommodate lithium metal for constructing a stable composite anode. Energy Materials. 2022; 2(6): 200039. http://dx.doi.org/10.20517/energymater.2022.53
Chicago/Turabian Style
Ding, Baichuan, Xufei An, Jing Yu, Wei Lv, Feiyu Kang, Yan-Bing He. 2022. "Metal nitride heterostructures capsulated in carbon nanospheres to accommodate lithium metal for constructing a stable composite anode" Energy Materials. 2, no.6: 200039. http://dx.doi.org/10.20517/energymater.2022.53
ACS Style
Ding, B.; An X.; Yu J.; Lv W.; Kang F.; He Y.B. Metal nitride heterostructures capsulated in carbon nanospheres to accommodate lithium metal for constructing a stable composite anode. Energy Mater. 2022, 2, 200039. http://dx.doi.org/10.20517/energymater.2022.53
About This Article
Copyright
Data & Comments
Data
 Cite This Article 13 clicks
Cite This Article 13 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.