Design of manganese dioxide for supercapacitors and zinc-ion batteries: similarities and differences
Abstract
Energy storage devices, e.g., supercapacitors (SCs) and zinc-ion batteries (ZIBs), based on aqueous electrolytes, have the advantages of rapid ion diffusion, environmental benignness, high safety and low cost. Generally, SCs provide excellent power density with the capability of fast charge/discharge, while ZIBs offer high energy density by storing more charge per unit weight/volume. Although the charge storage mechanisms are considered different, manganese dioxide (MnO2) has proven to be an appropriate electrode material for both SCs and ZIBs because of its unique characteristics, including polymorphic forms, tunable structures and designable morphologies. Herein, the design of MnO2-based materials for SCs and ZIBs is comprehensively reviewed. In particular, we compare the similarities and differences in utilizing MnO2-based materials as active materials for SCs and ZIBs by highlighting their corresponding charge storage mechanisms. We then introduce a few commonly adopted strategies for tuning the physicochemical properties of MnO2 and their specific merits. Finally, we discuss the future perspectives of MnO2 for SC and ZIB applications regarding the investigation of charge storage mechanisms, materials design and the enhancement of electrochemical performance.
Keywords
INTRODUCTION
In recent decades, electrochemical energy storage devices have been widely applied as power systems for a variety of applications ranging from portable electronics and electric vehicles to smart electric grids[1-5]. Due to their advantages of high energy density and long cycling lifespan without a memory effect, lithium-ion batteries (LIBs) are the most successful products, with the 2019 Nobel Prize in Chemistry awarded to John B. Goodenough, M. Stanley Whittingham and Akira Yoshino[6-11]. However, safety issues, such as fires and explosions, are always major concerns regarding the utilization of LIBs since their lithium salt-containing organic electrolytes are highly flammable[12-15]. To address this issue, one viable strategy is to develop aqueous-based devices, which are considered as promising alternatives due to their high safety and low cost[16-22].
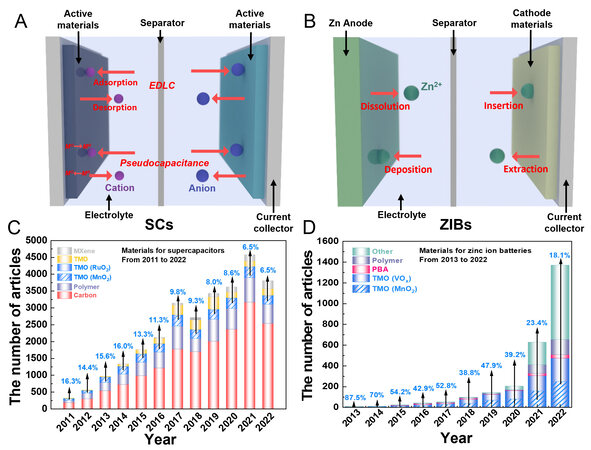
Presently, two types of aqueous-based devices, namely, supercapacitors (SCs) and zinc-ion batteries (ZIBs), as schematically illustrated in Figure 1A and B, have attracted tremendous attention owing to their unique features[20,23-29]. Typically, SCs exhibit ultralong cyclic stability, fast charge/discharge rates and high power density, thus explaining their broad applications in urban public transportation, aerospace, the military, and so on[30-33]. The charges are stored in SC materials based on two mechanisms: (1) adsorption/desorption of electrolyte ions on the electrode surface, excluding any redox reactions (electrochemical double-layer capacitance, EDLC); and (2) fast and reversible redox reactions on the electrode materials (pseudocapacitance). In contrast, ZIBs are considered promising candidates for grid-scale safe energy storage due to their low redox potential (-0.76 V vs. NHE), high theoretical capacity (820 mAh g-1) due to the metal Zn anode, intrinsic nonflammability, non-toxicity and high ionic conductivity of the aqueous electrolytes and low cost[34-38].
Figure 1. Schematic illustrations of (A) SC and (B) ZIB devices. (C) publication numbers of different materials, including MXenes, TMDs, carbon, polymers and TMOs (MnO2 and RuO2), for SCs in the period from 2011 to 2022. (D) publication numbers of different cathode materials, such as TMOs (VOx and MnO2), PBAs, polymers and other materials (including carbonaceous materials, TMDs and MXenes), for ZIBs in the period from 2013 to 2022. All data were obtained from the Web of Science. EDLC: electrochemical double-layer capacitance; SCs: supercapacitors; ZIBs: zinc-ion batteries.
In contrast to SCs, electrolyte ions (Zn2+) are inserted and extracted from the electrode materials, usually accompanied with phase transitions. Undoubtedly, the performance of both devices severely relies on their electrode materials[39-41]. Based on the statistics summarized in Figure 1C and D, it is very interesting to find that the commonly used electrode materials for SCs, including nanocarbon, conducting polymers, transition metal oxides (RuO2 and MnO2), transition metal dichalcogenides (TMDs) and transition metal carbides/nitrides (MXenes), are also suitable for ZIBs. Among these materials, the most studied one is manganese dioxide (MnO2) because of its natural abundance, non-toxicity, wide potential window and high theoretical capacitance/capacity[42-47]. For example, α-MnO2 nanoneedles synthesized by a
CRYSTAL STRUCTURE
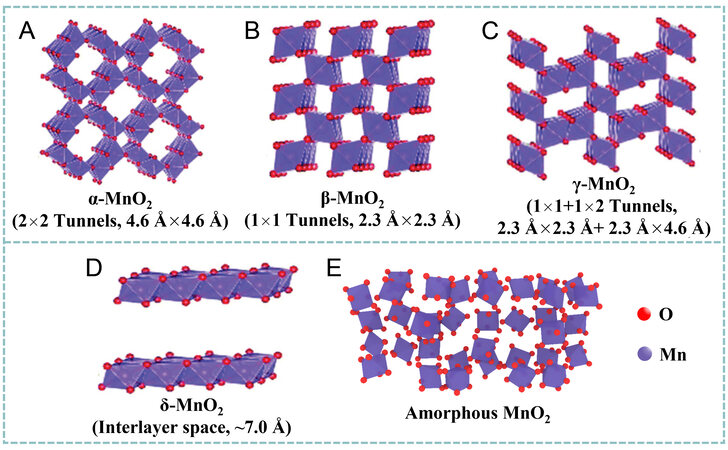
The commonly utilized MnO2 polymorphs in SCs and ZIBs can be classified into α-MnO2, β-MnO2,
Figure 2. Crystal structures of (A) α-, (B) β-, (C) γ-, (D) δ- and (E) amorphous MnO2. Reproduced with permission[26]. Copyright 2019, Royal Society of Chemistry.
SUPERCAPACITORS
Charge storage mechanisms
MnO2 is considered a pseudocapacitive material[57]. Traditionally, it is believed that the capacitance of MnO2 originates from surface redox reactions with a theoretical value of ~110 μF cm-2 based on a
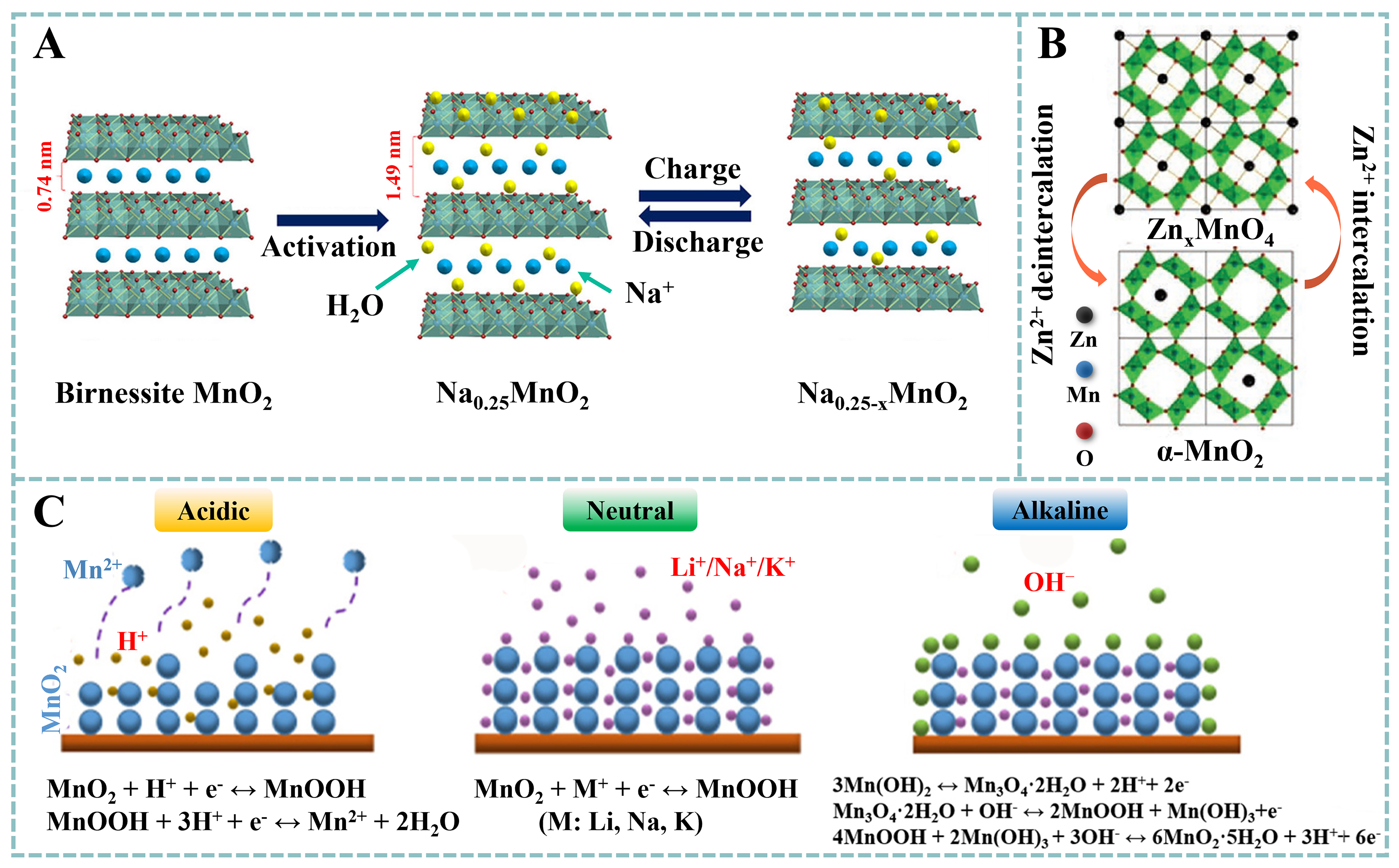
Figure 3. (A) Intercalation/deintercalation of Na+ using NaClO4 as the electrolyte. Reproduced with permission[60]. Copyright 2018, Elsevier. (B) charge storage mechanism of α-MnO2-based SC using ZnSO4 and MnSO4 as the electrolyte. Reproduced with permission[61]. Copyright 2020, Wiley-VCH. (C) redox reactions of MnO2 in acidic, neutral and alkaline electrolytes, respectively. Reproduced with permission[63]. Copyright 2019, Elsevier.
ZnxMnO2 - 2(x-y)e- ↔ ZnyMnO2 + (x-y)Zn2+ (1)
The electrochemical behavior of MnO2 is found to be heavily dependent on the pH of the electrolyte
In a neutral electrolyte:
2Mn3+ ↔ Mn4+ + Mn2+(aq) (2)
In an acidic electrolyte:
MnO2 + H+ + e- → MnOOH (3)
MnOOH + 3H+ + e- → Mn2+(aq) + 2H2O (4)
In an alkaline electrolyte:
MnO2 + H2O + e- → MnOOH + OH- (5)
MnOOH + H2O + e- → Mn(OH)2 + OH- (6)
3Mn(OH)2 ↔ Mn3O4·2H2O + 2H+ + 2e- (7)
Mn3O4·2H2O + OH- ↔ 2MnOOH + Mn(OH)3 + e- (8)
4MnOOH + 2Mn(OH)3 + 6OH- ↔ 6MnO2·5H2O + 3H2O + 6e- (9)
Overall, two main charge storage mechanisms are involved for MnO2 when adopted in aqueous SCs: (i) surface redox reactions Equation (10); and (ii) intercalation/deintercalation of cations Equation (11):
(MnO2)surface + M+ + e- ↔ (MnOOM)surface (10)
(MnO2)bulk + M+ + e- ↔ (MnOOM)bulk (11)
Performance enhancements
Nanostructure design
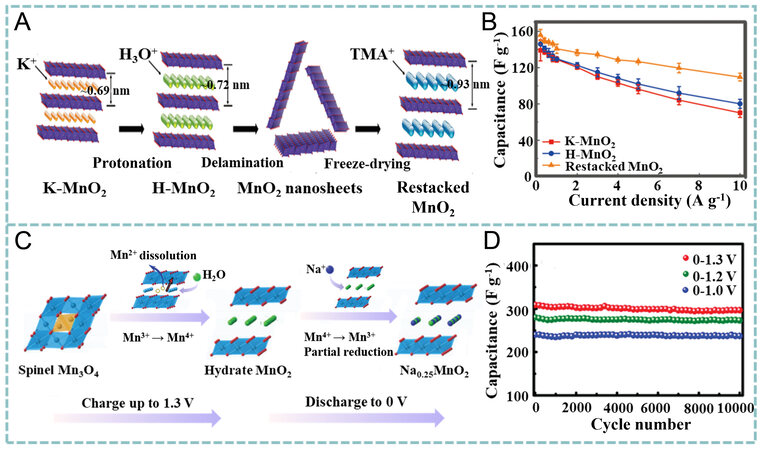
Enhancing the ion diffusion kinetics via nanostructural design is considered a feasible method to improve the capacitance of MnO2[70-77]. Xiong et al. prepared interlayer expanded MnO2 (0.93 nm) with intercalated tetramethylammonium ions (TMA+), following a two-step cation exchange [Figure 4A][78]. Density functional theory (DFT) calculations suggested that the expanded interlayer can weaken the interactions between the negatively charged MnO2 and K+ due to the decreased diffusion energy barrier, thereby accelerating ion diffusion during charge/discharge. A specific capacitance of 160 F g-1 was obtained for the restacked MnO2 in aqueous K2SO4 electrolytes at 0.2 A g-1, while at 10 A g-1, 70% capacitance was retained (110 F g-1). In contrast, in the cases of K- and H-MnO2 nanobelts, only 50% and 55% capacitance retentions were obtained, respectively, as the current density increased from 0.2 to
Figure 4. (A) Synthetic process of restacked MnO2. (B) rate capability in K2SO4 electrolyte at current densities from 0.2 to 10 A g-1. Reproduced with permission[78]. Copyright 2017, American Chemical Society. (C) structural evolution of Mn3O4 during electrochemical oxidation. (D) cyclic performance of Na0.5MnO2 nanowall arrays (NWAs) in different potential windows. Reproduced with permission[84]. Copyright 2017, Wiley-VCH.
Foreign ion/molecular pre-insertion
Since the charge storage capacity of MnO2 can be enhanced by the Mn4+/Mn3+ redox pair with cation adsorption (or intercalation)[79-81], it is speculated that the pre-insertion of cations in MnO2 could improve the specific capacitance by enhancing ion diffusion[81-83]. Following this guidance, Jabeen et al. prepared birnessite Na0.5MnO2 arrays on a carbon cloth by the electrochemical conversion of Mn3O4 in 10 M Na2SO4 via cyclic scanning at 10 mV s-1 within the potential window of 0 and 1.3 V (vs. Ag/AgCl) for 500 cycles
Defect engineering
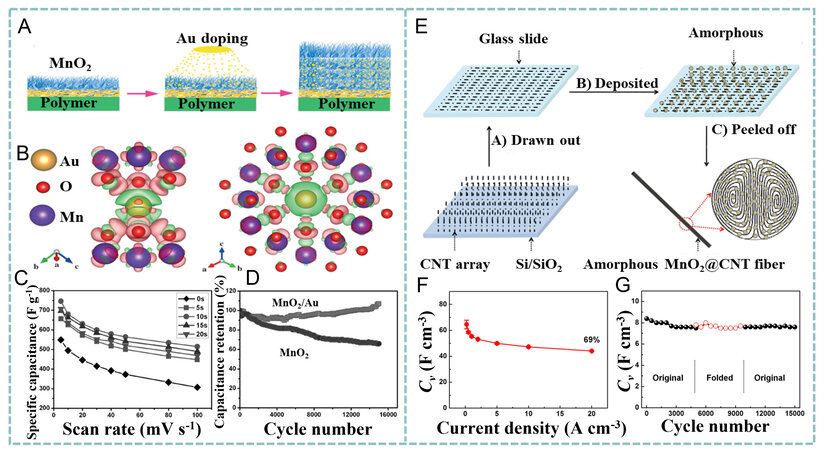
In addition to ion diffusion, the high electrical resistance of MnO2 needs to be lowered to achieve rapid electron transport during the redox reactions[85-87]. Defect engineering (heteroatom doping and the introduction of oxygen vacancies) has been demonstrated to be an effective strategy for improving the conductivity of MnO2[88-96]. Kang et al. designed a thick Au-doped MnO2 film (1.35 μm) by electrochemically depositing MnO2 and sputtering Au alternately to adjust the electronic structure of MnO2 [Figure 5A][97]. Au atoms distributed in the lattice of MnO2 with a total doping level of 9.9 at.% act as electron donors to induce a new state in the bandgap (~1.0 eV), thereby enhancing the overall conductivity, which is beneficial to the kinetics of the electrode reaction [Figure 5B]. As shown in Figure 5C, the specific capacitance of the
Figure 5. (A) Fabrication and (B) first-principle calculations of Au-doped MnO2. Differential charge densities of (left) Au-substituted and (right) Au-interstitial MnO2. Green indicates a loss of electrons and pink represents a gain of electrons. (C) specific capacitance of Au/MnO2 at different Au sputtering times. (D) cycling stability of pure MnO2 and Au-doped MnO2 electrodes with the same thickness of ~1.35 μm. Reproduced with permission[97]. Copyright 2013, Wiley-VCH. (E) fabrication of amorphous MnO2@MWCNT fibers. (F) CV of amorphous MnO2@MWCNT fiber electrode. (G) cycling and bending stability of fiber SC at 1 A cm-3. Reproduced with permission[100]. Copyright 2017, American Chemical Society.
Alternatively, Peng et al. hydrothermally built an oxygen vacancy-rich MnO2-x/reduced graphene oxide (rGO) composite in tetrahydrofuran using manganese carbonyl (Mn(CO)5) and GO as the precursors[99]. Benefiting from the good electronic conduction in the interconnected rGO networks and high redox activity of the partially reduced MnO2 nanoparticles, the vacancy-rich MnO2-x/rGO film showed a high specific capacitance of
4MnO4- + 3C + H2O → 4MnO2 + CO32- + 2HCO3- (12)
4MnO4- + 4H+ → 4MnO2 + 3O2 + 2H2O (13)
Benefiting from the abundant ion transport channels and the fast electron transport of the MWCNTs, the as-prepared amorphous MnO2@MWCNT fibers exhibited a capacitance of 60.8 F cm-3 at 0.2 A cm-3 with the calculated contribution of MnO2 as high as 615.2 F g-1 and excellent rate performance retaining 44.1 F cm-3 at 20 A cm-3 [Figure 5F]. The symmetric SC made of amorphous MnO2@MWCNT fibers delivered a volumetric capacitance (CV) of 10.9 F cm-3 at 0.1 A cm-3 and retained 6.9 F cm-3 at 5 A cm-3, together with an EV of 1.5 mWh cm-3 at the PV of 0.05 W cm-3. In addition, the fiber SC also presented outstanding stability over 15,000 charge/discharge cycles and mechanical robustness for 5000 bending/unbending cycles [Figure 5G].
Hybridization
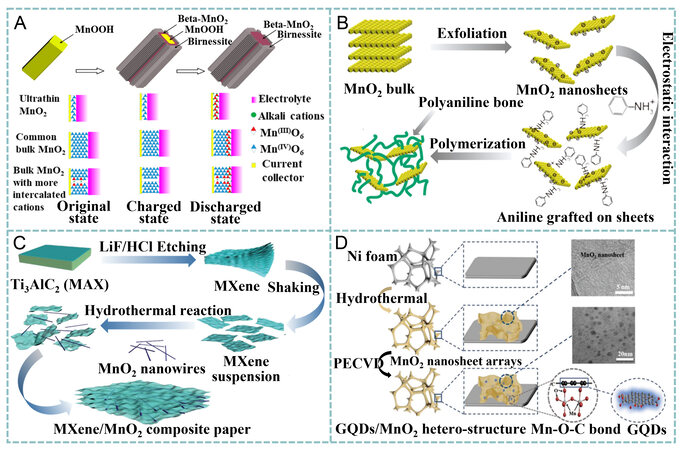
Combining the merits of two materials via hybridization is highly desired for improving the performance of MnO2[101-104]. Zhu et al. developed a core-shell structure by depositing δ-MnO2 nanosheets on the outer surface of β-MnO2 via two-step hydrothermal reactions using MnOOH nanowires as the self-sacrificial template and KMnO4 as the oxidant [Figure 6A][105]. It was found from their experiments that the content of Mn3+ played a critical role in improving the electrical conductivity of MnO2 due to the double-exchange interaction forming Mn3+-O-Mn4+. The as-prepared MnO2 with a Mn3+/Mn4+ ratio of 0.69 exhibited a Cg of 306 F g-1 at 0.25 A g-1, retaining 226 F g-1 at 64 A g-1 and 207 F g-1 after cycling at 2 A g-1 for 3000 times.
Figure 6. (A) Synthesis of β-MnO2/parallel birnessite core/shell nanorod and a diagram illustrating the enhanced utilization of Mn. Reproduced with permission[105]. Copyright 2018, American Chemical Society. (B) preparation of 3D network composed of MnO2 sheets and PANI chains. Reproduced with permission[106]. Copyright 2017, American Chemical Society. (C) fabrication of Ti3C2Tx/MnO2 NW composite paper. Reproduced with permission[107]. Copyright 2018, Wiley-VCH. (D) fabrication of GQD/MnO2 heterostructures. Reproduced with permission[113]. Copyright 2018, Wiley-VCH. GQDs: graphene quantum dots.
Recently, a hybrid paper electrode with high flexibility was proposed by Zhou et al. through vacuum infiltrating a homogeneous suspension of MXene (Ti3C2Tx) nanosheets and MnO2 nanowires
ZINC-ION BATTERIES
Charge storage mechanisms
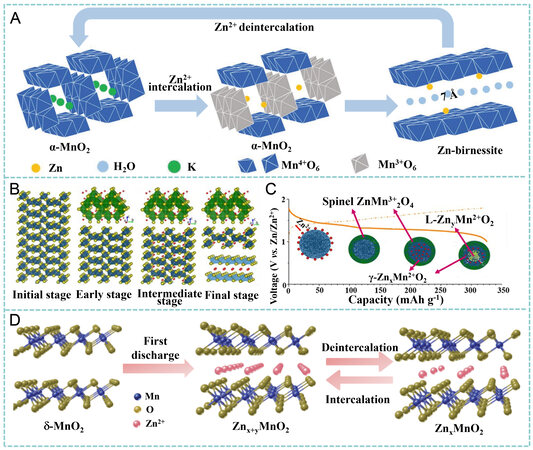
Distinct from those described for SCs above, the charge storage mechanisms of MnO2 in aqueous ZIBs are relatively complicated, possibly involving the insertion/extraction of Zn2+, the co-insertion/extraction of H+ and Zn2+ and the reversible dissolution-deposition of MnO2/Mn2+[50]. Typically, although MnO2 with tunnel structures that can accommodate Zn2+, such as α-MnO2 (2 × 2 tunnels), β-MnO2 (1 × 1 tunnels) and γ-MnO2 (1 × 2 and 1 × 1 tunnels), are considered as promising cathodes for ZIBs, they suffer from irreversible phase conversions to layered structure, spinel structure (ZnMn2O4) or both during discharge/charge, as shown in Figure 7A[114,115]. Alfaruqi et al. reported the application of a hydrothermally synthesized α-MnO2 cathode for rechargeable ZIBs using a ZnSO4 aqueous solution as the electrolyte within a cell voltage of 1.0-1.8 V[116].
Figure 7. (A) Phase transition between Zn-birnessite and α-MnO2. Reproduced with permission[114]. Copyright 2018, Wiley-VCH. (B and C) reaction pathway of Zn insertion in prepared γ-MnO2 cathode. Reproduced with permission[118]. Copyright 2015, American Chemical Society. (D) Zn2+ intercalation/deintercalation mechanism for orthorhombic MnO2. Reproduced with permission[120]. Copyright 2021, Royal Society of Chemistry.
Mn4+(s) + e- → Mn3+(s) (14)
2Mn3+(s) → Mn4+(s) + Mn2+(aq) (15)
Mn2+(aq) → Mn4+(s) + 2e- (16)
Distinctly, using ex-situ and synchrotron XRD and in-situ X-ray absorption near edge structure,
Zn2+ + 2e- + 2MnO2 → Zn2MnO4 (17)
nZn2+ + 2xe- + MnO2 → ZnnMnO2 (18)
δ-MnO2 with a typical layered structure has also been directly utilized as a cathode material for ZIBs.
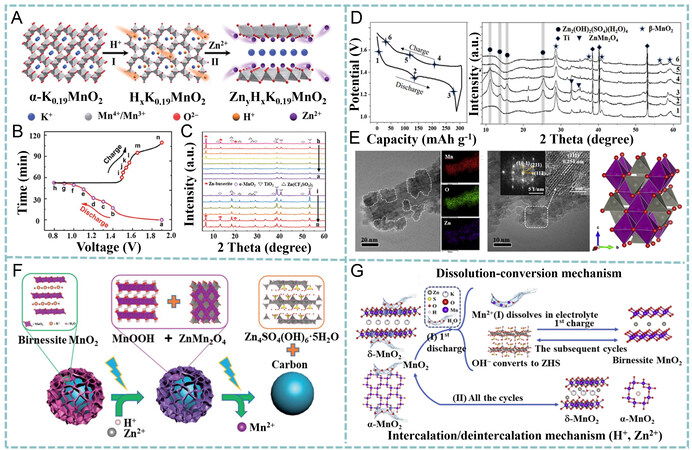
The co-intercalation of H+ and Zn2+ is also an important mechanism for MnO2 cathodes in ZIBs. Liu et al. investigated the charge storage process of tunnel-structured MnO2 nanotubes pre-intercalated by K+
Figure 8. (A) Co-intercalation of Zn2+ and H+ in α-K0.19MnO2. (B) voltage-time profile at 1 C and (C) corresponding XRD patterns
MnO2 + H+ + e → MnOOH (19)
4MnO2 + 2Zn2+ + 4e + H2O → Zn2Mn4O8·H2O (20)
2MnOOH + SO42 + 4Zn2+ + 7H2O + 2e → Zn4SO4(OH)6·5H2O + 2Mn2+ (21)
3Zn2Mn4O8·H2O + 8SO42 + 61H2O + 26Zn2+ + 12e → 8Zn4SO4(OH)6·5H2O + 12Mn2+ (22)
In contrast, Jin et al. unveiled the successive intercalation of Zn2+ and H+, respectively, corresponding to the high and low voltage plateaus by exploring the electrochemical behavior of δ-MnO2 in a Zn(TFSI)2-based aqueous electrolyte[124]. Bulky TFSI- (vs. SO42-) can decrease the number of water molecules surrounding the Zn2+ cation and reduce the solvation effect, thus facilitating Zn2+ transport and charge transfer. Therefore, in the Zn(TFSI)2-based electrolyte, the non-diffusion-controlled mechanism dominates the first step of fast Zn2+ storage in bulk δ-MnO2 without a significant phase transition Equation (23), while the
MnO2 + xZn2+ + 2xe- ↔ ZnxMnO2 (non-diffusion controlled) (23)
H2O ↔ H+ + OH- (24)
MnO2 + H+ + e- ↔ MnOOH (diffusion controlled) (25)
3Zn2+ + 6OH- + Zn(TFSI)2 + xH2O ↔ Zn(TFSI)2[Zn(OH)2]3·xH2O (26)
Typically, the dissolution of Mn2+ and the generation/decomposition of Zn4SO4(OH)6·H2O (ZSH) observed in ZnSO4 electrolytes are regarded as side reactions for capacity fade. However, a recent study performed by Guo et al. proposed a dissolution-deposition mechanism in a Zn//MnO2 battery Equations (27)-(30) and confirmed the capacity contribution from the reversibly formed ZSH[125]. As shown in Figure 8G, α-MnO2 (or -MnO2) reacted with H2O to produce ZSH and Mn2+ in the first discharge process and then ZSH reacted with Mn2+ to form birnessite-MnO2 in the first charge process. Such a dissolution-deposition mechanism dominated the subsequent energy storage processes, with the newly formed birnessite-MnO2 as a host material contributing most of the specific capacity. In contrast, the intercalation/deintercalation of H+/Zn2+ in residual undissolved MnO2 is considered to contribute negligible capacity.
3MnO2 + 6H2O + 6e- → 3Mn2+ + 12OH- (27)
3Mn2+ + 12OH- 6e- → 3birnessite-MnO2 + 6H2O (28)
3birnessite-MnO2 + 6H2O + 6e- ↔ 3Mn2+ + 12OH- (29)
12OH- + 2SO42- + 8Zn2+ + 8H2O ↔ 2Zn4(OH)6SO4·4H2O (30)
Performance enhancements
Foreign ion/molecular pre-insertion
One of the current issues for the development of Mn-based cathodes is the sluggish reaction kinetics caused by a high-energy barrier for Zn2+ migration due to the strong electrostatic interactions with the host material, as well as the serious structural transformation during cycling[64]. The incorporation of cations
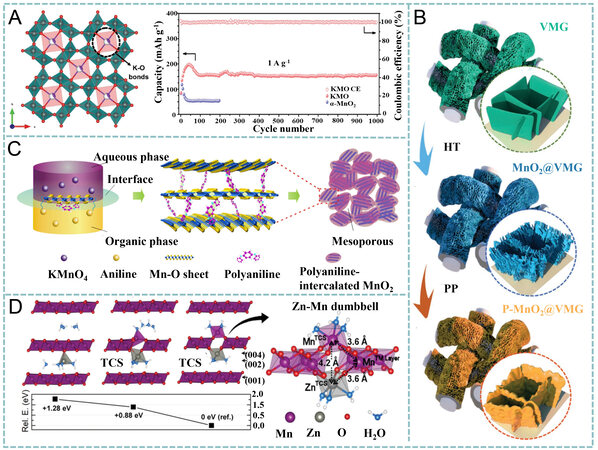
Figure 9. (A) Tunnel structure stabilized by K+ and cycling performance of KMO and α-MnO2 at 1 A g-1. Reproduced with permission[128]. Copyright 2019, Wiley-VCH. (B) synthesis of P-MnO2-x@VMG shell/core arrays. Reproduced with permission[130]. Copyright 2020, Wiley-VCH. (C) preparation and structural advantage of PANI-intercalated MnO2 nanolayers. Reproduced with permission[131]. Copyright 2018, Nature. (D) optimized structure of cw-MnO2 for Zn2+ intercalation and the corresponding relative energies. Reproduced with permission[54]. Copyright 2019, Royal Society of Chemistry. HT: hydrothermal method; TCS:
Alternatively, Huang et al. prepared mesoporous PANI-intercalated MnO2 nanosheets with a thickness of ~10 nm and expanded interlayer space of ~1.0 nm through the polymerization at the interface of the organic and aqueous phases [Figure 9C][131]. The oxidation-induced polymerization of aniline (in CCl4) and the reduction of MnO42- (in H2O) occurred simultaneously, thereby restricting the growth of MnO2 anisotropically and facilitating the layer-by-layer assembly of the 2D MnO2 and PANI. Since the guest polymer in the interlayer of the MnO2 nanosheets efficiently prevented phase transformation and strengthened the layered structure during repeated insertion/extraction of hydrated cations, a reversible discharge capacity of 280 mAh g-1 at 200 mA g-1 was achieved with 110 mAh g-1 retained even at 3 A g-1. Compared to its monovalent counterparts, Zn2+ requires high energy for desolvation at the
Defect engineering
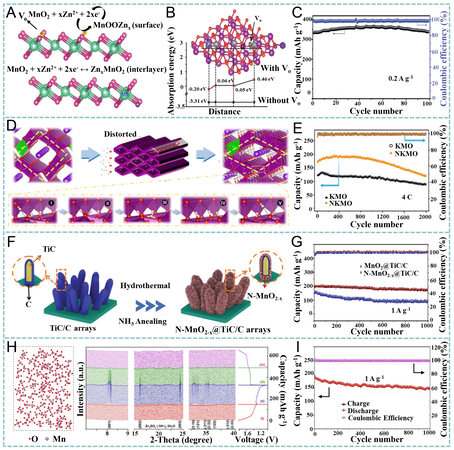
Defect engineering is another widely adopted method to enhance the performance of MnO2 by tuning the electronic structure, enhancing the structural robustness and adjusting the interaction between the host and Zn2+[134,135]. Oxygen vacancies (VO) have proven their capability in gauging the adsorption/desorption of
Figure 10. (A) Zn2+ storage mechanism for VO-MnO2. (B) comparison of adsorption energies for Zn2+ on δ-MnO2 and VO-MnO2. (C) cycling performance of VO-MnO2 at 0.2 A g-1. Reproduced with permission[136]. Copyright 2019, Wiley-VCH. (D) Zn2+ storage in
Alternatively, Wang et al. reported a Ce-doped MnO2 nanorod-like electrode material synthesized by a hydrothermal method[139]. Cerium doping induced a structural transformation of MnO2 from its original
Amorphous structures with disordered atomic arrangements and abundant structural defects that may lead to enhanced ion diffusion kinetics, improved capacity and alleviated volume expansion are considered to be promising cathode candidates for ZIBs[55,90]. Cai et al. demonstrated the feasibility of amorphous MnO2
Hybridization
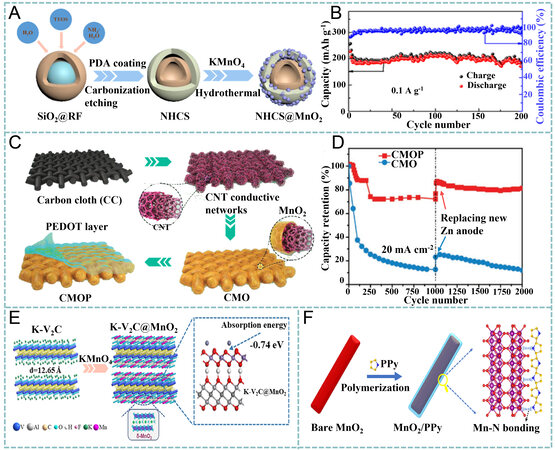
Hybridization has been confirmed as a useful method for improving the performance of MnO2 in ZIBs[142-146]. Li et al. anchored MnO2 particles on N-doped hollow carbon spheres (NHCSs@MnO2) via a direct reaction between carbon and KMnO4 [Figure 11A][147]. The hollow porous carbon nanospheres provided large interfaces, thus ensuring the fast transport of ions/electrons. The obtained NHCSs@MnO2 exhibited improved performance for Zn2+ storage in 2 M ZnSO4 and 0.1 M MnSO4 with an excellent reversible capacity of ~206 mAh g-1 and a retention ratio of 89.5% after 200 cycles at 0.1 A g-1 [Figure 11B]. Zhang et al. used a CNT network as a conductive scaffold on which conformable MnO2 sheath and rough poly(3, 4-ethylenedioxythiophene) (PEDOT) protective layers were deposited sequentially to form a
Figure 11. (A) Synthesis of SiO2@phenol-formaldehyde resin (RF), SiO2@RF@polydopamine (PDA), NHCSs and NHCSs@MnO2 composites using tetraethoxysilane (TEOS) as Si source. (B) cyclic performance of NHCSs@MnO2 at 100 mA g-1 for 200 cycles. Reproduced with permission[147]. Copyright 2020, Elsevier. (C) 3D structure of CNT conductive networks and fabrication of CMOP cathodes. (D) long-term cycling performance of CMO and CMOP electrodes. Reproduced with permission[148]. Copyright 2019,
Alternatively, Zhu et al. hybridized MnO2 nanosheets with a V2CTx MXene (K-V2C@MnO2) by K+ intercalation, followed by a hydrothermal growth strategy [Figure 11E][149]. It was found that the adsorbed K+ on the V2C surface facilitated the growth of K-birnessite MnO2, hydrogen bonds existed between
SUMMARY AND OUTLOOK
Summary
This review focuses on the applications of MnO2 for aqueous energy storage (SCs and ZIBs) and summarizes and compares the similarities and differences of the corresponding charge storage mechanisms and the principles for materials modification [Table 1].
Similarities and differences in using MnO2 as electrode material in SCs and ZIBs
| Classification | Mechanisms | Modification strategies |
| SCs | ☆ Surface redox reaction ☆ Bulk intercalation (no phase transition) | • Nanostructural design • Foreign ion/molecular pre-insertion • Defect engineering • Hybridization |
| ZIBs | ☆ Insertion/extraction of Zn2+ ☆ Co-insertion of H+ and Zn2+ ☆ Dissolution-deposition (with phase transition) | • Foreign ion/molecular pre-insertion • Defect engineering • Hybridization |
Charge storage mechanisms
In the case of SCs, the surface redox reactions and bulk intercalation of electrolyte ions are the two dominating mechanisms for charge storage in MnO2 Equations (10) and (11). The pH of the electrolyte severely affects the electrochemical reactions during charge/discharge. In an acidic solution, an irreversible reaction occurs between H+ and MnOOH, forming dissoluble Mn2+. In an alkaline solution, a passivation layer of Mn(OH)2 is generated as a result of the reaction between MnO2 and OH-. In a neutral electrolyte, the disproportionation of Mn3+ to dissoluble Mn2+ is usually observed. Considering the chemical stability of the zinc metal anode, a near-neutral electrolyte is usually selected (e.g., ZnSO4 aqueous solution) for ZIBs. Three possible mechanisms are proposed: (i) intercalation/deintercalation of Zn2+; (ii)
MnO2 modification methods
Low electrical conductivity, structural instability and slow electrode reaction kinetics are common issues of MnO2 as electrodes for energy storage. Based on our summary, similar strategies have been adopted for MnO2 modification in both SCs and ZIBs, including foreign ion/molecular pre-insertion, defect engineering, nanostructural design and hybridization. Typically, the pre-inserted ions/molecules in MnO2 facilitate the ion intercalation/deintercalation by expanding the interlayer spaces, shield the strong electrostatic interaction between intercalated ions and MnO2 and stabilize the crystal structure of MnO2 during ion intercalation/deintercalation. The introduction of oxygen vacancies and heteroatom doping (defect engineering) are effective methods to regulate the electron structure of MnO2, which significantly affect the electron transport during the redox reaction. Rational nanostructural design contributes to the accessibility of electrolyte ions to active sites, thereby improving the capacity of the electrode. Hybridizing with high conductive carbonaceous materials (e.g., CNTs and rGO) can efficiently improve the conductivity of bare MnO2, thus boosting the kinetics of the electrode reaction.
Outlook
Although significant efforts have been devoted to the investigation of MnO2 for potential applications in SCs and ZIBs and there are several prospective aspects that need to be considered:
(1) MnO2 is considered as one of the most promising candidates for SCs because of its low cost, earth abundance, high theoretical capacitance and environmental benignness. Nevertheless, the performance of MnO2 in SCs is far from satisfactory. An in-depth understanding of the underlying mechanisms and efficient strategies for performance improvements are highly required.
(2) To date, the mechanism for Zn2+ storage is still under debate and severely depends on the testing conditions. More efforts are required by employing advanced characterization techniques.
(3) The introduction of oxygen vacancies and heteroatoms is deemed to be an effective method to regulate the properties of MnO2 for enhancing the charge storage in both SCs and ZIBs. However, since MnO2 crystals are composed of MnO6 octahedral units, it remains necessary to quantitatively identify the concentration and position of oxygen vacancies and heteroatoms that play a critical role in governing MnO2 performance.
(4) The pre-intercalation of cations, water molecules and polymers has been demonstrated to be an effective strategy to enhance the capacitance/capacity and stability of MnO2. The mechanisms for performance enhancement need to be further investigated.
(5) Hybridization with conductive materials (e.g., graphene and MXenes) is considered a feasible method to efficiently improve the electron transfer/transport of MnO2. In addition, the interfacial properties between two materials are believed to contribute the performance enhancement. However, a detailed explanation of interface-dependent electrochemical behavior is still required.
(6) Finally, more attention should be paid to the performance decay mechanisms of MnO2 in SCs and ZIBs, which are the guidelines for future materials design.
DECLARATIONS
Authors’ contributionsPreparing the manuscript draft: Dai H, Zhou R
Writing-review: Zhang Z
Editing: Zhou J
Funding acquisition, supervision: Sun G
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by the National Natural Science Foundation of China (No. 21975123), Anhui Provincial Key R&D Program (No. 2022i01020021) and Six Talent Peaks Project in Jiangsu Province (No. XCL-024).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Gerard O, Numan A, Krishnan S, Khalid M, Subramaniam R, Kasi R. A review on the recent advances in binder-free electrodes for electrochemical energy storage application. J Energy Storage 2022;50:104283.
2. Yu C, Gong Y, Chen R, et al. A solid-state fibriform supercapacitor boosted by host-guest hybridization between the carbon nanotube scaffold and MXene nanosheets. Small 2018:e1801203.
3. Xiao J, Han J, Zhang C, Ling G, Kang F, Yang Q. Dimensionality, function and performance of carbon materials in energy storage devices. Adv Energy Mater 2022;12:2100775.
4. Liu Y, Xu J, Li J, et al. Pre-intercalation chemistry of electrode materials in aqueous energy storage systems. Coord Chem Rev 2022;460:214477.
5. Shin J, Lee J, Park Y, Choi JW. Aqueous zinc ion batteries: focus on zinc metal anodes. Chem Sci 2020;11:2028-44.
6. Dai H, Zhou J, Qin G, Sun G. Enhanced Jahn-Teller distortion boosts molybdenum trioxide’s superior lithium ion storage capability. Dalton Trans 2022;51:524-31.
7. Dai H, Wang L, Zhao Y, et al. Recent advances in molybdenum-based materials for lithium-sulfur batteries. Research 2021;2021:5130420.
8. Zhang X, Li Z, Luo L, Fan Y, Du Z. A review on thermal management of lithium-ion batteries for electric vehicles. Energy 2022;238:121652.
9. Rivera-barrera J, Muñoz-galeano N, Sarmiento-maldonado H. SoC estimation for lithium-ion batteries: review and future challenges. Electronics 2017;6:102.
10. Muralidharan N, Self EC, Dixit M, et al. Next-generation cobalt-free cathodes - a prospective solution to the battery industry’s cobalt problem. Adv Energy Mater 2022;12:2103050.
11. Li J, Fleetwood J, Hawley WB, Kays W. From materials to cell: state-of-the-art and prospective technologies for lithium-ion battery electrode processing. Chem Rev 2022;122:903-56.
12. Chao D, Zhou W, Xie F, et al. Roadmap for advanced aqueous batteries: from design of materials to applications. Sci Adv 2020;6:eaba4098.
13. Sui Y, Ji X. Anticatalytic strategies to suppress water electrolysis in aqueous batteries. Chem Rev 2021;121:6654-95.
14. Bin D, Wen Y, Wang Y, Xia Y. The development in aqueous lithium-ion batteries. J Energy Chem 2018;27:1521-35.
15. Jiang X, Chen Y, Meng X, et al. The impact of electrode with carbon materials on safety performance of lithium-ion batteries: a review. Carbon 2022;191:448-70.
16. Liu K, Liu Y, Lin D, Pei A, Cui Y. Materials for lithium-ion battery safety. Sci Adv 2018;4:eaas9820.
17. Su X, Wu Q, Li J, et al. Silicon-based nanomaterials for lithium-ion batteries: a review. Adv Energy Mater 2014;4:1300882.
19. Yang Z, Zhang J, Kintner-Meyer MC, et al. Electrochemical energy storage for green grid. Chem Rev 2011;111:3577-613.
20. Yong B, Ma D, Wang Y, Mi H, He C, Zhang P. Understanding the design principles of advanced aqueous zinc-ion battery cathodes: from transport kinetics to structural engineering, and future perspectives. Adv Energy Mater 2020;10:2002354.
21. Peng J, Zhang W, Wang S, et al. The emerging electrochemical activation tactic for aqueous energy storage: fundamentals, applications, and future. Adv Funct Mater 2022;32:2111720.
22. Lee B, Lee HR, Kim H, Chung KY, Cho BW, Oh SH. Elucidating the intercalation mechanism of zinc ions into α-MnO2 for rechargeable zinc batteries. Chem Commun 2015;51:9265-8.
23. Tang Y, Zheng S, Xu Y, Xiao X, Xue H, Pang H. Advanced batteries based on manganese dioxide and its composites. Energy Stor Mater 2018;12:284-309.
24. Qian J, Jin H, Chen B, et al. Aqueous manganese dioxide ink for paper-based capacitive energy storage devices. Angew Chem Int Ed Engl 2015;54:6800-3.
25. Pan H, Shao Y, Yan P, et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat Energy 2016:1.
26. Tang B, Shan L, Liang S, Zhou J. Issues and opportunities facing aqueous Zinc-ion batteries. Energy Environ Sci 2019;12:3288-304.
27. Ming J, Guo J, Xia C, Wang W, Alshareef HN. Zinc-ion batteries: materials, mechanisms, and applications. Mater Sci Eng R Rep 2019;135:58-84.
28. Fang G, Zhou J, Pan A, Liang S. Recent advances in aqueous Zinc-ion batteries. ACS Energy Lett 2018;3:2480-501.
29. Dong C, Xu F, Chen L, Chen Z, Cao Y. Design strategies for high-voltage aqueous batteries. Small Struct 2021;2:2100001.
30. Wang F, Wu X, Yuan X, et al. Latest advances in supercapacitors: from new electrode materials to novel device designs. Chem Soc Rev 2017;46:6816-54.
31. Augustyn V, Simon P, Dunn B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ Sci 2014;7:1597.
32. Huang J, Yuan K, Chen Y. Wide voltage aqueous asymmetric supercapacitors: advances, strategies, and challenges. Adv Funct Mater 2022;32:2108107.
33. Wang S, Li T, Yin Y, Chang N, Zhang H, Li X. High-energy-density aqueous zinc-based hybrid supercapacitor-battery with uniform zinc deposition achieved by multifunctional decoupled additive. Nano Energy 2022;96:107120.
34. Li H, Ma L, Han C, et al. Advanced rechargeable zinc-based batteries: recent progress and future perspectives. Nano Energy 2019;62:550-87.
35. Zhang K, Han X, Hu Z, Zhang X, Tao Z, Chen J. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem Soc Rev 2015;44:699-728.
36. Zhong C, Deng Y, Hu W, Qiao J, Zhang L, Zhang J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem Soc Rev 2015;44:7484-539.
37. He P, Chen Q, Yan M, et al. Building better Zinc-ion batteries: a materials perspective. EnergyChem 2019;1:100022.
38. Lv Y, Xiao Y, Ma L, Zhi C, Chen S. Recent advances in electrolytes for “beyond aqueous” Zinc-ion batteries. Adv Mater 2022;34:e2106409.
39. Zhou T, Zhu L, Xie L, et al. Cathode materials for aqueous Zinc-ion batteries: a mini review. J Colloid Interface Sci 2022;605:828-50.
40. Gao Y, Zhao L. Review on recent advances in nanostructured transition-metal-sulfide-based electrode materials for cathode materials of asymmetric supercapacitors. Chem Eng J 2022;430:132745.
41. Kumar S, Saeed G, Zhu L, Hui KN, Kim NH, Lee JH. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: a review. Chem Eng J 2021;403:126352.
42. Hu Y, Wu Y, Wang J. Manganese-oxide-based electrode materials for energy storage applications: how close are we to the theoretical capacitance? Adv Mater 2018;30:e1802569.
43. Kumar A, Sanger A, Kumar A, Mishra YK, Chandra R. Performance of high energy density symmetric supercapacitor based on sputtered MnO2 nanorods. ChemistrySelect 2016;1:3885-91.
44. Yu N, Yin H, Zhang W, Liu Y, Tang Z, Zhu M. High-performance fiber-shaped all-solid-state asymmetric supercapacitors based on ultrathin MnO2 nanosheet/carbon fiber cathodes for wearable electronics. Adv Energy Mater 2016;6:1501458.
45. Radhamani AV, Shareef KM, Rao MS. ZnO@MnO2 core-shell nanofiber cathodes for high performance asymmetric supercapacitors. ACS Appl Mater Interfaces 2016;8:30531-42.
46. Zhou D, Lin H, Zhang F, et al. Freestanding MnO2 nanoflakes/porous carbon nanofibers for high-performance flexible supercapacitor electrodes. Electrochim Acta 2015;161:427-35.
47. Cai K, Luo SH, Feng J, et al. Recent advances on spinel zinc manganate cathode materials for Zinc-ion batteries. Chem Rec 2022;22:e202100169.
48. Davoglio RA, Cabello G, Marco JF, Biaggio SR. Synthesis and characterization of α-MnO2 nanoneedles for electrochemical supercapacitors. Electrochim Acta 2018;261:428-35.
49. Guo C, Liu H, Li J, et al. Ultrathin δ-MnO2 nanosheets as cathode for aqueous rechargeable zinc ion battery. Electrochim Acta 2019;304:370-7.
50. Li Y, Zhang D, Huang S, Yang HY. Guest-species-incorporation in manganese/vanadium-based oxides: towards high performance aqueous Zinc-ion batteries. Nano Energy 2021;85:105969.
51. Zhang Z, Li W, Shen Y, et al. Issues and opportunities of manganese-based materials for enhanced Zn-ion storage performances. J Energy Storage 2022;45:103729.
52. Xie Q, Cheng G, Xue T, et al. Alkali ions pre-intercalation of δ-MnO2 nanosheets for high-capacity and stable Zn-ion battery. Mater Today Energy 2022;24:100934.
53. Wang M, Zheng X, Zhang X, et al. Opportunities of aqueous manganese-based batteries with deposition and stripping chemistry. Adv Energy Mater 2021;11:2002904.
54. Nam KW, Kim H, Choi JH, Choi JW. Crystal water for high performance layered manganese oxide cathodes in aqueous rechargeable zinc batteries. Energy Environ Sci 2019;12:1999-2009.
55. Wu Y, Fee J, Tobin Z, et al. Amorphous manganese oxides: an approach for reversible aqueous Zinc-ion batteries. ACS Appl Energy Mater 2020;3:1627-33.
56. Li S, Liu Q, Qi L, Lu L, Wang H. Progress in research on manganese dioxide electrode materials for electrochemical capacitors. Chinese J Anal Chem 2012;40:339-46.
57. Wang J, Dong S, Ding B, et al. Pseudocapacitive materials for electrochemical capacitors: from rational synthesis to capacitance optimization. Natl Sci Rev 2017;4:71-90.
58. Brousse T, Toupin M, Dugas R, Athouël L, Crosnier O, Bélanger D. Crystalline MnO2 as possible alternatives to amorphous compounds in electrochemical supercapacitors. J Electrochem Soc 2006;153:A2171.
59. Yan J, Fan Z, Wei T, Qian W, Zhang M, Wei F. Fast and reversible surface redox reaction of graphene-MnO2 composites as supercapacitor electrodes. Carbon 2010;48:3825-33.
60. Zhang Y, An Y, Jiang J, et al. High performance aqueous sodium-ion capacitors enabled by pseudocapacitance of layered MnO2. Energy Technol 2018;6:2146-53.
61. Chen Q, Jin J, Kou Z, et al. Zn2+ pre-intercalation stabilizes the tunnel structure of MnO2 nanowires and enables Zinc-ion hybrid supercapacitor of battery-level energy density. Small 2020;16:e2000091.
62. Tang X, Zhu S, Ning J, Yang X, Hu M, Shao J. Charge storage mechanisms of manganese dioxide-based supercapacitors: a review. New Carbon Mater 2021;36:702-10.
63. Guo W, Yu C, Li S, et al. Strategies and insights towards the intrinsic capacitive properties of MnO2 for supercapacitors: challenges and perspectives. Nano Energy 2019;57:459-72.
64. Kim SJ, Wu D, Sadique N, et al. Unraveling the dissolution-mediated reaction mechanism of α-MnO2 cathodes for aqueous Zn-ion batteries. Small 2020;16:e2005406.
65. Qiu C, Zhu X, Xue L, et al. The function of Mn2+ additive in aqueous electrolyte for Zn/δ-MnO2 battery. Electrochim Acta 2020;351:136445.
66. Julien CM, Mauger A. Nanostructured MnO2 as electrode materials for energy storage. Nanomaterials 2017;7:396.
67. Majumdar D. Review on current progress of MnO2-based ternary nanocomposites for supercapacitor applications. ChemElectroChem 2021;8:291-336.
68. Shin J, Seo JK, Yaylian R, Huang A, Meng YS. A review on mechanistic understanding of MnO2 in aqueous electrolyte for electrical energy storage systems. Int Mater Rev 2020;65:356-87.
69. Messaoudi B, Joiret S, Keddam M, Takenouti H. Anodic behaviour of manganese in alkaline medium. Electrochim Acta 2001;46:2487-98.
70. Roberts AJ, Slade RC. Effect of specific surface area on capacitance in asymmetric carbon/α-MnO2 supercapacitors. Electrochim Acta 2010;55:7460-9.
71. Wu B, Zhang G, Yan M, et al. Graphene scroll-coated α-MnO2 nanowires as high-performance cathode materials for aqueous Zn-ion battery. Small 2018;14:e1703850.
72. Wang J, Dong L, Xu C, Ren D, Ma X, Kang F. Polymorphous supercapacitors constructed from flexible three-dimensional carbon network/Polyaniline/MnO2 composite textiles. ACS Appl Mater Interfaces 2018;10:10851-9.
73. Fu Y, Gao X, Zha D, Zhu J, Ouyang X, Wang X. Yolk-shell-structured MnO2 microspheres with oxygen vacancies for high-performance supercapacitors. J Mater Chem A 2018;6:1601-11.
74. Fu W, Zhao E, Ren X, Magasinski A, Yushin G. Hierarchical fabric decorated with carbon nanowire/metal oxide nanocomposites for 1.6 V wearable aqueous supercapacitors. Adv Energy Mater 2018;8:1703454.
75. Qi H, Bo Z, Yang S, et al. Hierarchical nanocarbon-MnO2 electrodes for enhanced electrochemical capacitor performance. Energy Stor Mater 2019;16:607-18.
76. Chen L, Zhang M, Yang X, et al. Sandwich-structured MnO2@N-doped carbon@MnO2 nanotubes for high-performance supercapacitors. J Alloys Compd 2017;695:3339-47.
77. Han D, Jing X, Xu P, Ding Y, Liu J. Facile synthesis of hierarchical hollow ε-MnO2 spheres and their application in supercapacitor electrodes. J Solid State Chem 2014;218:178-83.
78. Xiong P, Ma R, Sakai N, Bai X, Li S, Sasaki T. Redox active cation intercalation/deintercalation in two-dimensional layered MnO2 nanostructures for high-rate electrochemical energy storage. ACS Appl Mater Interfaces 2017;9:6282-91.
79. Jabeen N, Xia Q, Savilov SV, Aldoshin SM, Yu Y, Xia H. Enhanced pseudocapacitive performance of α-MnO2 by cation preinsertion. ACS Appl Mater Interfaces 2016;8:33732-40.
80. Gao P, Metz P, Hey T, et al. The critical role of point defects in improving the specific capacitance of δ-MnO2 nanosheets. Nat Commun 2017;8:14559.
81. Chen S, Zhang M, Ma X, Li L, Zhou X, Zhang Z. Asymmetric supercapacitors by integrating high content Na+/K+-inserted MnO2 nanosheets and layered Ti3C2Tx paper. Electrochim Acta 2020;332:135497.
82. Zhao Q, Song A, Ding S, et al. Preintercalation strategy in manganese oxides for electrochemical energy storage: review and prospects. Adv Mater 2020;32:e2002450.
83. Peng H, Fan H, Sui J, Wang C, Zhang W, Wang W. Sodium in situ intercalated ultrathin δ-MnO2 flakes electrode with enhanced intercalation capacitive performance for asymmetric supercapacitors. ChemistrySelect 2020;5:869-74.
84. Jabeen N, Hussain A, Xia Q, Sun S, Zhu J, Xia H. High-performance 2.6 V aqueous asymmetric supercapacitors based on in situ formed Na0.5MnO2 nanosheet assembled nanowall arrays. Adv Mater 2017;29:1700804.
85. Jiang H, Wang Z, Yang Q, et al. A novel MnO2/Ti3C2Tx MXene nanocomposite as high performance electrode materials for flexible supercapacitors. Electrochim Acta 2018;290:695-703.
86. Chi HZ, Li Y, Xin Y, Qin H. Boron-doped manganese dioxide for supercapacitors. Chem Commun 2014;50:13349-52.
87. Choi C, Sim HJ, Spinks GM, Lepró X, Baughman RH, Kim SJ. Elastomeric and dynamic MnO2/CNT core-shell structure coiled yarn supercapacitor. Adv Energy Mater 2016;6:1502119.
88. Wang Y, Zhang Y, Gao Y, Sheng G, ten Elshof JE. Defect engineering of MnO2 nanosheets by substitutional doping for printable solid-state micro-supercapacitors. Nano Energy 2020;68:104306.
89. Wang J, Wang J, Liu H, et al. A highly flexible and lightweight MnO2/graphene membrane for superior Zinc-ion batteries. Adv Funct Mater 2021;31:2007397.
90. Tong H, Li T, Liu J, et al. Fabrication of the oxygen vacancy amorphous MnO2/Carbon nanotube as cathode for advanced aqueous Zinc-ion batteries. Energy Technol 2021;9:2000769.
91. Shi J, Wang S, Wang Q, et al. A new flexible Zinc-ion capacitor based on δ-MnO2@Carbon cloth battery-type cathode and MXene@Cotton cloth capacitor-type anode. J Power Sources 2020;446:227345.
92. Zhao L, Wang W, Zhao H, et al. Controlling oxygen vacancies through gas-assisted hydrothermal method and improving the capacitive properties of MnO2 nanowires. Appl Surf Sci 2019;491:24-31.
93. Yan L, Shen C, Niu L, et al. Experimental and theoretical investigation of the effect of oxygen vacancies on the electronic structure and pseudocapacitance of MnO2. ChemSusChem 2019;12:3571-81.
94. Zhai T, Xie S, Yu M, et al. Oxygen vacancies enhancing capacitive properties of MnO2 nanorods for wearable asymmetric supercapacitors. Nano Energy 2014;8:255-63.
95. Ou T, Hsu C, Hu C. Synthesis and characterization of sodium-doped MnO2 for the aqueous asymmetric supercapacitor application. J Electrochem Soc 2015;162:A5124-32.
96. Li J, Ren Y, Wang S, Ren Z, Yu J. Transition metal doped MnO2 nanosheets grown on internal surface of macroporous carbon for supercapacitors and oxygen reduction reaction electrocatalysts. Appl Mater Today 2016;3:63-72.
97. Kang J, Hirata A, Kang L, et al. Enhanced supercapacitor performance of MnO2 by atomic doping. Angew Chem Int Ed Engl 2013;52:1664-7.
98. Wang Z, Wang F, Li Y, Hu J, Lu Y, Xu M. Interlinked multiphase Fe-doped MnO2 nanostructures: a novel design for enhanced pseudocapacitive performance. Nanoscale 2016;8:7309-17.
99. Peng R, Wang H, Wei X, Wu Z, Yu P, Luo Y. One-step synthesis of vacancy-rich MnO2-x/reduced graphene oxide composite film for high electrochemical performance. ChemElectroChem 2019;6:1122-8.
100. Shi P, Li L, Hua L, et al. Design of amorphous manganese oxide@multiwalled carbon nanotube fiber for robust solid-state supercapacitor. ACS Nano 2017;11:444-52.
101. Gou L, Xue D, Mou K, et al. α-MnO2@In2O3 nanotubes as cathode material for aqueous rechargeable Zn-ion battery with high electrochemical performance. J Electrochem Soc 2019;166:A3362-8.
102. Zhang J, Li Y, Zhang Y, et al. The enhanced adhesion between overlong TiNxOy/MnO2 nanoarrays and Ti substrate: towards flexible supercapacitors with high energy density and long service life. Nano Energy 2018;43:91-102.
103. Xu J, Sun Y, Lu M, et al. Fabrication of hierarchical MnMoO4·H2O@MnO2 core-shell nanosheet arrays on nickel foam as an advanced electrode for asymmetric supercapacitors. Chem Eng J 2018;334:1466-76.
104. Shinde PA, Lokhande VC, Patil AM, Ji T, Lokhande CD. Single-step hydrothermal synthesis of WO3-MnO2 composite as an active material for all-solid-state flexible asymmetric supercapacitor. Int J Hydrog Energy 2018;43:2869-80.
105. Zhu S, Li L, Liu J, et al. Structural directed growth of ultrathin parallel birnessite on β-MnO2 for high-performance asymmetric supercapacitors. ACS Nano 2018;12:1033-42.
106. Liu N, Su Y, Wang Z, et al. Electrostatic-interaction-assisted construction of 3D networks of manganese dioxide nanosheets for flexible high-performance solid-state asymmetric supercapacitors. ACS Nano 2017;11:7879-88.
107. Zhou J, Yu J, Shi L, et al. A conductive and highly deformable all-pseudocapacitive composite paper as supercapacitor electrode with improved areal and volumetric capacitance. Small 2018;14:e1803786.
108. Chen Q, Meng Y, Hu C, et al. MnO2-modified hierarchical graphene fiber electrochemical supercapacitor. J Power Sources 2014;247:32-9.
109. Tan X, Liu S, Guo Q, et al. Synthesis and characterisation of amorphous MnO2/CNT via solid-state microwave for high-performance supercapacitors. Int J Energy Res 2020;44:4556-67.
110. Chen Y, Zhang X, Xu C, Xu H. The fabrication of asymmetry supercapacitor based on MWCNTs/MnO2/PPy composites. Electrochim Acta 2019;309:424-31.
111. Zhang QZ, Zhang D, Miao ZC, Zhang XL, Chou SL. Research progress in MnO2-carbon based supercapacitor electrode materials. Small 2018;14:e1702883.
112. Yu G, Hu L, Vosgueritchian M, et al. Solution-processed graphene/MnO2 nanostructured textiles for high-performance electrochemical capacitors. Nano Lett 2011;11:2905-11.
113. Jia H, Cai Y, Lin J, et al. Heterostructural graphene quantum dot/MnO2 nanosheets toward high-potential window electrodes for high-performance supercapacitors. Adv Sci 2018;5:1700887.
114. Song M, Tan H, Chao D, Fan HJ. Recent advances in Zn-ion batteries. Adv Funct Mater 2018;28:1802564.
115. Jia X, Liu C, Neale ZG, Yang J, Cao G. Active materials for aqueous Zinc ion batteries: synthesis, crystal structure, morphology, and electrochemistry. Chem Rev 2020;120:7795-866.
116. Alfaruqi MH, Gim J, Kim S, et al. Enhanced reversible divalent zinc storage in a structurally stable α-MnO2 nanorod electrode. J Power Sources 2015;288:320-7.
117. Lee B, Yoon CS, Lee HR, Chung KY, Cho BW, Oh SH. Electrochemically-induced reversible transition from the tunneled to layered polymorphs of manganese dioxide. Sci Rep 2014;4:6066.
118. Alfaruqi MH, Mathew V, Gim J, et al. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity Zinc-ion battery system. Chem Mater 2015;27:3609-20.
119. Deng Y, Wan L, Xie Y, Qin X, Chen G. Recent advances in Mn-based oxides as anode materials for lithium ion batteries. RSC Adv 2014;4:23914-35.
120. Li F, Liu Y, Wang G, et al. The design of flower-like C-MnO2 nanosheets on carbon cloth toward high-performance flexible Zinc-ion batteries. J Mater Chem A 2021;9:9675-84.
121. Liu G, Huang H, Bi R, Xiao X, Ma T, Zhang L. K+ pre-intercalated manganese dioxide with enhanced Zn2+ diffusion for high rate and durable aqueous Zinc-ion batteries. J Mater Chem A 2019;7:20806-12.
122. Liu M, Zhao Q, Liu H, et al. Tuning phase evolution of β-MnO2 during microwave hydrothermal synthesis for high-performance aqueous Zn ion battery. Nano Energy 2019;64:103942.
123. Li G, Huang Z, Chen J, et al. Rechargeable Zn-ion batteries with high power and energy densities: a two-electron reaction pathway in birnessite MnO2 cathode materials. J Mater Chem A 2020;8:1975-85.
124. Jin Y, Zou L, Liu L, et al. Joint charge storage for high-rate aqueous zinc-manganese dioxide batteries. Adv Mater 2019;31:e1900567.
125. Guo X, Zhou J, Bai C, Li X, Fang G, Liang S. Zn/MnO2 battery chemistry with dissolution-deposition mechanism. Mater Today Energy 2020;16:100396.
126. Peng H, Fan H, Yang C, Tian Y, Wang C, Sui J. Ultrathin δ-MnO2 nanoflakes with Na+ intercalation as a high-capacity cathode for aqueous Zinc-ion batteries. RSC Adv 2020;10:17702-12.
127. Sun T, Nian Q, Zheng S, Shi J, Tao Z. Layered Ca0.28MnO2·0.5H2O as a high performance cathode for aqueous Zinc-ion battery. Small 2020;16:e2000597.
128. Fang G, Zhu C, Chen M, et al. Suppressing manganese dissolution in potassium manganate with rich oxygen defects engaged high-energy-density and durable aqueous Zinc-ion battery. Adv Funct Mater 2019;29:1808375.
129. Zhai T, Wan L, Sun S, et al. Phosphate ion functionalized Co3O4 ultrathin nanosheets with greatly improved surface reactivity for high performance pseudocapacitors. Adv Mater 2017;29:1604167.
130. Zhang Y, Deng S, Pan G, et al. Introducing oxygen defects into phosphate ions intercalated manganese dioxide/vertical multilayer graphene arrays to boost flexible Zinc ion storage. Small Methods 2020;4:1900828.
131. Huang J, Wang Z, Hou M, et al. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous Zinc-ion battery. Nat Commun 2018;9:2906.
132. Zhang Q, Yang Z, Ji H, et al. Issues and rational design of aqueous electrolyte for Zn-ion batteries. SusMat 2021;1:432-47.
133. Wang D, Wang L, Liang G, et al. A superior δ-MnO2 cathode and a self-healing Zn-δ-MnO2 battery. ACS Nano 2019;13:10643-52.
134. Han M, Huang J, Liang S, et al. Oxygen defects in β-MnO2 enabling high-performance rechargeable aqueous zinc/manganese dioxide battery. iScience 2020;23:100797.
135. Zhao J, Xu Z, Zhou Z, et al. A safe flexible self-powered wristband system by integrating defective MnO2-x nanosheet-based Zinc-ion batteries with perovskite solar cells. ACS Nano 2021;15:10597-608.
136. Xiong T, Yu ZG, Wu H, et al. Defect engineering of oxygen-deficient manganese oxide to achieve high-performing aqueous Zinc ion battery. Adv Energy Mater 2019;9:1803815.
137. Xiong T, Lee WSV, Xue J. K+-intercalated MnO2 electrode for high performance aqueous supercapacitor. ACS Appl Energy Mater 2018; doi: 10.1021/acsaem.8b01160.
138. Zhao Q, Song A, Zhao W, et al. Boosting the energy density of aqueous batteries via facile grotthuss proton transport. Angew Chem Int Ed Engl 2021;60:4169-74.
139. Wang J, Sun X, Zhao H, et al. Superior-performance aqueous Zinc ion battery based on structural transformation of MnO2 by rare earth doping. J Phys Chem C 2019;123:22735-41.
140. Zhang Y, Deng S, Luo M, et al. Defect promoted capacity and durability of N-MnO2-x branch arrays via low-temperature NH3 treatment for advanced aqueous Zinc ion batteries. Small 2019;15:e1905452.
141. Cai Y, Chua R, Huang S, Ren H, Srinivasan M. Amorphous manganese dioxide with the enhanced pseudocapacitive performance for aqueous rechargeable Zinc-ion battery. Chem Eng J 2020;396:125221.
142. Chen X, Li W, Zeng Z, Reed D, Li X, Liu X. Engineering stable Zn-MnO2 batteries by synergistic stabilization between the carbon nanofiber core and birnessite-MnO2 nanosheets shell. Chem Eng J 2021;405:126969.
143. Wu F, Gao X, Xu X, et al. MnO2 nanosheet-assembled hollow polyhedron grown on carbon cloth for flexible aqueous Zinc-ion batteries. ChemSusChem 2020;13:1537-45.
144. Long J, Yang F, Cuan J, et al. Boosted charge transfer in twinborn α-(Mn2O3-MnO2) heterostructures: toward high-rate and ultralong-life Zinc-ion batteries. ACS Appl Mater Interfaces 2020;12:32526-35.
145. Chen J, Cheng F. Combination of lightweight elements and nanostructured materials for batteries. ACC Chem Res 2009;42:713-23.
146. Ling W, Wang P, Chen Z, et al. Nanostructure design strategies for aqueous Zinc-ion batteries. ChemElectroChem 2020;7:2957-78.
147. Li D, Gao Q, Zhang H, et al. MnO2 particles grown on the surface of N-doped hollow porous carbon nanospheres for aqueous rechargeable zinc ion batteries. Appl Surf Sci 2020;510:145458.
148. Zhang X, Wu S, Deng S, et al. 3D CNTs networks enable MnO2 cathodes with high capacity and superior rate capability for flexible rechargeable Zn-MnO2 batteries. Small Methods 2019;3:1900525.
149. Zhu X, Cao Z, Wang W, et al. Superior-performance aqueous zinc-ion batteries based on the in situ growth of MnO2 nanosheets on V2CTx MXene. ACS Nano 2021;15:2971-83.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Dai H, Zhou R, Zhang Z, Zhou J, Sun G. Design of manganese dioxide for supercapacitors and zinc-ion batteries: similarities and differences. Energy Mater 2022;2:200040. http://dx.doi.org/10.20517/energymater.2022.56
AMA Style
Dai H, Zhou R, Zhang Z, Zhou J, Sun G. Design of manganese dioxide for supercapacitors and zinc-ion batteries: similarities and differences. Energy Materials. 2022; 2(6): 200040. http://dx.doi.org/10.20517/energymater.2022.56
Chicago/Turabian Style
Dai, Henghan, Ruicong Zhou, Zhao Zhang, Jinyuan Zhou, Gengzhi Sun. 2022. "Design of manganese dioxide for supercapacitors and zinc-ion batteries: similarities and differences" Energy Materials. 2, no.6: 200040. http://dx.doi.org/10.20517/energymater.2022.56
ACS Style
Dai, H.; Zhou R.; Zhang Z.; Zhou J.; Sun G. Design of manganese dioxide for supercapacitors and zinc-ion batteries: similarities and differences. Energy Mater. 2022, 2, 200040. http://dx.doi.org/10.20517/energymater.2022.56
About This Article
Copyright
Data & Comments
Data
 Cite This Article 17 clicks
Cite This Article 17 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.