Accelerating redox kinetics by ZIF-67 derived amorphous cobalt phosphide electrocatalyst for high-performance lithium-sulfur batteries
Abstract
The feasibility of the commercialization of lithium-sulfur (Li-S) batteries is troubled by sluggish redox conversion kinetics and the shuttle effect of polysulfides. Herein, a zeolitic imidazolate framework derived amorphous CoP combined with carbon nanotubes conductive network composites (aCoP@CNTs) has been synthesized as an effective dual-electrocatalyst for accelerating the redox kinetics of polysulfides to prolong the lifespan of Li-S batteries. Compared with crystalline CoP, unsaturated Co atoms of aCoP@CNTs exhibit stronger chemical adsorption capacity for polysulfides and serve as catalytic centers to accelerate the conversion from soluble polysulfides to solid-state lithium sulfide. Meanwhile, the 3D porous conductive network not only facilitates ion/electron transportation but also forms a physical barrier to limit the migration of polysulfides. Benefiting from the above preponderances, the batteries with aCoP@CNTs modified interlayer exhibited excellent cycle stability (initial discharge capacity of 1227.9 mAh g-1 at 0.2 C), rate performance (795.9 mAh g-1 at 2.5 C), long-term cycle reliability (decay rate of 0.049% per cycle at 1 C over 1000 cycles), and superior high-loading performance (high initial discharge capacity of 886 mAh g-1 and 753.6 mAh g-1 at 1 C under high S loading of 3 mg cm-2 and 4 mg cm-2).
Keywords
INTRODUCTION
For the last few years, lithium-sulfur (Li-S) batteries have attracted widespread attention owing to their natural advantages of the high theoretical energy density of 2600 Wh kg-1 and theoretical specific capacity of 1675 mAh g-1, abundant raw material reserves, and environmentally friendly[1-3]. Nevertheless, the practical development of Li-S batteries still faces several fatal obstacles: (i) the insulator nature of sulfur and its discharge product Li2S2/Li2S leads to low utilization of active materials; (ii) soluble polysulfides (PSs) intermediates dissolved in the electrolyte and shuttled back and forth between the cathode and anode sides (the shuttle effect), which leads to the reduction of coulomb efficiency and the rapid decline of capacity; and (iii) the drastic volume change of sulfur cathode during cycling[4-6].
Considerable efforts have been made to conquer the above technical challenges, including designing sulfur host of rational structures with various functional materials[7-10], modifying separator functionalization[11-14], and optimizing electrolytes[15-17]. Among them, separator functionalization exhibits unique advantages due to its traits of facile preparation and direct PSs intercept; meanwhile, the multifunctional coating of separator severs as upper current collectors extend the path for ions migration and ensure fast ion transmission[18]. Up to now, a variety of functional materials have been introduced to modify separators for the enhancement of the electrochemical performance of Li-S batteries. The nanoscale nonpolar carbon material, such as porous carbon spheres, carbon nanotubes (CNTs)[19,20], carbon nanofiber (CNF)[21], graphene oxide (GO)[22] and metal-organic frameworks (MOFs) derived carbon[23,24], were widely studied as the functional coating to ease the shuttle effect of PSs due to high conductively and specific surface area via physical adsorption[25]. For example, Manthiram’s group applied multi-walled CNT to spread on one side of the conventional polypropylene (PP) separator by using a facile vacuum filtration method, the high specific surface area brought by MWCNT provides many adsorption sites for PSs; thus, the Li-S batteries with the modified separator retain 621 mAh g-1 after 300 cycles[26]. Zhou et al. prepared a GO-coated separator and used it in Li-S battery under a high sulfur loading of 4 mg cm-2, and the cells achieved a high initial capacity of 1006 mAh g-1 at 0.9 C with an attenuation rate of 0.1% per cycle after 300 cycles[27].
Unfortunately, the practical application of Li-S batteries demands high content of sulfur load into the cathode, and thus the nonpolar carbon material, which only possesses weak chemical interaction between PSs and the poor ability of electrocatalysis, cannot further inhibit the dissolution of PSs and enhance redox kinetics during discharge/charge process, resulting in disappointing long-term cycle stability[28,29]. Therefore, polar materials, which both possess the dual-function of robust chemical adsorption between sulfur species and enhancement of electrocatalysis kinetics for the redox process, have attracted extensive attention. For instance, metal oxides (Fe3O4, MnO2, Co3O4)[30-33], transition metal phosphide (FeP, CoP)[34,35], metal carbides (Fe3C, Ti3C2)[36,37], and metal selenide (ZnSe, FeSe2)[38,39] combined with a series of unique designed carbon construction have been deeply investigated. In particular, some specific amorphous metal compounds have been demonstrated to possess stronger catalytic ability than their crystalline state. Recent research has revealed that the unsaturated electronic configuration of amorphous metal compounds may improve the electronic conductivity, and the rich defect sites and dangling bonds could strengthen the chemical interaction with PSs, therefore effectively inhibiting the shuttle effect[40]. For example, Li et al. successfully prepared amorphous cobalt oxide nanosheets as cathode additives for Li-S battery, and the DFT calculations suggested the d orbital redistribution of amorphization CoO could further strengthen chemical bonding energy of PSs, and thus exhibited satisfactory rate performance and cycle stability[41]. Therefore, the amorphous metal compounds possess great potential in enhancing the chemical polar adsorption and redox rate of PSs in Li-S kinetics.
Herein, we report a ZIF-67 derived amorphous CoP combined with carbon nanotubes conductive network composites (aCoP@CNTs) as a functional electrocatalytic interlayer to restrict the shuttle effect and enhance the redox kinetics in Li-S battery. The embedded 3D network can not only effectively promote the transmission of electrons and reduce the impedance of the interface, but also serve as an ion-sieving layer and physical barrier to prevent the diffusion of PSs. In addition, the amorphous CoP uniformly dispersed on the carbon matrix could further fix PSs on the cathode side via robust Co-S chemical bonding interaction[42,43]. More importantly, results demonstrated that the amorphous CoP contributes to superior electrocatalysis kinetics redox for sulfur confinement compared with crystalline CoP. Therefore, benefitting from the synergistic effect of physical/chemical adsorption toward PSs and electrocatalysis function, the Li-S battery with electrocatalytic aCoP@CNTs separator demonstrates robust electrochemical performance with a good initial discharge capacity of 1014.4 mAh g-1 at 1 C, and excellent long-term cycle stability at high S loading at 1 C.
EXPERIMENTAL
Synthesis of ZIF-67@CNTs composites
50 mg Polyvinylpyrrolidone (PVP) and 30 mg CNTs treated with nitric acid were dispersed ultrasonically in 20 mL methanol for 1 h to form a uniform suspension. CoH12N2O12 (0.582 g, Aladdin, AR, 99%) was added to the above suspension and stirred for 1 h. Then 10 mL methanol containing 0.648 g of 2-methylimidazole (Aladdin, AR, 99%) was quickly poured into the above suspension. The solution was stirred for 20 min and aged 24 h. Finally, the ZIF-67@CNTs composites were collected via centrifugation, washed with ethanol four times, and vacuum-dried at 80 °C for a night.
Synthesis of aCoP@CNTs and cCoP@CNTs composites
aCoP@CNTs sample was obtained by an in-situ phosphating method. Briefly, the as-synthesized
Synthesis of aCoP@CNTs, cCoP@CNTs and KB modified separators
To fabricate the aCoP@CNTs/cCoP@CNTs modified separators, 50 wt% of the aCoP@CNTs/C composite was mixed with 40 wt% of Ketjen Black (KB) and 10 wt% of polyvinylidene difluoride (PVDF) in
Preparation of the KB/S cathode
The KB/S cathode was prepared via a traditional melt-diffusion method. A uniform mixture of sublimed sulfur and KB with a mass ratio of 7:3 was put into an autoclave and maintained at 155 °C for 12 h to obtain KB/S composites. The KB/S cathode was uniformly mixed with KB and PVDF in NMP and smooth coated on the aluminum foil. Finally, dried in vacuum at 60 °C for 10 h. The high-load sulfur electrode is prepared with the same KB/S composite material as above, and the cathodes under different S loading are obtained by adjusting the thickness of the scraper.
Preparation of Li2S6 and Li2S8 solution
Li2S6 electrolyte was synthesized by reacting lithium sulfide and sulfur powders in 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) (VDOL:VDME = 1:1) with a moral mass ratio of 5:1 under vigorous stirring for 48 h in the glovebox.
Li2S8 electrolyte was synthesized by magnetically stirring lithium sulfide and sulfur powders with a moral mass ratio of 7:1 in tetraglyme for 48 h in the glovebox.
Catalytic performance tests
aCoP@CNTs, cCoP@CNTs, and KB were mixed with PVDF in NMP to form a uniform slurry with a mass ratio of 4:1, then the slurry was coated on carbon-coated aluminum foil and dried under vacuum at 60 °C for 12 h. the electrodes without sulfur loading were cut into circular pieces of φ12 mm.
Li2S6-symmetric cells CV test: The symmetric cells were fabricated with the above identical electrode under an Ar atmosphere using 0.2 M Li2S6 electrolyte in the glovebox. The CV measurements voltage range was from -1.4 to 1.4 V with a scan rate of 0.01V s-1.
Nucleation and dissolution of Li2S test: The asymmetrical cells were assembled by the above electrode as a cathode, lithium foil as an anode, 20 μL Li2S8 electrolyte as catholyte, and 20 μL 1.0 M LiTFSI and 2.0 wt% LiNO3 dissolved in DME and DOL (VDOL:VDME = 1:1) solution as anolyte. For the nucleation test, the batteries were first galvanostatic discharged to 2.06 V with a current value of 0.112 mA, and then were potentiostatic discharged until the current value was close to 0.01 mA at 2.05 V. For the dissolution test, the batteries were first galvanostatic discharged to 1.8 V at 0.1 mA, and then were further potentiostatic discharged to 1.8 V at 0.01 mA to ensure Li2S is fully formed, finally, the batteries were potentiostatic charged until the current value is close to 0.01 mA at 2.4 V.
Cells assembly and electrochemical testing
Typically, Li-S coin cells (CR2032) were assembled with lithium foil as an anode, KB/S compound as cathode, and 1.0 M LiTFSI and 1.0 wt% LiNO3 dissolved in DME and DOL (VDOL:VDME = 1:1) as the electrolyte in O2 and H2O < 0.1 ppm circumstances. The diameter and the sulfur loading of the KB/S cathode are 12 mm and 0.8~1 mg cm-2. The amount of electrolyte is ~15 μL. The performance of Li-S battery at high sulfur loading of 3.1 and 4.06 mg cm-2 with electrolyte/ sulfur (E/S) ratio of ~5.0 µL mg-1 was also evaluated. The modified layer of the separator faces the cathode side. The different scan rates of cyclic voltammetry (CV, voltage windows of 1.7-2.8 V) and electrochemical impedance spectroscopy (EIS, frequency range from 0.01 Hz to 100 kHz, AC voltage amplitude of 5 mV.) dates were gauged on a CHI760E electrochemical workstation. The galvanostatic charge/discharge and cycle performance dates were collected on Land CT2001A battery test system with a voltage range of 1.7-2.8 V.
Materials characterization
The phase information of materials was analyzed by XRD (Cu Ka radiation with k = 1.5405 Å) in the angle range of 5° to 80°. The structure and morphologies of the materials were analyzed by SEM (Nova Nano SEM450, 15k eV) and TEM (JEOL JEM 2100F). The specific surface area and pore size of the samples were measured by N2 adsorption/desorption isotherms (77 K, Tristar 3000). XPS was performed on ESCALAB 250 to study the chemical valence state of the samples. The elemental distribution of the materials was analyzed by HRTEM and attached EDS equipment (Oxford Instruments and EDAX).
RESULTS AND DISCUSSION
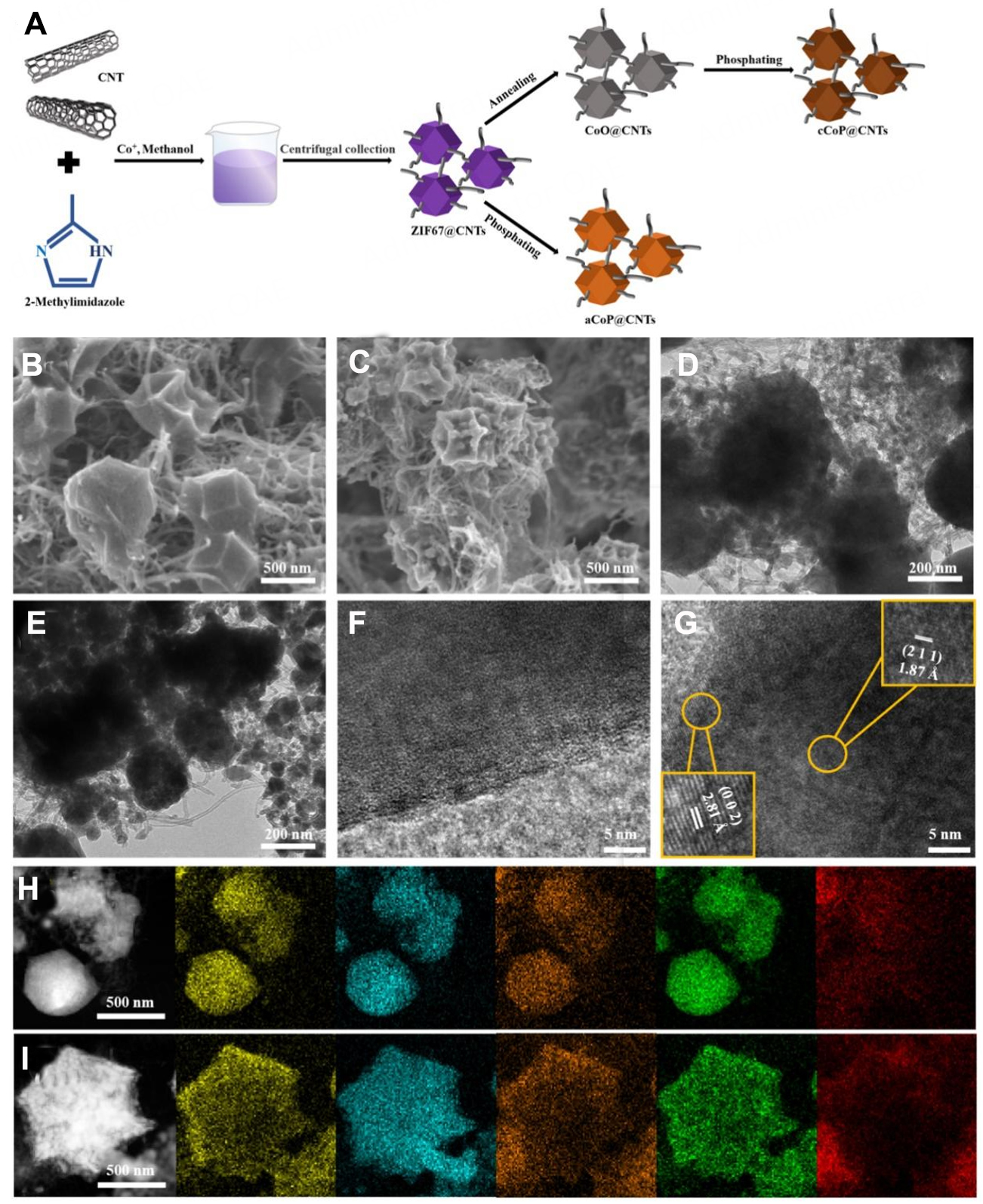
The ZIF-67@CNTs precursor was prepared by a modified precipitation method. As shown in Figure 1A, ZIF-67@CNTs precursor was first synthesized through the reaction of 2-methylimidazole in methanol and the mix suspension of Co+ and CNTs, and then the aCoP@CNTs was obtained through directly phosphating ZIF-67@CNTs precursor. While the cCoP@CNTs were synthesized via annealing to perform CoO@CNTs and then converted to cCoP@CNTs via phosphorization. SEM and TEM were used to deeply investigate the microstructure of obtained electrocatalyst. Supplementary Figure 1 shows morphologies of obtained ZIF-67@CNTs precursor, the CNT was well-fdistributed grown on the typical ZIF-67 crystal smooth polyhedron. After phosphating at 350 °C, no obvious change in the morphology [Figure 1B, Supplementary Figure 2], aCoP@CNTs maintains the classic structure of polyhedron, the amorphous CoP was attached to the ZIF-67 derived carbon skeleton with the size of 300~400 nm; meanwhile, the uniform distribution of CNTs would effectively ensure the conductivity of the electrocatalyst. SEM image of cCoP@CNTs in Figure 1C exhibits the similar construction to aCoP@CNTs. Further, the TEM image
Figure 1. (A) Diagrammatic sketch of synthesis route of aCoP@CNTs and cCoP@CNTs; SEM images of (B) aCoP@CNTs and (C) cCoP@CNTs; TEM images of (D) aCoP@CNTs and (E) cCoP@CNTs; HRTEM images of (F) aCoP@CNTs and (G) cCoP@CNTs; HAADF-STEM image and EDX mappings of (H) aCoP@CNTs and (I) cCoP@CNTs.
The XRD patterns of pure ZIF-67 and ZIF-67@CNTs in Supplementary Figure 3 show similar characteristic peaks of ZIF-67. Figure 2A shows XRD patterns of aCoP@CNTs and cCoP@CNTs samples after phosphidation. The intense diffraction peaks of the two samples at ~26° correspond to the characteristic peak of carbon. The characteristic peaks of cCoP@CNTs are well indexed to orthorhombic CoP
Figure 2. (A) XRD patterns of aCoP@CNTs and cCoP@CNTs; (B) full-scan XPS spectra of aCoP@CNTs; (C) Co 2p HRXPS spectra of aCoP@CNTs and cCoP@CNTs, HRXPS spectra of aCoP@CNTs: (D) P 2p; (E) N 1s; (F) C 1s.
To further verify the superiority of aCoP@CNTs modified separator in ion migration behavior, the impedance of stainless steel (SS)|modified separator|SS batteries at different temperatures were tested and obtained the corresponding ionic conductivity, as shown in Supplementary Figure 6 and Figure 3A. Specifically, the aCoP@CNTs exhibit the highest ionic conductivity of 0.999, 1.052 and 1.129 cm-1 at 30, 40 and 50 °C. Besides, the activation energy corresponding to the above battery is calculated by Arrhenius equation [Figure 3B][53]. The calculated activation energies of aCoP@CNTs, cCoP@CNTs, KB, and PP are 0.0316, 0.0392, 0.0534, and 0.0911 eV, respectively, which suggest that the introduction of amorphous CoP active sites on interconnect CNTs effectively improves the mobility of ions, benefiting the fast Li+ transmission.
Figure 3. (A) Ion conductivity of different modified separators; (B) activation energy of different materials derived from ionic conductivity; (C) CV curves of the Li2S6-symmetric cells with as-fabricated functional interlayer materials; the fitting curves of Li2S deposition on the surface of aCoP@CNTs (D), aCoP@CNTs (E) and KB (F); (G) CV curves with different scan rates of the Li-S batteries with aCoP@CNTs; (H) slope value of fitting linear curves of the peak currents versus square roots of scan rate; (I) contact angle of aCoP@CNTs and PP separators.
The Li2S6-symmetric cells were assembled to evaluate the catalytic potentiality of amorphous CoP particles for soluble PSs intermediates. Specifically, the interlayer materials serve as the identical working electrode and counter electrode, and 10 μL 1M Li2S6 solution act as electrolyte. CV profiles were acquired over the voltage range from -1.4 V to 1.4 V at a scan rate of 10 mV s-1. Figure 3C shows that the aCoP@CNTs symmetric cells exhibit the highest current density and the smallest voltage difference between symmetrical oxidation peaks and reduction peaks, implying the increase of catalytic activity for PSs conversion compared to the cCoP@CNTs electrode. The potentiostatic discharge testing of Li2S8 electrolytes on different interlayer materials further proves the superiority of the aCoP@CNTs in catalyzing Li2S nucleation and growth. Specifically, the aCoP@CNTs [Figure 3D] possess the highest current density, and shortest nucleation time of Li2S compared with cCoP@CNTs [Figure 3E] and KB [Figure 3F], the Li2S nucleation capacity of aCoP@CNTs (171.7 mAh g-1) was also much higher than that of cCoP@CNTs (130.7 mAh g-1) and KB (63.3 mAh g-1). Relatively, the dissolution of the Li2S test further confirmed the efficient selective catalysis of the amorphous CoP[54]. As shown in Supplementary Figure 7, contrasted with cCoP@CNTs and KB, aCoP@CNTs achieved a shorter dissolution time (2871 s) and a higher current peak, thus delivering a high dissolution capacity of Li2S (420.4 mAh g-1).
The Li+ ion diffusion behaviors of different materials were evaluated by deriving from the CV curves at scanning rates of 0.1 mV s-1 to 0.6 mV s-1. The intensity of redox peaks gradually becomes stronger with the increase in scan rates, and the potential difference between cathode peaks and anode peak decrease by degrees [Figure 3G, Supplementary Figures 8 and 9]. The fitting results of three peaks via Randles-Sevcik Equation: (1) Ip = 2.65 × 105n3/2aDLi+1/2v1/2CLi+ and shown in Figure 3H[55,56]. The slope of aCoP@CNTs was higher than aCoP@CNTs and KB in different peaks, confirming the better diffusion kinetics of aCoP@CNTs[57]. Galvanostatic cycling performance of Li symmetric batteries with aCoP@CNTs and PP was assembled to evaluate the interfacial stability and plating/stripping effect of the aCoP@CNTs on regulating the Li deposition of the anode. As shown in Supplementary Figure 10, the polarization for the cCoP@CNTs separator exhibit a sharp increase after the continuous Li plating/stripping process after 170 h owing to the growth of Li dendrites and serious side effects. Contrarily, the aCoP@CNTs separator maintained a flat plateau with a low overpotential of 25.9 mV even after 300 h, which proves a positive effect of the aCoP@CNTs on the regulation of Li deposition[58].
The wettability of aCoP@CNTs and PP separators to the electrolyte was characterized by a contact angle test. As exhibited in Figure 3I, after dropping the same volume of electrolyte on the surface of the separator, a large contact angle of 48.7° is observed on the surface of PP. Impressively, the contact angle of aCoP@CNTs decreased to 10.9°, much smaller than that of PP separator, indicating that the electrolyte can rapidly infiltrate the aCoP@CNTs coating.
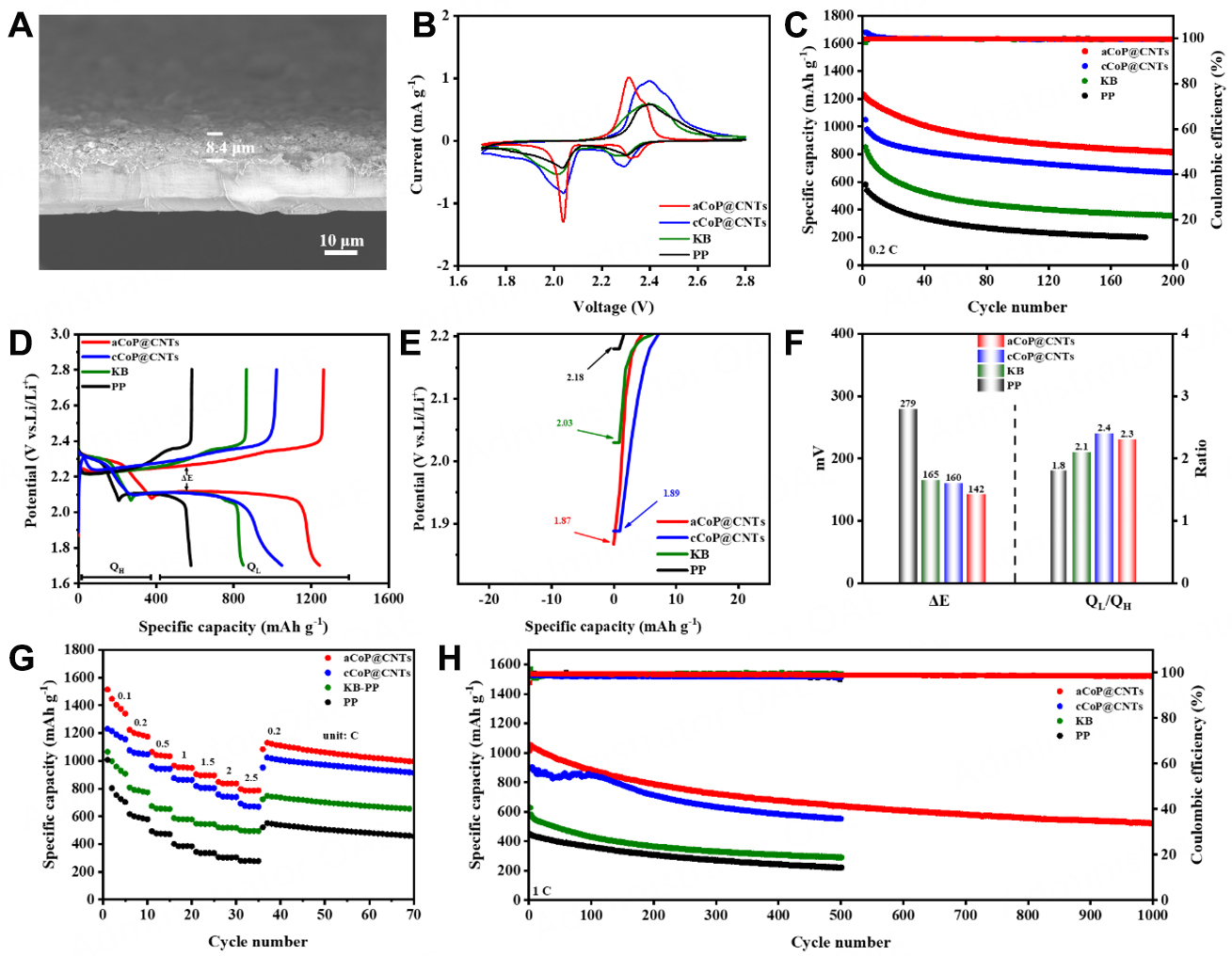
The cross-sectional image [Figure 4A] shows that the coating thickness of aCoP@CNTs modified interlayer is only about 8.4 µm and the average functional materials loading amount of only 0.17 mg cm-2 in each separator. The electrochemical performance corresponding to the above analysis of catalytic capacity was also characterized. Figure 4B shows the CV curves of the Li-S cells with three kinds of separators. All cells exhibit typical one anode peak and two cathode peaks, and the two reduction peaks located at ~2.30 V and ~2.06 V correspond to two reduction steps of S8 to Li2Sx (8 ≥ x ≥ 4) and Li2S4 to Li2S2/Li2S, respectively. The oxidation peak at ~2.40 V represents the conversion to elemental sulfur. In addition, the batteries with aCoP@CNTs show stronger and shaper current corresponding peaks than other cells, further indicating better redox reaction kinetics of PSs. Meanwhile, as shown in Supplementary Figure 11, the well-overlapped CV curves, except for the first cycle of aCoP@CNTs, proved good cycling reversibility. The cycle performance at 0.2 C in Figure 4C shows that the batteries with aCoP@CNTs modified separator delivered the highest initial discharge specific capacity of 1227.9 mAh g-1. After 200 cycles, the batteries remain a high reversible capacity of 814 mAh g-1, much higher than that of cCoP@CNTs and KB separators (665.5 and 357.3 mAh g-1, respectively). The first charge/discharge profiles of all cells at 0.2 C in Figure 4D exhibit two typical discharge plateaus, corresponding to the CV analysis. The initial potentials of the first charge profile were obtained in Figure 4E and could be regarded as the activation energy barrier of Li2S. The lowest potential (1.87 V) of aCoP@CNTs means the fast conversion of Li2S to PSs, demonstrating the outstanding electrocatalytic performance of amorphous CoP. Meanwhile, as shown in Figure 4D, the aCoP@CNTs batteries show the minimum value (142 mV) of a voltage difference (ΔE) compared to cCoP@CNTs
Figure 4. (A) Cross-sectional SEM image of the aCoP@CNTs modified separator; (B) CV curves of the cells with different separators under scan rate of 0.1 mV s-1; (C) cycling performance of the Li-S cells with different separators at 0.2 C; (D) discharge/charge profiles of cells with different separators and the corresponding discharge initial potential (E); (F) the histogram of polarization potential and
Beneficial from the synergistic effect of chemical limitations and excellent electrocatalyst capacity for PSs, the batteries with aCoP@CNTs also delivered satisfying rate performance with 1513.5, 1223.4, 1063.8, 965.5, 903.5, 848.8, 795.9 mAh g-1 at 0.1, 0.2, 0.5,1, 1.5, 2 and 2.5 C, respectively [Figure 4G]. Profiting from the robust chemical interaction with PSs and amorphous CoP, the shuttle effect has been greatly alleviated. When the current density was back to 0.2 C, the batteries still maintained a high reversible capacity of 1130.3 mAh g-1. However, the batteries with cCoP@CNTs, KB, and PP only remained 691.9, 498.1,
Simple visual PSs permeation experiments were used to intuitively observe the excellent adsorption capacity of aCoP@CNTs functional electrocatalyst for PSs, as shown in Figure 5A. The inner small bottle contains
Figure 5. (A) The visualized adsorption test of Li2S6; (B) UV-vis spectra after adsorption for 24 h; (C)long-term cycling performance of the Li-S cells with aCoP@CNTs modified separator under high sulfur loading; (D) top and side view of PSs molecule on aCoP surfaces; (E) adsorption energy between Li2Sx (x = 2,4 and 6) and aCoP@CNTs and cCoP @CNTs surface; (F) schematic diagram of the Li-S batteries with (right) and without (left) aCoP@CNTs modified separator.
The long cycle stability of Li-S cells under high sulfur loading was tested to evaluate the potential of the modified diaphragm in practical application, shown in Figure 5C. At the current density of 1 C, the batteries with aCoP@CNTs interlayer exhibit an excellent discharge specific capacity of 886 mAh g-1 and
Then, to further deepen the atomic-level insight into the adsorption behavior of crystalline and amorphous CoP for PSs. the adsorption properties of long-chain PSs (Li2Sx, x = 4, 6 and 8) on the (022) planes of crystalline and amorphous CoP were studied by DFT calculations. The most stable adsorption configurations of top and side views for long-chain PSs on crystalline and amorphous CoP surface are displayed in Figure 5D and Supplementary Figure 14. The specific calculation results are shown in Figure 5E. The aCoP@CNTs s (-1.91 eV for Li2S4, -2.17 eV for Li2S6, and -2.78 eV for Li2S8) render adsorption energy of more negative value for long-chain PSs than cCoP@CNTs (-1.28 eV for Li2S4, -1.31 eV for Li2S6 and -1.64 eV for Li2S8). Overall, the above DFT and experiment results demonstrated a strong interaction between PSs and amorphous CoP. Therefore, as shown in Figure 5F, the outstanding performance could be attributed to the merits of the robust physico-chemical adsorption and electrocatalyst capacity of amorphous CoP. Specifically, the cross-linked stacked CNTs act as an excellent conductive network and provide rich ion/electronic transmission; additionally, CNTs can also serve as physical barriers to restrict the shuttle effect. Furthermore, the amorphous CoP supported on CNTs network provides rich unsaturated dangling bonds and strong Co-S bond interaction for PSs, which promotes the conversion of PSs and further limits the shuttle effect.
CONCLUSIONS
In summary, we have successfully fabricated aCoP@CNTs and cCoP@CNTs electrocatalysts as functional separators for high-stability Li-S batteries and demonstrated that the unsaturated Co in amorphous CoP could rapidly adsorb PSs and promote the conversion reaction of soluble PSs to solid Li2S. Meanwhile, the in-situ introduction of CNTs could effectively improve the conductivity of the catalytic interface and remedy the low crystallinity of carbon after annealing at a lower temperature, ensuring the electrical conductivity, and enlarging the specific surface area of the separator. Therefore, the Li-S batteries with a functional aCoP@CNTs modified separator exhibit high initial discharge capacity, excellent rate performance, and good long-term stability under high sulfur loading. This work provides a novel perspective for the introduction of amorphous materials as the key components and highlights its unlimited possibilities for practical application in high-performance Li-S batteries.
DECLARATIONS
Authors’ contributionsConceived the study, designed the experiments and wrote the manuscript: Feng J, Song J
Carried out the synthesis and characterization: Feng J, Li J, Zhang H, Liu W, Lin Z, Wang T, Zhao X, Wang F
Gave advice to the research: Wang T, Sun B
Supervision, review and editing: Zhao X, Wang F, Song J
All authors discussed the results and commented on the manuscript.
Availability of data and materialsMore data on the results of our finding can be found in the Supplementary Material.
Financial support and sponsorshipSong J. would like to acknowledge the financially supported by the Natural Science Foundation of Shandong Province, China (ZR2021QE192), the Postdoctoral Science Foundation of China (2018M63074), Qingdao Postdoctoral Applied Research Project and Source Innovation Project of Qingdao (19-6-2-19-cg). Wang F. thanks the support of Shandong Provincial Natural Science Foundation (ZR2018JL021) and Key Research and Development Program of Shandong Province (2019GGX102067). Zhao X. thanks the support of the Natural Science Foundation of Hebei Province (B2019204009).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
1. Zhou G, Chen H, Cui Y. Formulating energy density for designing practical lithium-sulfur batteries. Nat Energy 2022;7:312-9.
2. Hu Y, Chen W, Lei T, et al. Strategies toward high-loading lithium-sulfur battery. Adv Energy Mater 2020;10:2000082.
3. Yang X, Li X, Adair K, et al. Structural design of lithium-sulfur batteries: from fundamental research to practical application. Electrochem Energy Rev 2018;1:239-93.
4. Li G, Wang S, Zhang Y, et al. Revisiting the role of polysulfides in lithium-sulfur batteries. Adv Mater 2018;30:e1705590.
5. Song J, Guo X, Zhang J, et al. Rational design of free-standing 3D porous MXene/rGO hybrid aerogels as polysulfide reservoirs for high-energy lithium-sulfur batteries. J Mater Chem A 2019;7:6507-13.
6. He J, Bhargav A, Manthiram A. Molybdenum boride as an efficient catalyst for polysulfide redox to enable high-energy-density lithium-sulfur batteries. Adv Mater 2020;32:e2004741.
7. Xie Y, Cao J, Wang X, Li W, et al. MOF-derived bifunctional Co0.85Se nanoparticles embedded in n-doped carbon nanosheet arrays as efficient sulfur hosts for lithium-sulfur batteries. Nano Lett 2021;21:8579-86.
8. Song C, Jin Q, Zhang W, et al. Prussian blue analogs derived Fe-Ni-P@nitrogen-doped carbon composites as sulfur host for high-performance lithium-sulfur batteries. J Colloid Interf Sci 2021;595:51-8.
9. Yu Y, Yan M, Dong W, et al. Optimizing inner voids in yolk-shell TiO2 nanostructure for high-performance and ultralong-life lithium-sulfur batteries. Chem Eng J 2021;417:129241.
10. Yu P, Feng L, Ma D, et al. Template-free self-caging nanochemistry for large-scale synthesis of sulfonated-graphene@sulfur nanocage for long-life lithium-sulfur batteries. Adv Funct Mater 2021;31:2008652.
11. Song X, Wang S, Chen G, et al. Fe-N-doped carbon nanofiber and graphene modified separator for lithium-sulfur batteries. Chem Eng J 2018;333:564-71.
12. Li C, Liu R, Xiao Y, et al. Recent progress of separators in lithium-sulfur batteries. Energy Storage Mater 2021;40:439-60.
13. Feng J, Li Y, Yuan J, et al. Energy-saving synthesis of functional CoS2/rGO interlayer with enhanced conversion kinetics for high-performance lithium-sulfur batteries. Front Chem 2022;9:830485.
14. Li Q, Liu Y, Yang L, et al. N, O co-doped chlorella-based biomass carbon modified separator for lithium-sulfur battery with high capacity and long cycle performance. J Colloid Interf Sci 2021;585:43-50.
15. Shin H, Baek M, Gupta A, et al. Recent progress in high donor electrolytes for lithium-sulfur batteries. Adv Energy Mater 2020;10:2001456.
16. Yang Q, Deng N, Chen J, et al. The recent research progress and prospect of gel polymer electrolytes in lithium-sulfur batteries. Chem Eng J 2021;413:127427.
17. Zhou Z, Sun T, Cui J, et al. A homogenous solid polymer electrolyte prepared by facile spray drying method is used for room-temperature solid lithium metal batteries. Nano Res 2021.
18. Tran M X, Enggar Anugrah Ardhi R, Liu G, et al. Plasma-polymerized C60-coated CNT interlayer with physical and chemical functions for lithium-sulfur batteries. Chem Eng J 2020;401:126075.
19. Zheng M, Chi Y, Hu Q, et al. Carbon nanotube-based materials for lithium-sulfur batteries. J Mater Chem A 2019;7:17204-41.
20. Huo J, Shen Z, Cao X, et al. Macro/micro-environment regulating carbon-supported single-atom catalysts for hydrogen/oxygen conversion reactions. Small 2022;18:2202394.
21. Li J, Zhang H, Luo L, et al. Blocking polysulfides with a Janus Fe3C/N-CNF@RGO electrode via physiochemical confinement and catalytic conversion for high-performance lithium-sulfur batteries. J Mater Chem A 2021;9:2205-13.
22. Jing F, Ma Z, Wang J, et al. Oxygen vacancy inducing phase transition during charge storage in MnOx@rGO supercapacitor electrode. Chem Eng J 2022;435:135103.
23. Fan L, Wu H, Wu X, et al. Fe-MOF derived jujube pit like Fe3O4/C composite as sulfur host for lithium-sulfur battery. Electrochim Acta 2019;295:444-51.
24. Cao X, Huo J, Li L, et al. Recent advances in engineered ru-based electrocatalysts for the hydrogen/oxygen conversion reactions. Adv Energy Mater 2022;12:2202119.
25. Liu H, Liu X, Li W, et al. Porous carbon composites for next generation rechargeable lithium batteries. Adv Energy Mater 2017;7:1700283.
26. Chung S, Manthiram A. High-performance Li-S batteries with an ultra-lightweight MWCNT-coated separator. J Phys Chem Lett 2014;5:1978-83.
27. Zhou G, Li L, Wang D, et al. A flexible sulfur-graphene-polypropylene separator integrated electrode for advanced Li-S batteries. Adv Mater 2015;27:641-7.
28. Chen Y, Wang T, Tian H, et al. Advances in lithium-sulfur batteries: from academic research to commercial viability. Adv Mater 2021;33:2003666.
29. Ma L, Yu L, Liu J, et al. Construction of Ti4O7/TiN/carbon microdisk sulfur host with strong polar N-Ti-O bond for ultralong life lithium-sulfur battery. Energy Storage Mater 2022;44:180-9.
30. Lu K, Zhang H, Gao S, et al. Manipulating polysulfides conversion with strongly coupled Fe3O4 and nitrogen doped carbon for stable and high capacity lithium-sulfur batteries. Adv Funct Mater 2019;29:1807309.
31. Li Q, Ma Z, Zhao J, et al. A flexible self-supporting ultralong MnO2 nanowires-expanded graphite nanosheets current collector with enhanced catalytic reaction kinetics for high-loading lithium-sulfur batteries. J Power Sources 2022;521:230929.
32. Wang S, Hou X, Zhong Z, et al. Co3O4-NP embedded mesoporous carbon rod with enhanced electrocatalytic conversion in lithium-sulfur battery. Sci Rep 2018;8:16133.
33. Xu K, Liang X, Wang L, et al. Tri-functionalized polypropylene separator by rGO/MoO2 composite for high-performance lithium-sulfur batteries. Rare Metals 2021;40:2810-8.
34. Xia G, Ye J, Zheng Z, et al. Catalytic FeP decorated carbon black as a multifunctional conducting additive for high-performance lithium-sulfur batteries. Carbon 2021;172:96-105.
35. Zhang H, Xin S, Li J, et al. Synergistic regulation of polysulfides immobilization and conversion by MOF-derived CoP-HNC nanocages for high-performance lithium-sulfur batteries. Nano Energy 2021;85:106011.
36. Zhang M, Guo Y, Wei Y, et al. Integrating conductivity and active sites: Fe/Fe3C@GNC as an trapping-catalyst interlayer and dendrite-free lithium host for the lithium-sulfur cell with outstanding rate performance. J Mater Chem A 2020;8:8987-19000.
37. Zhou H, Sui Z, Amin K, et al. Investigating the electrocatalysis of a Ti3C2/carbon hybrid in polysulfide conversion of lithium-sulfur batteries. ACS Appl Mater Interfaces 2020;12:13904-13.
38. Sun W, Li Y, Liu S, et al. Mechanism investigation of iron selenide as polysulfide mediator for long-life lithium-sulfur batteries. Chem Eng J 2021;416:129166.
39. Li X, Su J, Li Z, et al. Revealing interfacial space charge storage of Li+/Na+/K+ by operando magnetometry. Sci Bull 2022;67:1145-53.
40. Liu S, Li Y, Zhang C, et al. Amorphous TiO2 nanofilm interface coating on mesoporous carbon as efficient sulfur host for Lithium-Sulfur batteries. Electrochim Acta 2020;332:135458.
41. Li R, Rao D, Zhou J, et al. Amorphization-induced surface electronic states modulation of cobaltous oxide nanosheets for lithium-sulfur batteries. Nature Commun 2021;12:3102.
42. Chen X, Ding X, Wang C, et al. A multi-shelled CoP nanosphere modified separator for highly efficient Li-S batteries. Nanoscale 2018;10:13694-701.
43. Li C, Xi Z, Guo D, et al. Chemical immobilization effect on lithium polysulfides for lithium-sulfur batteries. Small 2018;14:1701986.
44. Jin R, Zhai Q, Wang Q, et al. Amorphous transition metal sulfides anchored on amorphous carbon-coated multiwalled carbon nanotubes for enhanced lithium-ion storage. Chem Eur J 2017;23:14056-63.
45. Liu L, Li Y, Zhang Y, et al. CoP@C with chemisorption-catalysis effect toward lithium polysulfides as multifunctional interlayer for high-performance lithium-sulfur batteries. Electrochim Acta 2022;419:140391.
46. Qin B, Cai Y, Wang P, et al. Crystalline molybdenum carbide-amorphous molybdenum oxide heterostructures: in situ surface reconfiguration and electronic states modulation for Li-S batteries. Energy Storage Mater 2022;47:345-53.
47. Ye Z, Jiang Y, Qian J, et al. Exceptional adsorption and catalysis effects of hollow polyhedra/carbon nanotube confined CoP nanoparticles superstructures for enhanced lithium-sulfur batteries. Nano Energy 2019;64:103965.
48. Zhang D, Luo Y, Wu B, et al. A heterogeneous FeP-CoP electrocatalyst for expediting sulfur redox in high-specific-energy lithium-sulfur batteries. Electrochim Acta 2021;397:139275.
49. Xu Y, Mo Y, Tian J, et al. The synergistic effect of graphitic N and pyrrolic N for the enhanced photocatalytic performance of nitrogen-doped graphene/TiO2 nanocomposites. Appl Catal B Environ 2016;181:810-7.
50. Nita C, Fullenwarth J, Monconduit L, et al. Eco-friendly synthesis of SiO2 nanoparticles confined in hard carbon: a promising material with unexpected mechanism for Li-ion batteries. Carbon 2019;143:598-609.
51. Zhou W, Zhao D, Wu Q, et al. Amorphous CoP nanoparticle composites with nitrogen-doped hollow carbon nanospheres for synergetic anchoring and catalytic conversion of polysulfides in Li-S batteries. J Colloid Interf Sci 2021;603:1-10.
52. Sun R, Bai Y, Luo M, et al. A enhancing polysulfide confinement and electrochemical kinetics by amorphous cobalt phosphide for highly efficient lithium-sulfur batteries. ACS Nano 2021;15:739-50.
53. Zhou Q, Yang X, Xiong X, et al. A solid electrolyte based on electrochemical active Li4Ti5O12 with PVDF for solid state lithium metal battery. Adv Energy Mater 2022;12:2201991.
54. He J, Bhargav A, Manthiram A. Covalent organic framework as an efficient protection layer for a stable lithium-metal anode. Angew Chem Int Ed 2022;61:e202116586.
55. Zhu Q, Xu H, Shen K, et al. Efficient polysulfides conversion on Mo2CTx MXene for high-performance lithium-sulfur batteries. Rare Metals 2021;41:311-8.
56. Li J, Song J, Luo L, et al. Synergy of MXene with Se infiltrated porous N-doped carbon nanofibers as janus electrodes for high-performance sodium/lithium-selenium batteries. Adv Energy Mater 2022;12:2200894.
57. Ruan J, Sun H, Song Y, et al. Constructing 1D/2D interwoven carbonous matrix to enable high-efficiency sulfur immobilization in
58. Castillo J, Qiao L, Santiago A, et al. Perspective of polymer-based solid-state Li-S batteries. Energy Mater 2022;2:200003.
59. Cai M, Zhang H, Zhang Y, et al. Boosting the potassium-ion storage performance enabled by engineering of hierarchical MoSSe nanosheets modified with carbon on porous carbon sphere. Sci Bull 2022;67:933-45.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Feng J, Li J, Zhang H, Liu W, Lin Z, Wang T, Sun B, Zhao X, Wang F, Song J. Accelerating redox kinetics by ZIF-67 derived amorphous cobalt phosphide electrocatalyst for high-performance lithium-sulfur batteries. Energy Mater 2023;3:300001. http://dx.doi.org/10.20517/energymater.2022.62
AMA Style
Feng J, Li J, Zhang H, Liu W, Lin Z, Wang T, Sun B, Zhao X, Wang F, Song J. Accelerating redox kinetics by ZIF-67 derived amorphous cobalt phosphide electrocatalyst for high-performance lithium-sulfur batteries. Energy Materials. 2023; 3(1): 300001. http://dx.doi.org/10.20517/energymater.2022.62
Chicago/Turabian Style
Feng, Junan, Jiayi Li, Hongwei Zhang, Wendong Liu, Zenghui Lin, Tianyi Wang, Bing Sun, Xiaoxian Zhao, Fengyun Wang, Jianjun Song. 2023. "Accelerating redox kinetics by ZIF-67 derived amorphous cobalt phosphide electrocatalyst for high-performance lithium-sulfur batteries" Energy Materials. 3, no.1: 300001. http://dx.doi.org/10.20517/energymater.2022.62
ACS Style
Feng, J.; Li J.; Zhang H.; Liu W.; Lin Z.; Wang T.; Sun B.; Zhao X.; Wang F.; Song J. Accelerating redox kinetics by ZIF-67 derived amorphous cobalt phosphide electrocatalyst for high-performance lithium-sulfur batteries. Energy Mater. 2023, 3, 300001. http://dx.doi.org/10.20517/energymater.2022.62
About This Article
Copyright
Data & Comments
Data
 Cite This Article 19 clicks
Cite This Article 19 clicks

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.