Air-exposed lithium metal as a highly stable anode for low-temperature energy storage applications
Abstract
The demand for cryogenic applications has resulted in higher requirements for the low-temperature performance of energy storage systems. Lithium-metal batteries are the most promising energy storage systems. Lithium-metal anodes have the merits of high capacity and low potential. However, at low temperatures, especially sub-zero, the formation of lithium dendrites seriously hinders their applications. Herein, distinct from the traditional strategies of separating lithium metal from oxygen substances, we propose a new strategy to suppress dendrites by exposing lithium metal to air for short periods to generate a controlled oxidative protective layer in situ that is compact, homogeneous and mainly composed of Li3N, Li2O, LiOH and Li2CO3. Symmetrical and full cells are assembled. The air-pretreated Li metal symmetrical cell exhibits an excellent lifespan of up to 4500 h (1 mA cm-2) at 30 °C and also shows a smaller voltage polarization of 20 mV at 1.0 mA cm-2 at -20 °C. Importantly, the full cell using the air-pretreated Li metal as an anode and NCM811 as a cathode can charge-discharge normally at -20 and -40 °C. This work provides an efficient and facile approach for developing superior lithium-metal batteries for future utilization at a wide range of temperatures.
Keywords
INTRODUCTION
Lithium-metal batteries (LMBs) are considered to be the most promising candidates for next-generation electrochemical energy storage technology, attributed to their merits, which include the high capacity
The main components of a SEI are lithium-based compounds, such as LiF, Li2CO3, LiOH, Li2O and Li3N[14]. Typically, the SEI compositions are highly dependent on the additives in the electrolyte, such as LiNO3, fluoroethylene carbonate, H2O and CO2, which are added to regulate metallic Li deposition[15]. Nevertheless, these additives are continuously consumed and exhausted rapidly because their solubility in the widely applied carbonate electrolytes is low[16]. For low-temperature applications, inspired by the merits of the surface protection layer and artificial SEI, we exposed Li metal directly to air and an air-pretreated layer (APL) formed on the surface. Li metal can directly/indirectly react with N2, O2, H2O and CO2 and form lithium-based compounds (Li3N, Li2O, LiOH and Li2CO3), which are the main components of the SEI. These in situ formed compounds guarantee the strong adhesion between Li metal and the APL, as well as the interactions between the components in the APL. Among the formed compounds, Li3N can function as a Li+ conductor (10-3 S cm-1)[17,18], the density functional theory-calculated Young’s moduli of Li2CO3 is
The performance of LMBs must be sensitive to the exposure time in air because the thickness of the protective layer increases with exposure time; thus, the thickness of the APL should be a balance of resistivity and conductivity. Thus, the main problem is controlling the exposure time. Li metal is treated in air with a humidity of ~40% for 10 min, 30 min, 1 h and 2 h, respectively. We then evaluated their electrochemical performance at both room (30 °C) and low temperatures (-20 and -40 °C).
RESULTS AND DISCUSSION
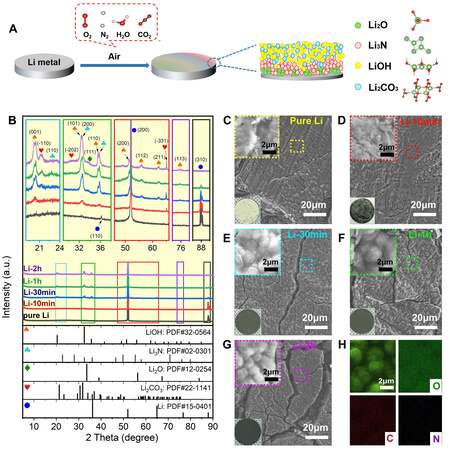
As depicted in Figure 1A, when exposed to air, the highly active Li metal reacts with N2, O2, H2O and CO2 directly/indirectly and then the APL forms on the surface of the Li metal[21]. Figure 1B shows the X-ray diffraction (XRD) for pure Li, Li-10 min, Li-30 min, Li-1 h and Li-2 h. The XRD peaks 36.19°, 51.97° and 87.89° of pure Li are corresponding to the (110), (200) and (220) facets (JCPDS No. 15-0401), respectively. After exposing Li metal to the air, with increasing exposure time, the peaks of pure Li became weakened gradually, while the peaks of Li compounds became stronger and stronger. The weak peak of the Li2O (111) facet at 33.61° was observed in Li-30 min, Li-1 h and Li-2 h, indicating the existence of Li2O, which may locate underneath the formed layer and direct contact with Li metal. There are two peaks at ~32.00°, the peak at 32.48° is the (101) facet of LiOH and the peak at 32.53° is the (200) facet of Li3N. The Li3N (200) peak appears first, then the LiOH (101) peak becomes stronger gradually with increasing exposure time and the same applies to the two peaks at ~36.00°. Finally, the peaks of LiOH and Li2CO3 became the most obvious among the formed compounds and it can be inferred that LiOH and Li2CO3 exist at the outermost region of the APL. Li3N should be located in the middle or interior of the APL because Li3N is unstable in air. In addition, Li2CO3 can react with Li metal under the internal operating conditions of the battery[20]. The possible distribution of the lithium-based compounds in the protection layer is shown in Figure 1A.
Figure 1. (A) Schematic synthesis of in-situ protection layer on lithium metal through exposure to air. (B) X-ray diffraction pattern of pure Li, Li-10 min, Li-30 min, Li-1 h and Li-2 h. SEM images of (C) pure Li, (D) Li-10 min, (E) Li-30 min, (F) Li-1 h and (G) Li-2 h. Insets show high-magnification images and digital photographs. (H) EDX mapping of in-situ protection layer.
To obtain the morphological variations of the APL on Li metal, the surface was observed through scanning electron microscopy (SEM) analysis [Figure 1C-G]. The surface of pure Li metal is a relative plane and with increasing exposure time, the surface becomes rougher with raised bulges observed in the zoomed-in area. The enriched cracks indicate the formation of a large amount of lithium-based compounds, because the molecular volumes (cell volume) of Li3N, Li2O, LiOH and Li2CO3 are 44.88, 25.27, 56.89 and 122.9 ų, respectively, and that of a Li atom is 42.9 ų. The molecular volumes of Li3N, LiOH and Li2CO3 are larger than that of a single lithium atom. Therefore, with the accumulation of these compounds on the Li metal substrate, the inner stress of the APL increases, cracks appear and the APL of Li-1 h and Li-2 h tends to peel off. As shown in the digital photos in the insets of Figure 1C-G, the pure Li metal has a bright metallic luster, the majority of the
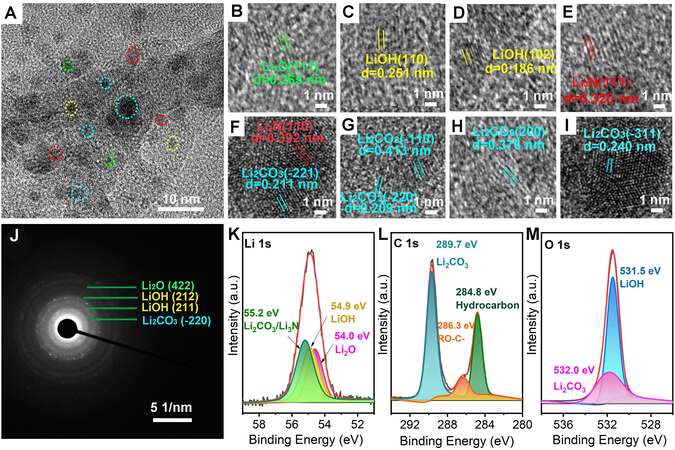
To further investigate the components of the APL, we performed high-resolution transmission electron microscopy(HRTEM) tests [Figure 2A-I] to reveal that the APL mainly contained nanocrystals of lithium-based compounds. The crystalline grains were identified. The lattice fringe with the interplanar spacing of 0.265 nm was identified to be the (111) crystal plane of
Figure 2. (A) TEM image, (B-I) HRTEM images, (J) SAED and XPS spectra of in-situ protection layer. XPS spectra of (K) Li 1s, (L) C 1s and (M) O 1s.
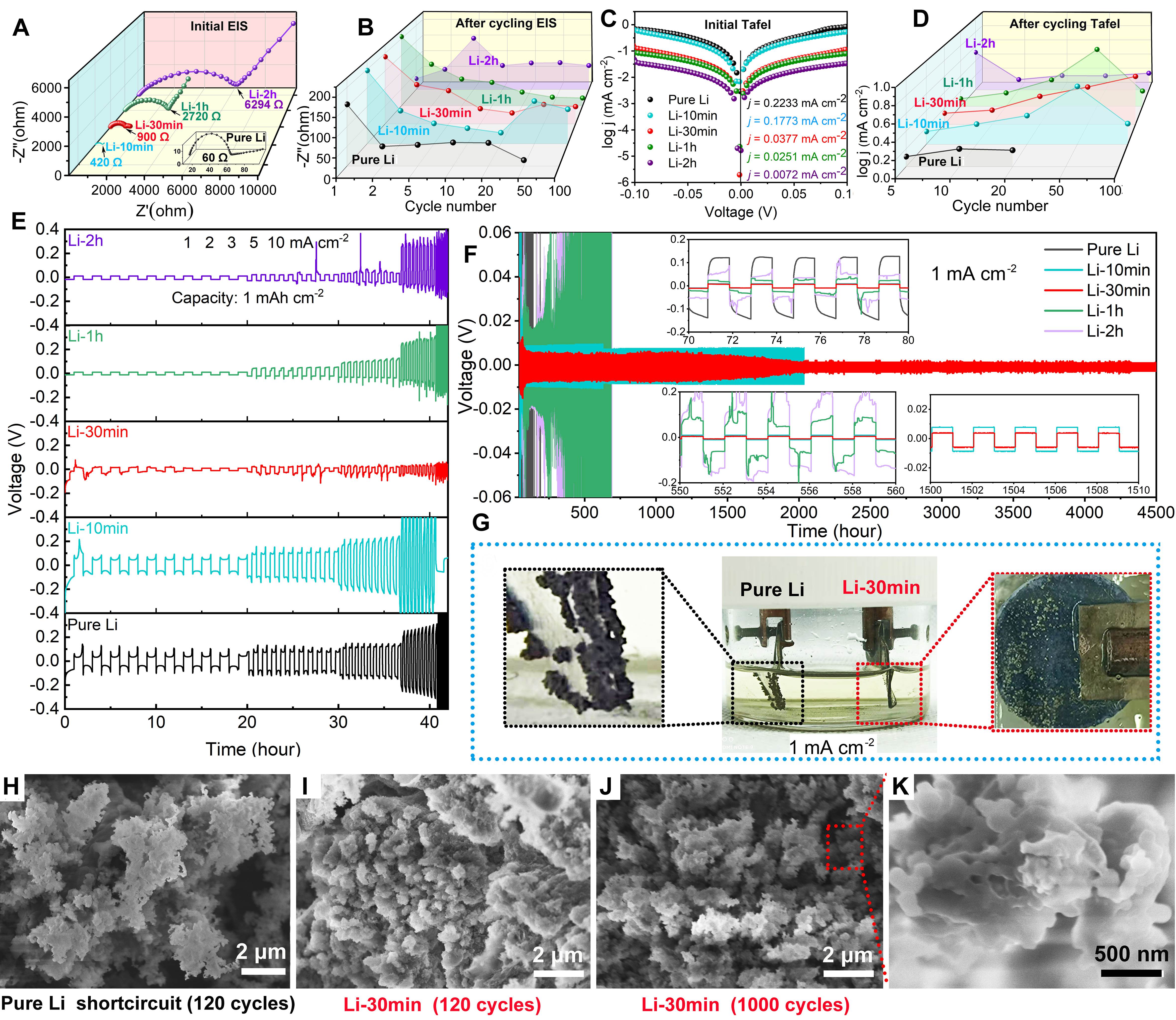
Based on the above characterization, although the components of the in-situ APL have been revealed, it is also meaningful to confirm its dendrite suppression ability. First, the electrochemical performance was evaluated carefully at room temperature [Figure 3] using symmetrical cells. The initial electrochemical impedance spectroscopy (EIS) results reveal the low conductivity of APL [Figure 3A], with charge transfer resistance (Rct) values of 60, 420, 900, 2720 and 6294 Ω for pure Li, Li-10 min, Li-30 min, Li-1 h and Li-2 h, respectively. The Rct increased drastically with the exposure time. The EIS plots after cycling (1st, 2nd, 5th, 10th, 20th, 50th and 100th) were acquired, with the Rct value tending to reduce with cycling, as shown in Figure 3B. The Rct of all samples decreased beneath 200 Ω after the 1st cycle, which could be due to the uniform deposition of Li metal in the APL after cycling. Conductive pathways were then constructed, revealing that the electrochemical performance was only partially determined by the initial resistance. Figure 3C and D show the Tafel plots for evaluating the exchange current density. Pure Li exhibits the largest exchange current density; after cycling, the value of all samples tends to increase, which corresponds to the EIS results. The above data reveal that the conductivity of the APL was enhanced after cycling.
Figure 3. (A) EIS plots, (B) Rct variations after cycling, (C) Tafel plots and (D) exchange current density variations after cycling of pure Li, Li-10 min, Li-30 min, Li-1 h and Li-2 h. (E) Corresponding voltage-time profiles of Li plating/stripping at current densities of 1, 2, 3, 5 and 10 mA cm-2 in symmetrical cells. (F) Long cycling voltage-time profiles at 1 mA cm-2. Insets show selected voltage-time profiles.
Figure 3E shows the voltage profiles of the symmetrical cells during Li plating and stripping with increasing current densities of 1, 2, 3, 5 and 10 mA cm-2 at a fixed capacity of 1 mAh cm-2. Li-10 min exhibits almost the same plots as pure Li but with a larger voltage range. With the exposure time increasing to greater than or equal to 30 min, the voltage range was kept in the range of -50-50 mV at 1 mA cm-2. Among all the samples, Li-30 min exhibited the best voltage control ability and when the current density switched to
As four main Li compounds were detected, it is therefore imperative to test the dendrite-suppression performance of each compound. Li3N, Li2O, LiOH, and Li2CO3 were applied to modify Li metal by cold pressing. Their voltage profiles during Li plating and stripping with increasing current densities of 1, 2, 3, 5 and 10 mA cm-2 at a fixed capacity of 1 mAh cm-2 are shown in Supplementary Figure 1. Among them, Li2O and LiOH exhibit better dendrite suppression, together with the good mechanical performance of Li2CO3 and superior ion conductivity of Li3N, thereby enabling a synthetic effect.
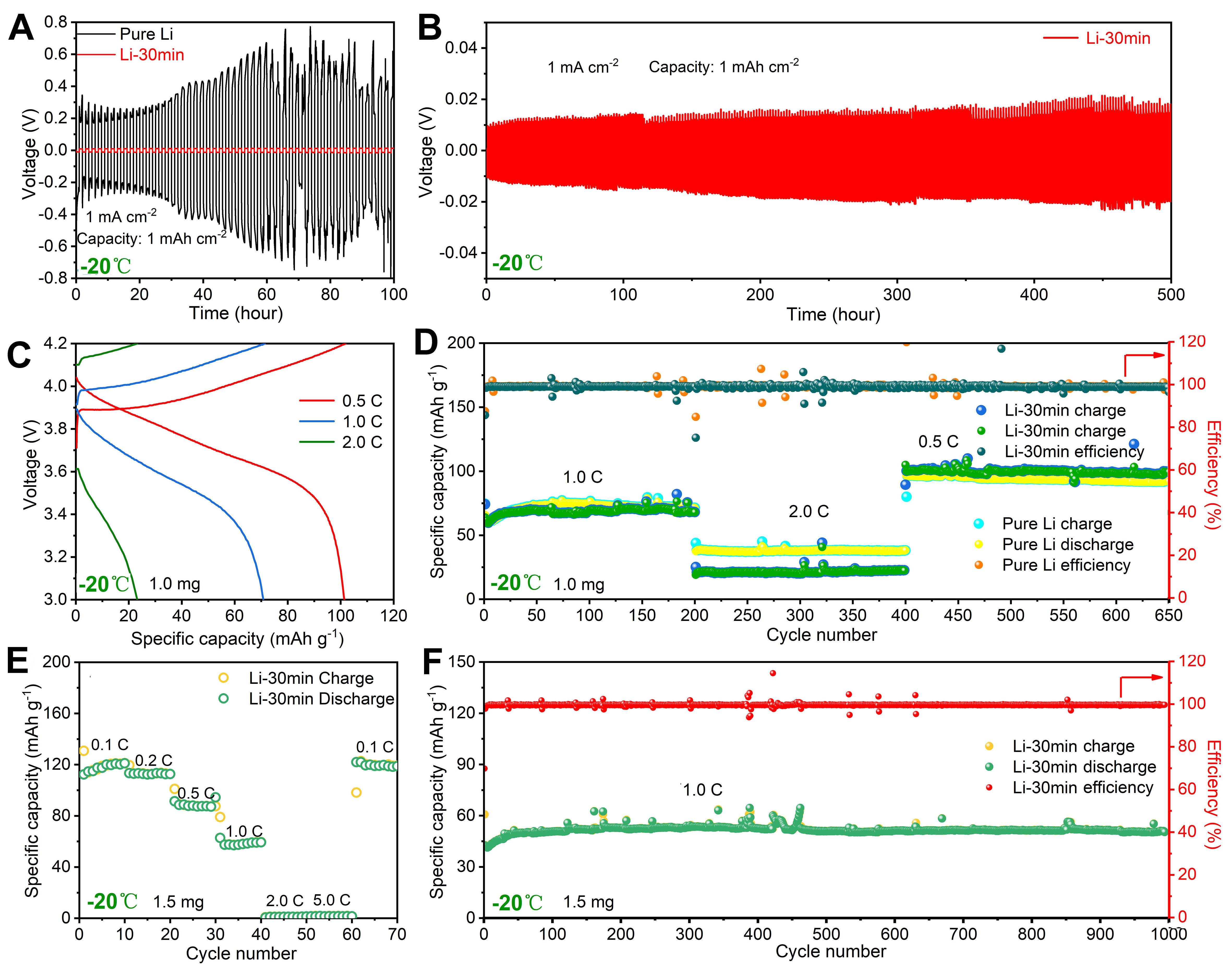
The energy storage performance of Li-30 min|NCM8111mg and pure Li|NCM8111mg cells was determined at 30, -20 and -40 °C [1.0 M LiPF6 in EC:DEC (1:1 v/v) as the electrolyte]. The voltage of pure Li symmetrical cell reaches ~500 mV at 1 mA cm-2 at -20 °C and has a short circuit after 70 h. The corresponding voltage of the Li-30 min symmetrical cell is ~20 mV [Figure 4A]. The Li-30 min symmetrical cell shows stable Li plating-stripping for more than 500 h and exhibits excellent dendrite suppression at low temperatures [Figure 4B]. Under 30 °C [Supplementary Figure 2], the capacities of Li-30 min|NCM8111mg at 0.1 C, 0.2 C, 0.5 C, 1.0 C, 2.0 C, 5.0 C and 10.0 C are 208, 205, 196, 184, 140, 100 and 41 mAh g-1, respectively, while those of pure Li|NCM8111mg are 173, 154, 146, 137, 117, 93 and 54 mAh g-1, respectively, Li-30 min|NCM8111mg, respectively, exhibiting higher capacity compared to pure Li|NCM8111mg. This is because the APL of Li-30 min provides the required components for the SEI, while the extra capacity cost to generate the continuous formation SEI films in pure Li|NCM8111mg, but at 10 C, the capacity of pure Li |NCM8111mg becomes higher due to the inferior conductivity of the APL on Li-30 min hindering the formation of smooth Li. As a result, the APL performs well at low rates. At 30 °C [Supplementary Figure 3], a long cycling (300 cycles) test was performed, with the per cycle capacity decay of Li-30 min|NCM8111mg being 0.05%, while that of pure Li|NCM8111mg is 0.12%.
Figure 4. (A) Voltage-time profiles of Li-30 min and pure Li symmetrical cells. (B) Long cycling voltage-time profiles at 1 mA cm-2.
The charge-discharge curves of Li-30 min|NCM8111mg at 30, -20 and -40 °C are shown in
CONCLUSION
Exposing pure Li to air alters the Li deposition behavior and enables the dendrite-free growth of Li metal at moderate current densities, thereby enhancing the cycling stability and extending the cycle life for high-capacity Li metal batteries at a wide temperature range. In contrast to typical efforts made to protect Li metal from oxidation, we exposed Li to air and made it oxidize. This strategy has the advantages of being extremely low cost, green and has been shown to be applicable to various metal batteries (e.g., Na-, K- and Zn-metal batteries) at wide temperatures.
Experimental section
Materials: Lithium oxide (Li2O, 99.99%, Aladdin), lithium nitride (Li3N, 99.9%, Aladdin), lithium carbonate (Li2CO3, 99.5%, Macklin) and lithium hydroxide (LiOH, 99%, J&K). All chemicals were used as received.
Preparation of air-pretreated Li metal: Li metal was exposed to air (humidity of 40%). Four samples were then obtained and all samples were named according to their air exposure time, which are Li-10 min,
Structural characterization: X-ray diffraction (XRD) patterns were obtained by using an X’Pert PRO MDP with Cu Kα radiation (λ = 1.5405 Å) with 40 mA and 40 kV. X-ray photoelectron spectroscopy (XPS) was collected at an Escalab 250Xi spectrophotometer. Field emission scanning electron microscopy (FESEM) images were obtained using a JEOL JSM-7001F scanning electron microscope operating at 15 kV. Transmission electron microscopy (TEM) images were obtained using a JEOL JEM-2100F electron microscope operating at 200 kV. Thermogravimetric analysis (TGA) data were collected using a DTA-60 (Shimadzu), annealed under air/N2 atmosphere at 10 °C min-1.
Electrochemical measurements: The CR2032 coin-type cells were assembled in an argon-filled glove box. The electrolyte consisted of a solution of 1.0 M LiPF6 in EC:DEC (1:1 v/v) and the amount of electrolyte used for the batteries was 40 μL. The cells were cycled galvanostatically in the voltage range of 3.0-4.2 V with a battery testing system (Neware Electronic Co., China). Electrochemical impedance spectroscopy (EIS) was conducted by a bio-logic VMP3 electrochemical workstation.
DECLARATIONS
Authors’ contributionsCompleting the experiments and preparing the manuscript draft: Zheng S
Discussing and revising the manuscript: Geng H, Eliseeva SN
Writing-review and editing, funding acquisition, supervision: Wang B
Availability of data and materialsNot applicable.
Financial support and sponsorshipWe acknowledge financial support from National Natural Science Foundation of China (No. 52172250).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
Supplementary MaterialsREFERENCES
1. Xu H, Li Y, Zhou A, et al. Li3N-modified garnet electrolyte for all-solid-state lithium metal batteries operated at 40 °C. Nano Lett 2018;18:7414-8.
2. Wang SH, Yin YX, Zuo TT, et al. Stable Li metal anodes via regulating lithium plating/stripping in vertically aligned microchannels. Adv Mater 2017;29:1703729.
3. Ji X, Hou S, Wang P, et al. Solid-state electrolyte design for lithium dendrite suppression. Adv Mater 2020;32:e2002741.
4. Hu Z, Li G, Wang A, Luo J, Liu X. Recent progress of electrolyte design for lithium metal batteries. Batteries Supercaps 2020;3:331-5.
5. Bi Z, Guo X. Solidification for solid-state lithium batteries with high energy density and long cycle life. Energy Mater 2022;2:200011.
6. Meyerson ML, Papa PE, Heller A, Mullins CB. Recent developments in dendrite-free lithium-metal deposition through tailoring of micro- and nanoscale artificial coatings. ACS Nano 2021;15:29-46.
7. Wondimkun ZT, Tegegne WA, Shi-kai J, et al. Highly-lithiophilic Ag@PDA-GO film to suppress dendrite formation on Cu substrate in anode-free lithium metal batteries. Energy Stor Mater 2021;35:334-44.
8. Xiao Y, Xu R, Xu L, Ding J, Huang J. Recent advances in anion-derived SEIs for fast-charging and stable lithium batteries. Energy Mater 2022;1:100013.
9. Gupta A, Manthiram A. Designing advanced lithium-based batteries for low-temperature conditions. Adv Energy Mater 2020;10:2001972.
10. Liu F, Wang L, Zhang Z, et al. A mixed lithium-ion conductive Li2S/Li2Se protection layer for stable lithium metal anode. Adv Funct Mater 2020;30:2001607.
11. Wang D, Liu Y, Li G, Qin C, Huang L, Wu Y. Liquid metal welding to suppress Li dendrite by equalized heat distribution. Adv Funct Mater 2021;31:2106740.
12. Luo Y, Li T, Zhang H, et al. Endogenous symbiotic Li3N/cellulose skin to extend the cycle life of lithium anode. Angew Chem Int Ed 2021;60:11718-24.
13. Liu S, Xia X, Deng S, et al. In Situ solid electrolyte interphase from spray quenching on molten Li: a new way to construct high-performance lithium-metal anodes. Adv Mater 2019;31:e1806470.
14. Wu J, Ihsan-ul-haq M, Chen Y, Kim J. Understanding solid electrolyte interphases: advanced characterization techniques and theoretical simulations. Nano Energy 2021;89:106489.
15. Zhang H, Luo J, Qi M, et al. Enabling lithium metal anode in nonflammable phosphate electrolyte with electrochemically induced chemical reactions. Angew Chem Int Ed 2021;60:19183-90.
16. Fu L, Wang X, Wang L, et al. A salt-in-metal anode: stabilizing the solid electrolyte interphase to enable prolonged battery cycling. Adv Funct Mater 2021;31:2010602.
17. Ma G, Wen Z, Wu M, et al. A lithium anode protection guided highly-stable lithium-sulfur battery. Chem Commun 2014;50:14209-12.
18. Park K, Goodenough JB. Dendrite-suppressed lithium plating from a liquid electrolyte via wetting of Li3N. Adv Energy Mater 2017;7:1700732.
19. Zhai P, Liu L, Gu X, Wang T, Gong Y. Interface engineering for lithium metal anodes in liquid electrolyte. Adv Energy Mater 2020;10:2001257.
20. Han B, Zhang Z, Zou Y, et al. Poor stability of Li2CO3 in the solid electrolyte interphase of a lithium-metal anode revealed by cryo-electron microscopy. Adv Mater 2021;33:e2100404.
21. Zhang Y, Wang W, Tang H, et al. An ex-situ nitridation route to synthesize Li3N-modified Li anodes for lithium secondary batteries. J Power Sources 2015;277:304-11.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zheng S, Geng H, Eliseeva SN, Wang B. Air-exposed lithium metal as a highly stable anode for low-temperature energy storage applications. Energy Mater 2022;2:200042. http://dx.doi.org/10.20517/energymater.2022.66
AMA Style
Zheng S, Geng H, Eliseeva SN, Wang B. Air-exposed lithium metal as a highly stable anode for low-temperature energy storage applications. Energy Materials. 2022; 2(6): 200042. http://dx.doi.org/10.20517/energymater.2022.66
Chicago/Turabian Style
Zheng, Shumin, Haitao Geng, Svetlana N. Eliseeva, Bao Wang. 2022. "Air-exposed lithium metal as a highly stable anode for low-temperature energy storage applications" Energy Materials. 2, no.6: 200042. http://dx.doi.org/10.20517/energymater.2022.66
ACS Style
Zheng, S.; Geng H.; Eliseeva SN.; Wang B. Air-exposed lithium metal as a highly stable anode for low-temperature energy storage applications. Energy Mater. 2022, 2, 200042. http://dx.doi.org/10.20517/energymater.2022.66
About This Article
Copyright
Data & Comments
Data
 Cite This Article 11 clicks
Cite This Article 11 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.