Recent progress of multilayer polymer electrolytes for lithium batteries
Abstract
The significant market for electric vehicles and portable electronic devices is driving the development of high-energy-density solid-state lithium batteries. However, the solid electrolyte is still the main obstacle to the development of solid-state lithium batteries, mainly due to the lack of a single solid electrolyte that is compatible with both high-voltage cathodes and lithium metal anodes. These problems can potentially be solved with multilayer electrolytes. The property of each layer of the electrolyte can be tuned separately, which not only meets the different needs of the cathode and anode but also makes up for the shortcomings of each layer of the electrolyte, thereby achieving good mechanical properties and chemical and electrochemical stability. This review first presents a brief introduction to homogeneous single-layer electrolytes. The design principles of multilayer polymer electrolytes and the application of these principles using examples from recent work are then introduced. Finally, several suggestions as guides for future work are given.
Keywords
INTRODUCTION
Lithium anodes are used in high-energy-density batteries because of their high theoretical capacity and low reduction potential[1]. By combining a lithium metal anode and a high-voltage cathode, the energy density of a lithium battery can exceed 260 Wh kg-1[2], which is appropriate for electric vehicles[3]. However, as anodes, alkali metals are faced with key problems, such as dendrite growth and side reactions with electrolytes, which lead to potential safety hazards[4,5]. Polymers are considered promising electrolytes to address the safety problems of lithium batteries[6]. Generally, polymer electrolytes can be divided into solid polymer electrolytes (SPEs) and gel polymer electrolytes (GPEs). Fillers and additives can also be added to the polymer matrix to make composite polymer electrolytes. The investigation of SPEs started with
An ideal polymer electrolyte should possess high ionic conductivity (> 10-4 S cm-1 at room temperature) and lithium-ion mobility, excellent chemical and electrochemical stability, compatibility with high-voltage cathodes (> 4.5 V vs. Li/Li+) and lithium anodes and resistance to the volume change of the electrode and lithium dendrite formation. However, integrating all these required properties into polymer electrolytes remains a challenging task. Common polymer electrolytes, such as polyethers, polyesters, polyacrylonitrile, fluoropolymers and their copolymers, cannot easily meet the above requirements simultaneously, so the emergence of multilayer electrolytes is imperative.
Multilayer polymer electrolytes (MLPEs), first reported in the 2000s[11], refer to two or more polymer electrolytes with different components that are superposed by ex-situ or in-situ preparation methods. An oxidation-resistant/soft polymer is used for the cathode side, a reduction-resistant/high-strength polymer is used for the anode side and a polymer with high ionic conductivity can be used as the intermediate layer. This architecture ideally takes full advantage of each polymer electrolyte and makes up for their shortcomings to achieve excellent mechanical, chemical and electrochemical properties. As a result, significant achievements in the development of MLPEs, including research publications[12,13] and patents[14], have been reported in recent years. For example, Zheng et al. introduced a PEO-Li6.4La3Zr1.4Ta0.6O12 (LLZTO)-ionic liquid (IL) transition layer at the P(VEC-co-EGDMA) (vinyl ethylene carbonate (VEC) as a monomer and poly (ethylene glycol) dimethacrylate (PEGDMA) as a cross-linking point) electrolyte/cathode interface through a solution-casting method to achieve a double-layer structure and improve the interfacial contact[15]. Li et al. designed a PEO-based multilayer electrolyte structure[16]. The sacrificial layer on the anode side could accommodate lithium deposition and hinder lithium dendrite growth[16].
This review first presents an introduction to the different types of single-layer polymer electrolytes and their advantages and disadvantages. We then discuss the design principles of MLPEs, introduce the application of these principles, present recent progress in MLPEs and conclude with the development prospects of MLPEs. This review will aid the further development of MLPEs and promote their application in the future.
SINGLE-LAYER POLYMER ELECTROLYTES: ADVANTAGES AND DISADVANTAGES
Common solid and quasi-solid polymer electrolytes can be divided into the following categories according to the functional groups/chemical bonds that interact with lithium ions: polyethers, polyesters (ester bonds in the main or side chain), fluoropolymers, polyacrylonitrile, hydrogen bonding materials and others. The different functional groups of polymers determine their performance as electrolytes regarding conductivity, oxidation and reduction resistance, flexibility and strength. These different kinds of polymer electrolytes are briefly described below.
Polyethers
The research of SPEs began with polyethers. Polyether-based SPEs, such as PEO, poly(propylene oxide), poly(ethylene glycol) methyl ether acrylate and poly(dioxolane), have strong electron-donating ether oxygen groups (-C-O-C-) and have good electrochemical compatibility with lithium metal. After adding lithium salts or additives, an ionic conductivity of 10-4 S cm-1 can be achieved at room temperature[17]. In addition, polyethers usually have good flexibility and fit well with the electrode interface[18]. However, the oxidation resistance of polyether-based SPEs is poor because the α-H of polyether is easy to lose and they cannot be coupled with high-voltage cathodes, such as LiNi1/3Co1/3Mn1/3O2 (NCM) and LiCoO2 (LCO). Moreover, their low mechanical strength makes them unable to effectively block lithium dendrites[19].
Polyesters
Polyesters are polymers that contain -COO-, where the electron-withdrawing -C=O groups coordinate with Li+ to ensure the solvation and transportation of Li+. Their highest occupied molecular orbital (HOMO) energy level is lower than polyether, so they have a wider electrochemical window. The ester group in the polyester can be in the main or side chain. Polycarbonates are a popular type of main-chain polyester. Although some polycarbonates, such as poly(ethylene carbonate), poly(propylene carbonate) (PPC) and poly(vinyl carbonate), have high a lithium-ion transference number (tLi+) and high electrochemical stability[20-22], they also have shortcomings, such as poor mechanical properties, insufficient flexibility and poor compatibility with lithium anodes[23,24]. For other polycarbonates, such as poly(trimethylene carbonate) and poly(ε-caprolactone) (PCL), the ion conductivity at room temperature is too low and the Coulombic efficiency and capacity retention of the resulting batteries are poor[25].
Ester side groups can be introduced into the polymer chain to coordinate with Li+. The most common ones are butyl polyacrylate (PBA) and polymethyl methacrylate (PMMA), both of which can resist high voltages and the latter is stable to lithium metal[26,27]. However, their ionic conductivity is so low that they can only be used in the form of GPEs with liquid electrolytes. Moreover, there is the issue of poor mechanical strength, with PBA being too soft to form a self-standing electrolyte and PMMA being hard and brittle after film formation[28].
Fluoropolymers and polynitriles
Strong electron-absorbing functional groups, such as F and cyano (C≡N) groups, in the polymer chain can lower the HOMO of SPEs and endow them with high electrochemical oxidation stability. Both polyvinylidene fluoride (PVDF) and PVDF hexafluoropropylene copolymers (HFPs) have high thermal stability and mechanical strength and a wide electrochemical window[29,30]; however, their high crystallinity is not conducive to ionic conductivity and their interfacial stability with lithium metal still needs to be improved[31]. Moreover, polynitriles contain C≡N groups, which can coordinate with the metal ions of high-voltage cathodes to alleviate the oxidation process at the electrolyte/cathode interface. The most common polynitrile is polyacrylonitrile (PAN), which has the advantages of oxidation resistance and high-temperature resistance and can be coupled with high-voltage cathodes[32]. However, the C≡N group undergoes a continuous side reaction with lithium metal, forming a thick solid electrolyte interphase (SEI) film with the main composition of Li3N, thereby seriously damaging the electrolyte/anode interface and ultimately leading to limited battery performance[33].
Hydrogen bonding materials
Hydrogen bonding materials, such as polyalcohols and polyamines, can form a hydrogen bond network and dissolve a large amount of lithium salt. Polyvinyl alcohol (PVA) is a type of polyalcohol that contains hydroxyl and acetate side groups. It has good chemical stability, hydrophilicity and mechanical strength[34]. However, plasticizers must be added to achieve reasonable ionic conductivity; moreover, they are unstable when in contact with lithium metal[35]. Similarly, polyethyleneimine is a type of polyamine with a structure and Li-ion transport mechanism similar to PEO. Li+ ions coordinate and hop between different nitrogen sites in the main chain, branched chain and terminal group. The amine group reduces the binding energy of lithium ions and anions and improves the conductivity[36], but its poor mechanical performance hinders its practical application. A comparison of the electrochemical and mechanical properties of different polymer electrolytes is presented in Table 1.
Comparison of electrochemical and mechanical properties of different polymer electrolytes
| Component | Ionic conductivity (S cm-1) | Electrochemical window (V) | Strength (MPa) | Strain (%) | References | |
| Polyether | PEO/LLZO | 5.6 × 10-4 (60 °C) | 4.75 | 1.0 (shear) | 2092 | [37,38] |
| Polyester | PPC-LiTFSI/PPC-LiTFSI-SN | 5.77 × 10-4 (55 °C) | 4.7 | 30.3 | 56 | [39] |
| Fluoropolymer | PVDF-LiBOB | 7.0 × 10-5 (25 °C) | 4.7 | 3.5 | 210 | [40,41] |
| Polynitrile | PAN-LiClO4 | 1.8 × 10-5 (60 °C) | 4.9 | 7.7 | 264 | [42] |
| Polyalcohol | (PVA-PEG) + NaNO3 | 4.32 × 10-6 (20 °C) | 4.0 | 1.39 | 68 | [43,44] |
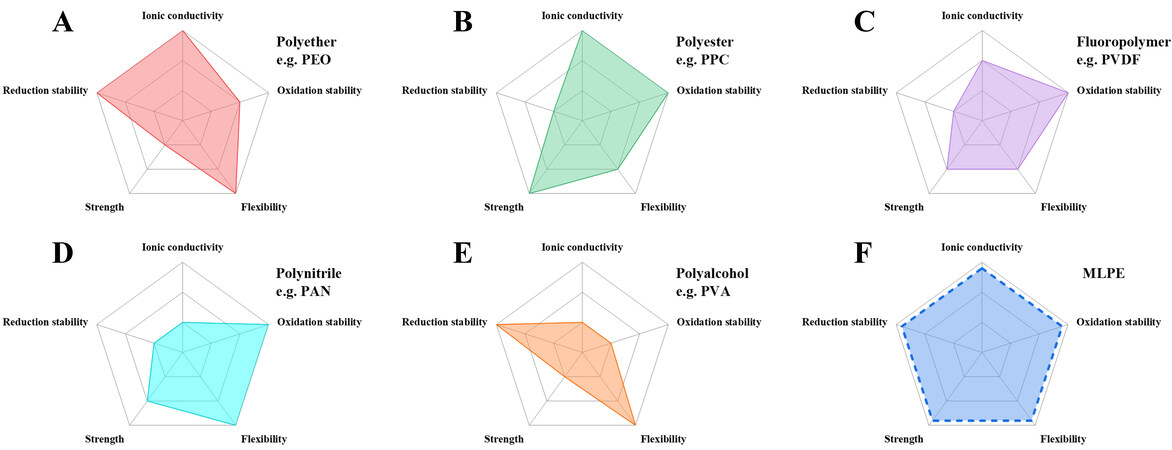
Even though significant progress has been achieved for the above-mentioned polymer systems, it is difficult for a single-layer electrolyte to simultaneously meet the requirements of high conductivity, compatibility with high-voltage cathodes and lithium anodes (oxidation and reduction stability), resistance to lithium dendrites (strength) and the volume change of the cathode (flexibility), as summarized in Figure 1. Interestingly, some of these requirements conflict with each other, e.g., oxidation and reduction stability, strength and flexibility. Therefore, to achieve a good combination, two or more component polymer electrolytes can be stacked to prepare MLPEs, as shown in Figure 1F. MLPEs can be divided into all-solid-state and quasi-solid-state electrolytes. Next, we introduce the design principles that should be followed to develop desirable MLPEs.
DESIGN PRINCIPLES OF MLPEs
MLPEs have the potential to meet the conflicting demands mentioned above and also improve battery safety. A desirable MLPE should follow the four design principles of high ionic conductivity, oxidation and reduction resistance, good flexibility and strength and compatibility with different polymer matrices.
Design of MLPEs with high ionic conductivity
An electrolyte must have excellent ionic conductivity and electronic insulation to reduce self-discharge. The room-temperature ionic conductivity generally needs to reach the practical value of 10-4 S cm-1 to enable the normal charge and discharge of the battery. In this aspect, the design principles of MLPEs and SPEs are similar, i.e., improving the ionic conductivity by appropriately selecting polymer matrices, lithium salts, plasticizers and fillers.
To obtain high conductivity, the matrix of MLPEs needs to have electron-donating groups, such as ether
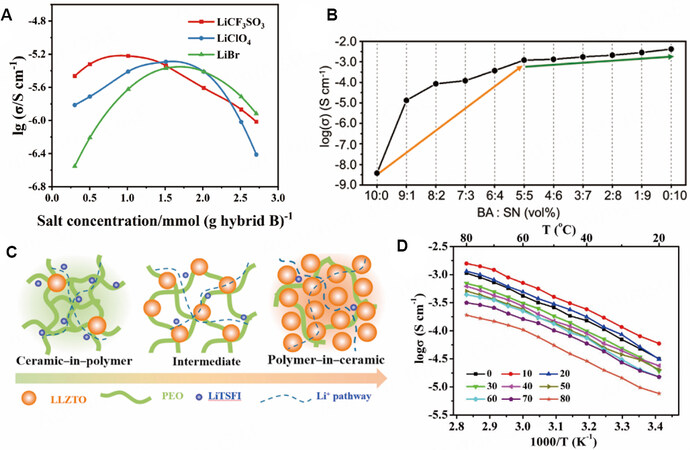
Lithium salts are the source of the charge carrier, Li+, in MLPEs, so their selection directly affects the ionic conductivity. The conductivity of an SPE or MLPE can be improved by selecting an appropriate type and content of lithium salt. To achieve high conductivity, the anions and cations in the lithium salt must be easily dissociated, i.e., the dissociation energy of the lithium salt should be low. Moreover, the high solubility in the electrolyte ensures a sufficient Li-ion concentration. One of the common types of lithium salt is lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), which has a large anion radius and is composed of N atoms with strong electronegativity and two S atoms connected with strong electron-withdrawing groups (-CF3)[48]. Increasing the concentration of lithium salts properly can increase the number of carriers in MLPEs to improve the ionic conductivity. However, too much lithium salt will hinder Li-ion conduction because of the increased viscosity and decreased Li-ion mobility. As a result, conductivity-salt concentration plots usually exhibit a maximum value, as shown in Figure 2A[49]. For LiCF3SO3, when the concentration is
Figure 2. Methods to improve ionic conductivity of SPEs. (A) Relationship between content of different lithium salts and Li-ion conductivity. Reproduced with permission[49]. Copyright, Elsevier 2003. (B) Influence of SN plasticizer content on ionic conductivity. Reproduced with permission[50]. Copyright, Springer Nature 2022. (C) Schematic images of transfer process of composite electrolytes with different contents of ceramic fillers. (D) Arrhenius plots of (1-x) wt.%[PEO8-LiTFSI]-x wt.% LLZTO composite electrolyte. Reproduced with permission[52]. Copyright, Elsevier 2018.
Plasticizers can be added to MLPEs to lower the glass transition point, promote the movement of chain segments and then improve the ionic conductivity. Plasticizers can generally be divided into three categories: plastic crystals[50]; organic solvents[26]; ILs[51]. For example, as shown in Figure 2B, when the content of succinonitrile (SN), a plastic-crystal plasticizer, increases to 50 vol.%, the conductivity of the gel electrolyte rapidly increases from 10-6 to 1 mS cm-1 (orange arrow), proving that the use of a plastic crystal plasticizer can effectively provide fast lithium-ion transport channels[50].
Fillers in MLPEs can also improve the ionic conductivity of the battery by reducing the crystallinity of the polymer matrix through interaction with the polymer matrix and lithium salt. Fillers can be divided into two types, namely, in-active and active fillers. In-active fillers are usually lithium-free inorganic solid materials (e.g., Al2O3, SiO2 and TiO2), which can effectively reduce the crystallinity of the polymer matrix, improve the mobility of the chain segment and enhance the Li-ion conductivity. It is noteworthy that some in-active fillers can be used as Lewis bases to complex with Li ions to improve the dissociation of lithium salts and can also be used as Lewis acids to combine with anions to increase the Li-ion migration number of polymer electrolytes. Active fillers generally include solid-state electrolyte materials [e.g., LLZTO,
Design of MLPEs with good oxidation and reduction stability
The MLPEs used in batteries with high-voltage cathodes and lithium metal anodes should exhibit good oxidation and reduction resistance. On the cathode side, the large contact area between the electrolyte and the cathode makes MLPEs susceptible to oxidative decomposition with the catalytic effect of transition metal ions or conductive carbon[45,53]. In addition, some functional groups (C-O and C-H) have poor stability under high voltages, which can lead to problems such as unstable contact of the cathode/electrolyte interface, capacity attenuation and so on. For the anode side, the continual reduction of the polymer may cause the volume change of the polymer, leading to the formation of a thick SEI layer with poor Li-ion conduction. The oxidation and reduction stability can be effectively enhanced by combining appropriate polymer matrices and lithium salts or adding fillers.
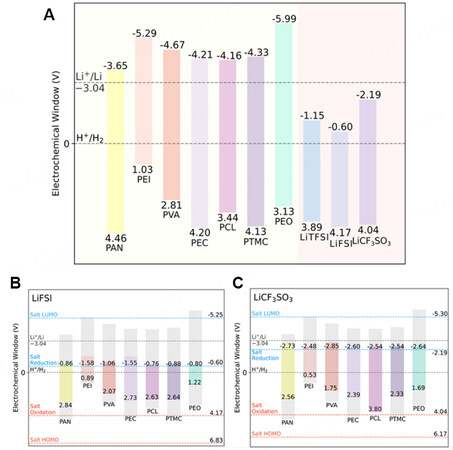
The theoretical calculation of molecular orbitals provides a useful guide to developing polymers with low HOMO energy and high lowest unoccupied molecular orbital (LUMO) energy. The gap between the HOMO and LUMO can be used to estimate the electrochemical stability window (ESW) of the electrolyte. A polymer with a lower HOMO energy level will have stronger oxidation resistance, so it is more suitable for coupling with a high-voltage cathode. Similarly, the higher the LUMO energy level, the stronger the reduction resistance of the polymer and there will be no continuous side reaction when contacting the lithium anode. The ESWs of several pristine polymers are shown in Figure 3A[54]. PAN exhibits one of the widest ESWs (~8.11 V vs. SHE) and has the highest oxidation potential (4.46 V vs. SHE). The calculation of HOMO and LUMO energy levels provides an intuitive basis for the design of MLPEs.
Figure 3. (A) Redox potentials for typical pristine polymers and lithium salts. (B) Redox potentials of (B) polymer/LiFSI and (C) polymer/LiCF3SO3 complexes. Reproduced with permission[54]. Copyright, ACS 2020.
Figure 3A also shows the redox potentials of three kinds of common lithium salts (i.e., LiTFSI, lithium bis(fluorosulfonyl) imide (LiFSI) and LiCF3SO3), which also play an important role in improving the oxidation and reduction resistance of MLPEs. When the polymer interacts with lithium salts, the redox potential changes. Unfortunately, this interaction usually narrows the ESW of the electrolyte, as proved by Marchiori et al. who used density functional theory to compare the ESWs for pristine polymers, salts and the corresponding polymer/salt complexes[54]. For example, the PEO/LiFSI complex decreases the ESW of 9.12 V in pristine PEO to 2.02 V (vs. SHE, as shown in Figure 3B). The less positive oxidation potential of the polymer/salt complexes compared to the pristine materials was attributed to the structural reorganization after oxidation with the counter anion. They also found that the polymer/LiCF3SO3 complexes displayed the largest ESW among the considered complexes (as shown in Figure 3B and C), with an order of polymer/LiCF3SO3 > polymer/LiTFSI > polymer/LiFSI. This ESW assessment is helpful for designing desirable polymer/salt complexes for MLPEs.
Moreover, the filler itself, whether organic or inorganic, has good electrochemical stability. Organic fillers can be added to MLPEs to participate in the formation of the cathode electrolyte interphase (CEI) layer on the cathode surface and improve the stability of the cathode/MLPE interface[55]. On the anode side, the improvement mechanism of reduction stability is due to the bond formation or interaction between the functional groups of the organic fillers and polymer matrices, which raises the LUMO energy of the polymers[56]. For ceramic fillers, the strategy is to take advantage of the Lewis acid-base interaction between the anions, ceramic particles and polymer chain to modify the chemical environment of the polymer matrix, thereby inhibiting the reductive decomposition of the polymer matrix at low voltages and facilitating the dissociation of lithium salts[57,58].
Design of flexible and strong MLPEs
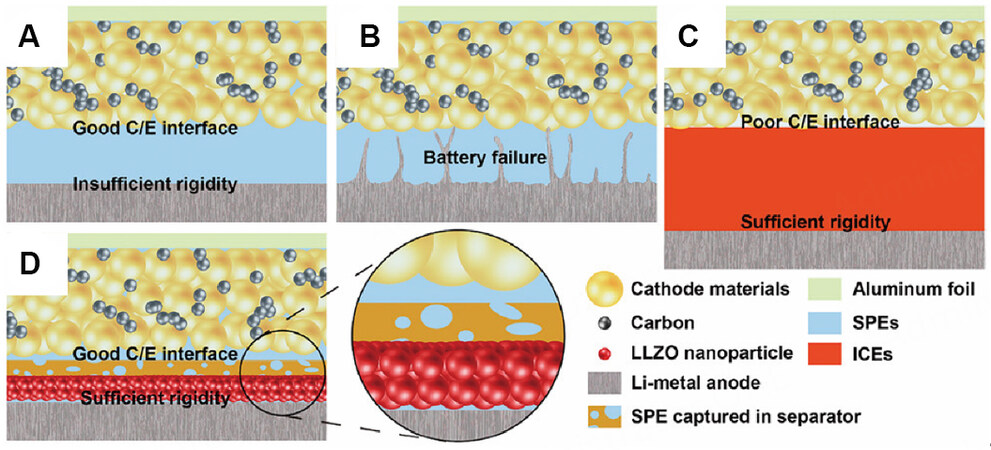
Poor interfacial contact between an MLPE and cathode usually causes large interfacial resistance, which may even prevent the battery from operating. Moreover, the volume change of the active material in the cathode caused by ion insertion/extraction leads to strain accumulation near the interfaces. Therefore, it is necessary to realize full and close interfacial contact between the cathode and electrolyte to enhance the electrochemical performance of polymer electrolytes. The SPE shown in Figure 4A has low interfacial resistance, but lithium dendrites will form due to its low mechanical strength [Figure 4B]. In contrast, the mechanical properties of inorganic ceramic electrolytes (ICEs) are sufficient to resist lithium dendrites, but the contact between rigid ICEs and electrodes is poor, resulting in high interfacial resistance [Figure 4C][59]. Duan et al. used dense LLZO electrolytes on the lithium metal side and poly(ethylene glycol) methyl ether acrylate electrolytes with almost no LLZO on the cathode side [Figure 4D][60]. The soft polymer electrolyte layer prepared by in-situ polymerization was in full contact with the active material, resulting in a good electrode/electrolyte interface and the promotion of the continuous transmission of Li ions.
Figure 4. Schematic of solid-state Li battery with (A and B) conventional SPEs, (C) ICEs and (D) MLPEs. Reproduced with permission[60]. Copyright, ACS 2018.
To solve the problem of the polymer electrolyte/lithium anode interface, attention should not only be given to the formation of a stable SEI layer, and it should also be ensured that the MLPE has high mechanical properties. Only when the shear modulus of the electrolyte material (> 6 GPa) is 1.8 times that of lithium metal (~4.25 GPa) can it effectively suppress dendritic growth on the surface of a lithium anode[61,62]. A polymer with good strength and high reduction resistance was selected as the matrix of a polymer electrolyte to form a stable anode/electrolyte interface, thus hindering the growth of lithium dendrites[63]. On this basis, a small amount of filler/skeleton can be added to the polymer electrolyte matrix to increase its elastic modulus. However, the increase in the elastic modulus of the MLPE leads to a decrease in the interfacial contact[64]. Therefore, it is of great importance to increase the compatibility of the interface while preserving the sufficient mechanical strength of the MLPE.
Design of MLPEs with good compatibility between two polymer electrolytes
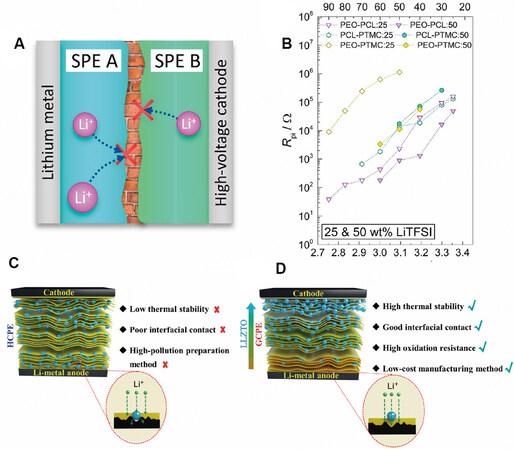
The polymer matrix in different layers in MLPEs may be different, so the interface between individual polymers cannot be ignored because of its high impedance. Previous studies have shown that due to the coordination strength difference or immiscibility between the two polymer bodies, the resistance of the polymer-polymer interface can be very large and even dominate the total resistance of the whole battery
Figure 5. (A) Schematic diagram of interface between two SPEs. (B) Polymer interfacial resistance (Rpi) with different Li salt contents at temperatures ranging from 25 to 90 °C. Reproduced with permission[65]. Copyright, ACS 2021. Schematics of (C) homogeneous composite polymer electrolyte and (D) gradient composite polymer electrolyte. Reproduced with permission[66]. Copyright, John Wiley and Sons 2021.
The second method is to use the same polymer matrix and different plasticizers. A quasi-double-layer polymer electrolyte was prepared by adding two plasticizers, i.e., propylene carbonate (PC) and diethylene glycol dimethyl ether (DGM), to the same matrix of PVDF/LLZO/LiTFSI. The same basic components, which can eliminate the contact interface of different electrolytes, reduce the interfacial resistance[56].
The final method involves obtaining a filler concentration gradient within the MLPE. As shown in Figure 5C and D, LLZTO fillers with a concentration gradient change the traditional design of double-layer polymer electrolytes and have the following advantages: (1) the continuous change of composition and structure eliminates all macroscale interfaces; (2) the high and the low LLZTO content sides meet the needs of high-voltage cathodes and lithium metal anodes, respectively; and (3) the residual and thermal stresses of the interface are reduced and the good mechanical properties of the electrolyte are ensured[66]. The following section introduces the application of these four design principles with some of the recent progress for MLPEs and highlights the electrolyte design, preparation methods and electrochemical properties.
LITHIUM METAL BATTERIES BASED ON MLPEs
Preparation protocols for MLPEs
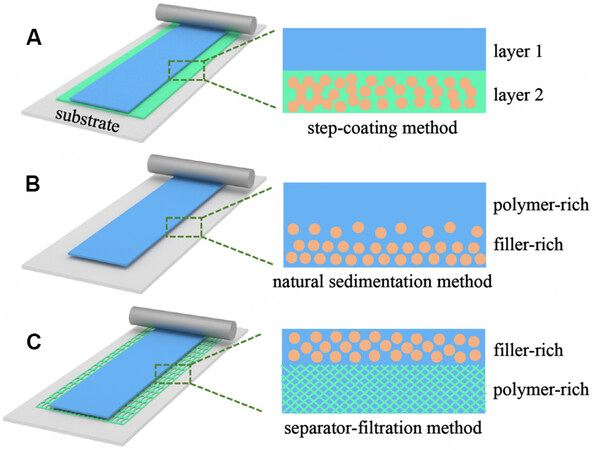
So far, researchers have developed a variety of methods to prepare MLPEs, which can be mainly divided into step-coating, natural sedimentation and separator-filtration methods, as shown in Figure 6. In the step-coating method, different polymer solutions consisting of different polymer matrices, lithium salts or different fillers are first prepared. These solutions are then coated stepwise on a cathode or anode substrate to make the MLPEs with the structure shown in Figure 6A. This method is versatile, straightforward and effective in preparing MLPEs to meet the different demands of high-voltage cathodes and lithium metal anodes. However, the disadvantage of this approach is that a new interlayer interface is introduced and the compatibility between layers needs to be considered.
Figure 6. Preparation of MLPEs: (A) step-coating method; (B) natural sedimentation method; (C) separator-filtration method.
The natural sedimentation and separator-filtration methods are usually used to prepare MLPEs with the same polymer matrix and different fillers. In the natural settlement method, a polymer-filler mixture is coated directly on the substrate. A gradient distribution of the filler is achieved through a standing step by taking advantage of the density difference between the polymer and the filler to have the filler settle with gravity, as shown in Figure 6B. In this manner, a rigid filler-rich layer helps suppress lithium dendrite formation and a soft polymer-rich layer helps to make good contact with the cathode. This method has few process steps, but the distribution of the filler is related to the particle size, solution viscosity, coating thickness and standing time, resulting in higher requirements for the raw materials and process control. To gain improved process control, the separator-filtration method is used, where a polymer-filler mixture is coated on a separator by reasonably selecting the pore size of the separator and the diameter of the filler. After settling, a heterogeneous structure, as displayed in Figure 6C, is achieved, with a filler-rich layer above the separator and a polymer-rich layer within the separator. This method can effectively avoid the introduction of the new interface and the compatibility problem, but it needs a separator, which may complicate the electrolyte structure and affect the ionic conductivity.
MLPEs using different polymer matrices
In combination with the design principles described above, the most common MLPE involves combining two polymer electrolytes with high ionic conductivity. The one on the cathode side is soft and oxidation resistant, while the one on the anode side is hard and reduction resistant. They are usually prepared by a two-step method via an ex-situ or in-situ route.
The ex-situ two-step process refers to the preparation of two kinds of solid electrolyte membranes by tape-casting and then combining the two membranes into a whole by hot pressing or other methods. The compatibility and adhesion between the two polymer matrices need to be high to reduce the interfacial impedance between them. For example, Yao et al. prepared LATP-reinforced PVDF-HFP-based composite electrolytes on the cathode side and PEO-LATP composite electrolytes on the anode side[67]. The MLPE was then formed by hot pressing at 60 °C with the help of the internal pressure of the battery itself. As shown in Figure 7, PEO has good film-forming properties and excellent stability to lithium metal, while PVDF-HFP can be coupled with the NCM111 cathode because of its excellent oxidation resistance. The interfacial resistance between them can also be reduced by placing it at 60 °C. The tLi+ reached 0.43 and the capacity of NCM111/MLPE/Li was still 105 mAh g-1 after 100 cycles at 0.2 C.
Figure 7. Schematic diagram of preparation method of (A) PEO SPE, (B) PEO-LATP electrolyte and (C) MLPE via an ex-situ two-step process. Reproduced with permission[67]. Copyright, ACS 2021.
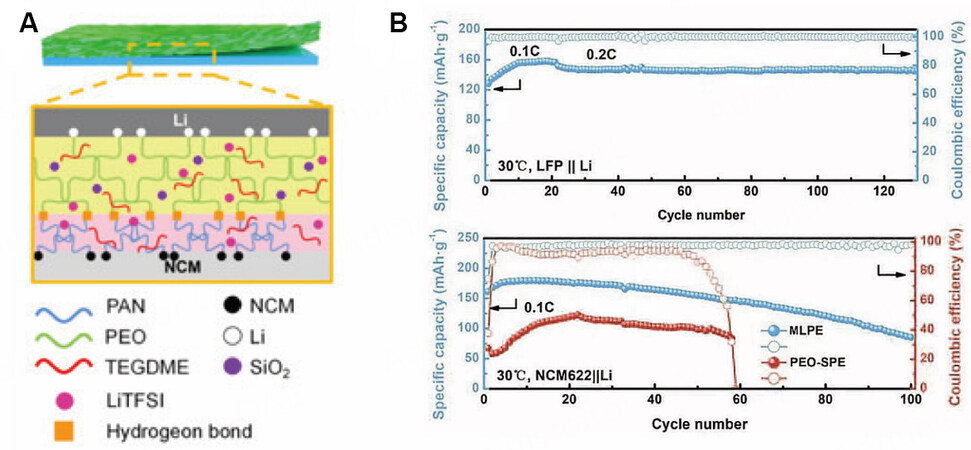
The in-situ two-step synthesis method covers the second polymer precursor or solution onto the surface of the first layer and forms an integrated structure through photocuring or solvent volatilization, respectively. This method ensures good interfacial contact while avoiding mutual penetration between the two layers.
Figure 8. (A) Schematic of MLPE prepared via an in-situ two-step synthesis method. (B) Electrochemical performance of NCM622/Li using MLPE and PEO-SPE. Reproduced with permission[33]. Copyright, MDPI 2022.
Nevertheless, the interface between the two electrolytes cannot be ignored when two different polymers are used as the matrix. Considering the design principles discussed above, it may be desirable to use a single polymer as the matrix and change the type and/or content of lithium salt, plasticizer and ceramic filler to reduce the impedance between the interfaces and improve the ion transport efficiency.
Same polymer matrix and different lithium salts or additives
As described above, the choice of lithium salt is of particular importance in designing MLPEs. Specifically, the lithium salt is related to ionic conductivity, electrochemical stability, interface formation and lithium dendrite propagation[68]. The lithium salt also plays an important role in controlling the ESW of MLPEs. There are two mechanisms to broaden the ESW. One is to adjust the frontier orbital energy level of electrolytes through thermodynamics. The interaction between the polymer segment, salt (cation and anion) and additives can change the HOMO and/or LUMO of the electrolyte[69]. The other mechanism is to control the formation of the dynamically stable interphase (i.e., CEI and SEI) on the electrode surface to suppress direct reactions between the electrolyte and electrode materials. Anions that migrate to the electrode participate in the formation of a stable CEI and SEI.
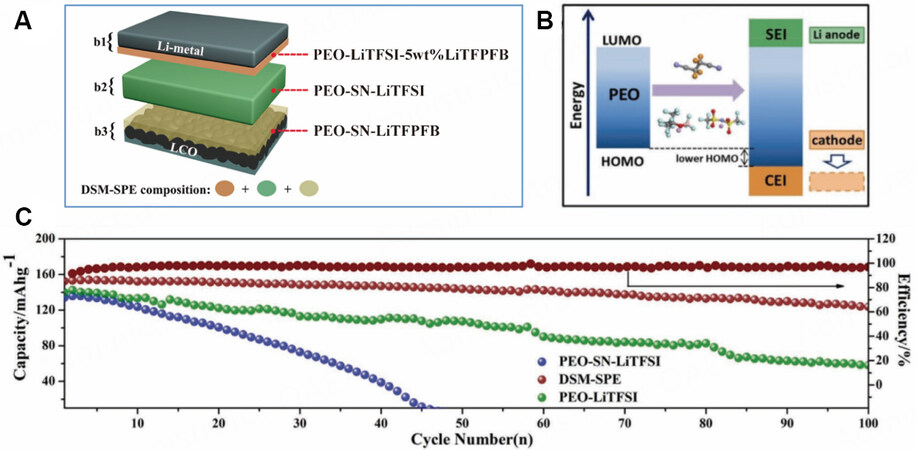
MLPEs with the same polymer matrix can be prepared by changing the types of lithium salts. Wang et al. designed a three-layer polymer solid electrolyte, as shown in Figure 9[70]. The anode-side component was PEO-LiTFSI-lithium trifluoro(perfluoro-tert-butyloxyl) borate) (LiTFPFB), the cathode-side component was PEO-SN-LiTFPFB and the intermediate layer was PEO-SN-LiTFSI. LiTFPFB on both electrode sides helps to form a stable contact layer while preventing side reactions between SN and Li and widening the HOMO-LUMO gap of the electrolyte [Figure 9B]. Based on the complexation reaction between lithium salts and the polymer matrix, the ether oxygen donates the lone electron pairs to the Li ions of the lithium salts. Thus, the α-H of PEO is not easy to lose, leading to the lowering of the HOMO energy level of PEO. The electrolytes on the cathode and anode sides were prepared by an in-situ method to reduce the interfacial impedance with the electrode. As a result, the Li/Li symmetric batteries could cycle for more than 600 h at a current density of 0.25 mAh cm-2. The high-voltage LiCoO2/Li battery could be cycled 100 times at 0.5 C with a discharge capacity of 119 mAh g-1 and maintain a capacity retention rate of 83.3% at a rate of 0.1 C [Figure 9C].
Figure 9. (A) Schematic of MLPE with different lithium salts. (B) Schematic of widened ESW of PEO matrix with interaction of lithium salts. (C) Cycling performance of LiCoO2/Li batteries with PEO-SN-LiTFSI, PEO-LiTFSI and MLPE at 0.1 C (1 C = 155 mAh g-1) with a voltage range of 2.5-4.3 V at 60 °C. Reproduced with permission[70]. Copyright, John Wiley and Sons 2019.
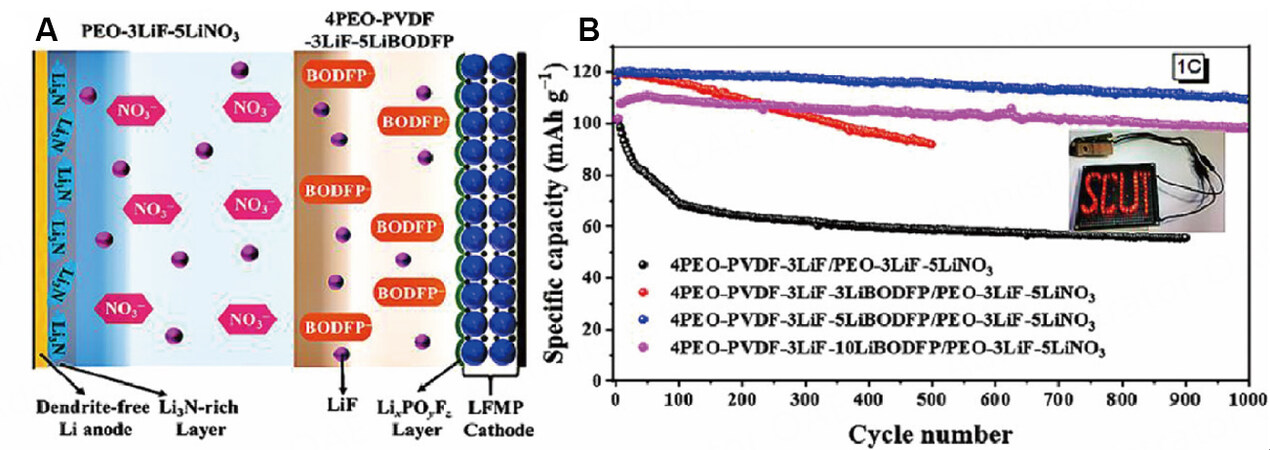
In addition, additives can be introduced into MLPEs to adjust the composition and structure of the CEI and/or SEI layers. Li et al. first reported a PEO-based heterogeneous MLPE containing a lithium bisoxalatodifluorophosphate (LiBODFP) additive on the cathode side[71]. LiBODFP can induce the formation of a stable CEI layer, prevent continuous decomposition and resist a high voltage of 5.0 V vs. Li/Li+. Simultaneously, the LiF on the LiFexMn1-xPO4 (LFMP, 0 < x ≤ 0.5) cathode side can stabilize the PEO matrix and LLZTO can reduce the crystallinity of the polymer to improve the ionic conductivity and enhance the mechanical strength. Furthermore, the additive of LiNO3 on the anode side promotes stable SEI formation to protect lithium from dendrite formation [Figure 10A]. After the LFMP/Li battery was cycled 1000 times in the range of 2.5-4.5 V vs. Li/Li+ at 1 C, the capacity retention rate was still 90.6%, as shown in Figure 10B.
Figure 10. (A) Schematic of MLPE with different additives. (B) Cycling performance of LFMP@C/Li cells with MLPE at 1 C in the voltage range of 2.5-4.5 V. Reproduced with permission[71]. Copyright, Elsevier 2022.
Same polymer matrix and different fillers
The design of MLPEs can be realized by using different contents of fillers. Generally, increasing the filler content in the polymer electrolyte can effectively improve the mechanical properties and inhibit the growth of Li dendrites. Moreover, a polymer layer with a high filler content can possess a wide ESW, while a polymer layer with less filler content can provide good interfacial contact and help to release the capacity of the electrode. Therefore, through the reasonable design of MLPEs, both short-circuit resistance and good interfacial contact can be achieved simultaneously. Therefore, researchers have attempted to prepare MLPEs with asymmetric filler content by step-coating, natural sedimentation and separator-filtration methods.
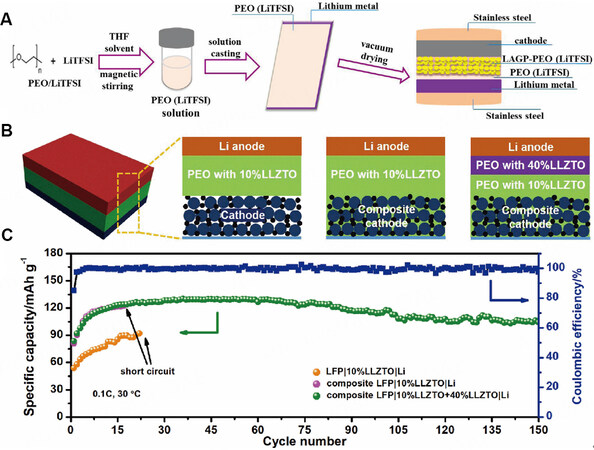
The step-coating method includes an ex-situ and in-situ route. Wang et al. employed PEO as a matrix to design an MLPE based on LAGP and PEO through an ex-situ step-coating method[72], as shown in Figure 11A. The optimized LAGP-PEO (LiTFSI) layer exhibits good electrochemical stability (> 5.12 V vs. Li/Li+), which means that this polymer layer can match with the high-voltage cathode material (LFMP). Moreover, the PEO (LiTFSI) layer exhibits good interfacial contact and mechanical properties, which can effectively improve the capacity retention rate and discharge-specific capacity of the half-cells. Under the combination of the use of these two layers, the all-solid-state half-cell delivered a discharge capacity as high as 160.8 mAh g-1 at 50 °C. In another work, Bi et al. developed an in-situ step-coating method to prepare a MLPE composed of PEO and LLZTO filler [Figure 11B][73]. In this MLPE, PEO with 10 wt.% LLZTO near the cathode side exhibits the optimum ionic conductivity while PEO with 40 wt.% LLZTO near the anode side possesses excellent mechanical properties for inhibiting the growth of lithium dendrites. Such a solid-state battery design presented a discharge capacity of 129 mAh g-1 and a cycle life of over 150 cycles [Figure 11C].
Figure 11. (A) Preparation of Li/LFMP cells with MLPE through an ex-situ step-coating method. Reproduced with permission[72]. Copyright, ACS 2017. (B) Schematic of three kinds of battery configurations: LFP/10%LLZTO/Li; composite LFP/10%LLZTO/Li; composite LFP/MLPE/Li. (C) Cycling performance for these three batteries at 30 °C. Reproduced with permission[73]. Copyright, Elsevier 2021.
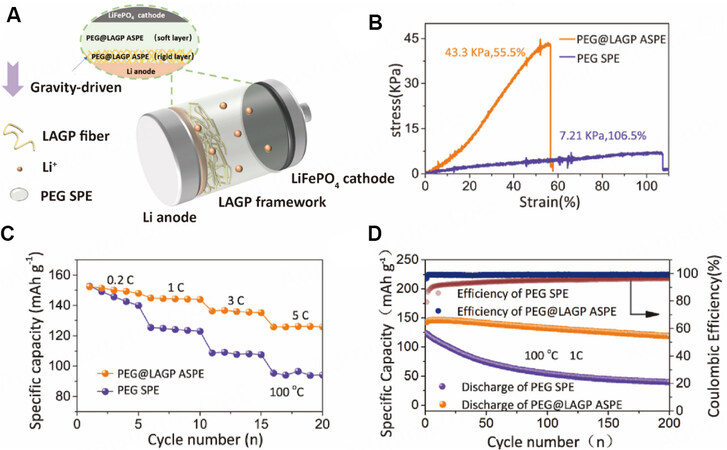
The natural sedimentation method is a simple and operable method for the in-situ preparation of MLPEs. Yang et al. designed a gravity-driven MLPE, as shown in Figure 12A[74]. The rigid layer consisting of a polymer-embedded 3D continuous LAGP framework efficiently increases the tLi+ and critical current density. The soft poly(ethylene glycol) methyl methacrylate layer prepared by thermal polymerization has good interfacial contact with the cathode. These two layers are naturally layered by gravity during polymerization. The electrolyte showed a tensile strength of up to 43.3 kPa, as shown in Figure 12B, while LFP/PEG@LAGP/Li cells provided significantly improved electrochemical performance compared to LFP/PEG/Li cells at high current density and 100 °C [Figure 12C and D].
Figure 12. (A) Schematic diagram of gravity-driven MLPE structure. (B) Stress-strain curves of PEG SPE and PEG@LAGP MLPE.
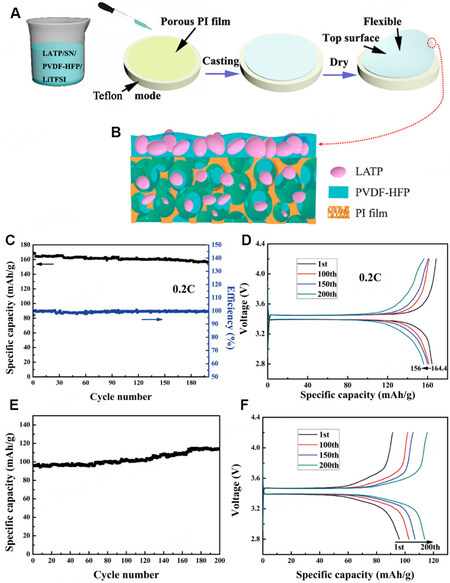
A membrane with micropores can allow the permeation of the polymer to the lower layer while leaving the ceramic fillers in the upper layer to form an MLPE. Gai et al. prepared an MLPE using a porous polyimide film, as shown in Figure 13A and B[75]. PVDF-HFP and LATP are used as the polymer matrix and ceramic filler, respectively, and SN is added to achieve high conductivity (1.28 × 10-4 S cm-1). The LFP/MLPE/Li battery exhibits a reversible discharge capacity of 156 mAh g-1 after 200 cycles at a rate of 0.2 C and the Coulombic efficiency is greater than 98% [Figure 13C and D]. It is noteworthy that the battery can work at
Figure 13. (A and B) Schematic diagrams of fabrication process of MLPE with separator-filtration. (C and E) Long-term cycling performance of LFP/MLPE/Li cell for 0.2 C at (C) 25 and (E) 4 °C. (D and F) Voltage-capacity profiles of 1st, 100th, 150th and 200th cycles for 0.2 C at (D) 25 and (F) 4 °C. Reproduced with permission[75]. Copyright, ACS 2019.
The above-mentioned methods can also be combined to prepare MLPEs. For example, Deng et al. combined the step-coating and natural sedimentation methods[66]. They first settled LLZTO in (poly(ethylene glycol) diacrylate (PEGDA) under gravity and cured it once under ultraviolet light. Next, when PEGDA without LLZTO was added, the LLZTO content in the MLPE after complete curing showed a gradient distribution, as shown in Figure 5D. The as-prepared MLPEs did not have any macroscale interfaces and exhibited reduced residual and thermal stress with phase separation avoided during charging and discharging.
It is noteworthy that there is some discrepancy in which side of the MLPEs, the filler-concentrated side or the polymer-rich side, faces the cathode in the above examples. In Refs.[66,72,75], the filler-concentrated side was chosen to face the cathode, while in Refs.[73,74], the polymer-rich side was chosen. The former group claims that the porous filler-concentrated side with a wide ESW is oxidation resistant to the cathode and the thin dense polymer-rich side provides good interfacial contact to reduce Li dendrite growth. In contrast, the latter group states that the filler-concentrated side brings enhanced mechanical strength and Li+ transference number to resist lithium dendrites, while the soft polymer-rich layer makes good interfacial contact with cathodes. Different MLPE arrangements can be selected according to different considerations, e.g., the specific electrode configuration and demands of the battery.
Quasi-solid-state multilayer GPEs
Despite the flexibility of MLPEs provided by the polymer matrix, there may still exist inherent gaps and unstable connection reactions at the solid-solid interface between the electrolyte and the electrode. Accordingly, a small amount of liquid electrolyte can be added to form a multilayer gel polymer electrolyte (MLGPE) for potential comprehensive performance advantages, including high ionic conductivity, good interfacial contact and a wide operating temperature range. Similar to the classification of MLPEs, this section classifies MLGPEs according to their components and divides them into MLGPEs with different polymer matrixes, namely, MLGPEs with the same polymer matrix and different plasticizers and MLGPEs with the same polymer matrix and different fillers.
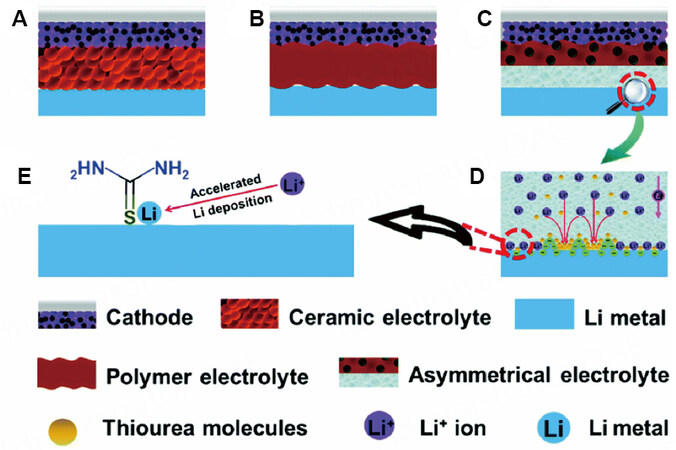
Different layers of MLGPEs may have different polymer matrices. For instance, Wang et al. coated a soft PEO-LLZO composite electrolyte on one side of a separator facing the cathode to guarantee good interfacial compatibility and coated a thin PVDF-HFP gel-electrolyte with thiourea (THU) additive on the other side towards the Li metal[76]. The separator contained a large number of submicron-connected pores to adsorb liquid [Figure 14]. The THU additive on the anode side could accelerate the speed of lithium adsorption on the anode, thereby forming a flat SEI to inhibit the formation of lithium dendrites and promote the electrochemical kinetic process. Compared with a pure ceramic electrolyte [Figure 14A] and a polymer electrolyte [Figure 14B], the 45 μm-thick MLGPEs showed improved electrochemical performance. The LFP/MLGPE/Li battery showed stable cycling performance over 200 cycles at 1 C with a Coulombic efficiency of 99.6%.
Figure 14. Schematic comparison of solid-state Li batteries with three different electrolytes: (A) an inorganic ceramic electrolyte; (B) a typical polymer electrolyte; (C) MLGPE. (D and E) Schematic description of effect of THU additive in MLGPE on Li deposition. Reproduced with permission[76]. Copyright, Royal Society of Chemistry 2020.
Low anodic reactivity and high voltage compatibility can be achieved by adding different kinds of plasticizers to the same polymer matrix. Pan et al. added different plasticizers, namely, PC and DGM, to a PVDF/LLZO/LiTFSI composite[56]. The side with the PC faces the cathode because the in-situ-polymerized PC can serve as a CEI film and enhance the antioxidation ability. In contrast, on the anode side, the nucleophilic substitution of DGM with PVDF increases the reduction stability and suppresses the formation of lithium dendrites. Moreover, because of the use of the same matrix, there is no interfacial resistance of different electrolytes, so the MLGPE exhibits an ionic conductivity of 1.3 × 10-4 S cm-1. The cycling stability of NCM811/MLGPE/Li and NCM811/MLGPE/hard carbon batteries is better than that with GPE added with PC and DGM only. The capacity retention rate of NCM811/MLGPE/hard carbon batteries was 80.2% after 200 cycles at 1 C and 25 °C.
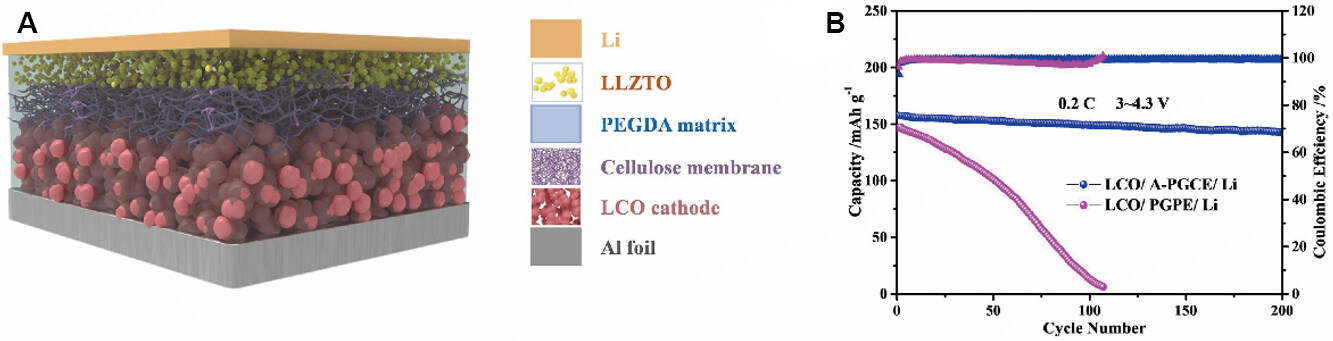
MLGPEs can also be prepared by controlling the filler content at different positions in the same polymer matrix. Cai et al. developed a simple and environmentally-friendly in-situ polymerization method by dropping a mixture of PEGDA monomer[77], liquid electrolyte and LLZTO ceramic particles on a cellulose membrane and using gravity to allow it to settle naturally [Figure 15A]. After thermal polymerization, an MLGPE rich in LLZTO at the top could be obtained. The LLZTO-rich side faced the lithium anode during cell assembly to take advantage of its improved mechanical properties to inhibit the growth of Li dendrites. Subsequently, the MLGPE showed an ionic conductivity of 1.25 × 10-3 S cm-1 and tLi+ of 0.57. The LCO/MLGPE/Li cell showed an initial discharge capacity of 157.6 mAh g-1 in the voltage range of 3.0 to
Figure 15. (A) Schematic of LCO/MLGPE/Li cells via an in-situ fabrication process. (B) Long-term cycling performance of cell at 0.2 C and room temperature. Reproduced with permission[77]. Copyright, Elsevier 2022.
Regarding the MLGPE preparation, some MLGPEs may traditionally need an additional ex-situ step to incorporate plasticizers by immersing the dry film into the liquid electrolyte. In this method, toxic organic solvents are employed to dissolve polymers and lithium salts, which are then removed by environmentally unfriendly evaporating and/or heating. In addition, the residual solvent in the MLGPE may react with the electrode, resulting in the degradation of battery performance. Furthermore, many recent studies have attempted an in-situ polymerization technology, which directly converts liquid precursors into solid electrolytes by crosslinking polymer monomers under controlled conditions. This not only eliminates the use of polluting and toxic organic solvents but also reduces the electrolyte/electrode interfacial resistance by injecting the liquid precursor directly into the battery during cell assembly[78]. The in-situ polymerization also helps to improve the mechanical strength of polymer electrolytes[18]. In addition, the mechanical strength of MLGPEs with ceramic fillers can also be significantly improved. Therefore, in-situ polymerized MLGPEs reinforced with mechanically strong ceramic fillers are especially promising as serious electrolyte contenders for future high-energy-density lithium batteries.
CONCLUSION AND PERSPECTIVES
This review has systematically summarized the typical polymer electrolytes and their advantages and disadvantages, introduced the concept and design principles of MLPEs and presented the recent progress in MLPEs and MLGPEs from the aspects of matrices, additives, lithium salts and ceramic fillers. MLPEs have the potential to meet the mechanical properties and voltage requirements of the cathode and anode while simultaneously having high conductivity and good electrochemical performance. We propose the following directions as being worthy of future research.
In-situ preparation process
The preparation process of MLPEs is mainly divided into two methods: ex-situ and in-situ. Although the
Computation-guided polymer design
Though the present review of the recent progress in MLPEs has mainly focused on experimental work, theoretical computation is expected to have a profound influence on exploring the properties of MLPEs and designing new MLPEs. For instance, one of the advantages of MLPEs is their wide ESW. The experimental determination of the ESW (e.g., optical absorption spectra and cyclic voltammetry) is normally time-consuming and provides insufficient electronic information, such as the polymer band edges. Density functional theory (DFT) has been used to unravel the role of the polymer matrix in determining the ESW of polymer electrolytes[80]. With the rapid development of computing power, future work can be directed to the utilization of computation to understand the complex interplay of other factors affecting the ESW of MLPEs, such as the chemical and morphological variations present in the polymer matrix, interactions between the polymer matrix and the lithium salts, and the evolution of the electrode/electrolyte interfaces. As an example, Yuan et al. used a multi-physical field model to simulate the change in the growth direction and shape of lithium dendrites by adding heterogeneous reinforcements to the polymer matrix and comprehensively studied the factors controlling the dendrite inhibition effect[81].
More recently, the field of polymer informatics has emerged, which aims to accelerate polymer property prediction and design via surrogate machine learning models that are built on reliable previous data. An online platform known as the Polymer Genome has been set up by Doan Tran[82] to incorporate various models and datasets. This paradigm shift based on high-throughput computation and data science approaches promises to speed up polymer-matrix screening, novel polymer discovery and even the optimization of composition and experimental conditions. Recent work by Liu et al. compares various machine learning models based on a dataset from both literature and independently conducted experiments and finds that the random forest model is the best among six (i.e., linear regression, ridge regression, lasso regression, random forest, decision tree and a support vector machine) in terms of accuracy and predictive ability[83]. This data science approach could be extended to include other types of polymer matrices and commonly used salts, even bi- and tri-salt components.
Evaluation of interfacial compatibility between two polymers
For multilayer electrolytes, the commonly used evaluation indicators, such as the electrochemical window, lithium-ion transference number, cycling stability, and so on, mainly come from the electrochemistry community. To set up a more comprehensive evaluation system, indicators from other communities are needed. One of the greatest differences between MLPEs and homogeneous electrolytes is that the interface between different layers cannot be ignored. The interfacial resistance may play a decisive role in the overall resistance of the battery, so the compatibility between interfaces is worth optimizing and evaluating. In previous studies, the polymer-polymer interface impedance has been separated and the impedance can be related to the frequency through DRT and other methods to more accurately describe and evaluate the interfacial resistance. Simultaneously, the concept of the solubility parameter is proposed for the evaluation of the interfacial compatibility of two polymers, which is originally a parameter characterizing the polymer-solvent interaction. This concept can be applied to describe the polymer-polymer interaction. When the solubility parameters of the two materials are similar, they can be blended and have good co-solubility. It is noteworthy that the solubility parameter can also evaluate the affinity between the plasticizer and polymer matrix, and thus a better combination can be selected to prepare MLPEs.
Given the molar gravitational constant (F, J1/2 cm3/2 mol-1) and internal pressure square root (λ, J1/2 cm-3/2) of the common groups in the polymer and the molar volume of the repeating unit (Vm, cm3 mol-1), the density (ρ, g cm-3) and the molar mass (M0, g mol-1) of the polymer, the internal pressure square root and solubility parameter (Δ, J1/2 cm-3/2) of the polymer can be calculated from[84]:
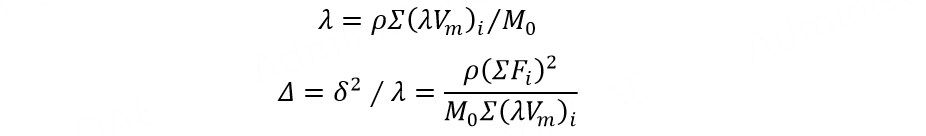
where δ is the cohesive energy density of the polymer, J1/2 cm-3/2.
Two kinds of polymer can be applied to the adjacent layers of electrolytes if the difference between their solubility parameters is less than 3. For example, the solubility parameters of PCL and poly(ethylene adipate) are 18.0 and 18.1 J1/2 cm-3/2, respectively, meaning that the co-solubility of these two kinds of polymer is good and the impedance of the polymer-polymer interface is small.
DECLARATIONS
Authors’ contributionsConceptualization: Wu S, Duan H
Data curation, formal analysis, writing - original draft: Wu S, Zhang N
Resources, technical support: Zheng H, Li G, Liu H
Funding acquisition, supervision: Duan H
Writing - review and editing: Wu S, Zhang N, Duan H
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was financially supported by the National Natural Science Foundation of China (no. 51972211).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participate.Not applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
3. Zheng H, Li G, Liu J, et al. A rational design of garnet-type Li7La3Zr2O12 with ultrahigh moisture stability. Energy Stor Mater 2022;49:278-90.
4. Zou C, Yang L, Luo K, et al. In situ formed protective layer: toward a more stable interface between the lithium metal anode and
5. Zhou D, Shanmukaraj D, Tkacheva A, Armand M, Wang G. Polymer electrolytes for lithium-based batteries: advances and prospects. Chem 2019;5:2326-52.
7. Fenton D, Parker J, Wright P. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973;14:589.
8. Feuillade G, Perche P. Ion-conductive macromolecular gels and membranes for solid lithium cells. J Appl Electrochem 1975;5:63-9.
9. Quartarone E, Mustarelli P. Electrolytes for solid-state lithium rechargeable batteries: recent advances and perspectives. Chem Soc Rev 2011;40:2525-40.
10. Zou C, Yang L, Zang Z, et al. LiAlO2-coated LiNi0.8Co0.1Mn0.1O2 and chlorine-rich argyrodite enabling high-performance all-solid-state lithium batteries at suitable stack pressure. Ceram Int 2023;49:443-9.
11. Aldissi M. Multi-layered polymer electrolytes towards interfacial stability in lithium ion batteries. J Power Sources 2001;94:219-24.
12. Zhou W, Huang Q, Xie X, et al. Research progress of polymer electrolyte for solid state lithium batteries. Energy Stor Sci Technol 2022;11:1788-805.
13. Wang H, Liu J, He J, et al. Pseudo-concentrated electrolytes for lithium metal batteries. eScience 2022;2:557-65.
14. Ko D, Wook Y, Kyung D, et al. Polymer electrolyte having multi-layer structure, and all-solid battery comprising same; 0. Available from: https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2017074116 [Last accessed on 7 Feb 2023].
15. Zheng X, Wu J, Wang X, Yang Z. Cellulose-reinforced poly(cyclocarbonate-ether)-based composite polymer electrolyte and facile gel interfacial modification for solid-state lithium-ion batteries. Chem Eng J 2022;446:137194.
16. Li X, Zheng Y, Fullerton WR, Li CY. Multilayered solid polymer electrolytes with sacrificial coating for suppressing lithium dendrite growth. ACS Appl Mater Interfaces 2022;14:484-91.
17. Tao X, Liu Y, Liu W, et al. Solid-state lithium-sulfur batteries operated at 37 °C with composites of nanostructured Li7La3Zr2O12/carbon foam and polymer. Nano Lett 2017;17:2967-72.
18. Zhang Y, Lu W, Cong L, et al. Cross-linking network based on Poly(ethylene oxide): solid polymer electrolyte for room temperature lithium battery. J Power Sources 2019;420:63-72.
19. Yang L, Wang Z, Feng Y, et al. Flexible composite solid electrolyte facilitating highly stable “soft contacting” Li-electrolyte interface for solid state lithium-ion batteries. Adv Energy Mater 2017;7:1701437.
20. Kimura K, Matsumoto H, Hassoun J, Panero S, Scrosati B, Tominaga Y. A quaternaryPoly(ethylene carbonate)-lithium bis(trifluoromethanesulfonyl)imide-Ionic liquid-silica fiber composite polymer electrolyte for lithium batteries. Electrochim Acta 2015;175:134-40.
21. Kimura K, Yajima M, Tominaga Y. A highly-concentrated poly(ethylene carbonate)-based electrolyte for all-solid-state Li battery working at room temperature. Electrochem Commun 2016;66:46-8.
22. Chai J, Liu Z, Ma J, et al. In situ generation of poly (vinylene carbonate) based solid electrolyte with interfacial stability for LiCoO2 lithium batteries. Adv Sci 2017;4:1600377.
23. Mindemark J, Lacey MJ, Bowden T, Brandell D. Beyond PEO-alternative host materials for Li+-conducting solid polymer electrolytes. Prog Polym Sci 2018;81:114-43.
24. Arrese-igor M, Martinez-ibañez M, Pavlenko E, et al. Toward high-voltage solid-state Li-metal batteries with double-layer polymer electrolytes. ACS Energy Lett 2022;7:1473-80.
25. Mindemark J, Törmä E, Sun B, Brandell D. Copolymers of trimethylene carbonate and ε-caprolactone as electrolytes for lithium-ion batteries. Polymer 2015;63:91-8.
26. Wu S, Zheng H, Tian R, Hei Z, Liu H, Duan H. In-situ preparation of gel polymer electrolyte with glass fiber membrane for lithium batteries. J Power Sources 2020;472:228627.
27. Guo Y, Ouyang Y, Li D, Wei Y, Zhai T, Li H. PMMA-assisted Li deposition towards 3D continuous dendrite-free lithium anode. Energy Stor Mater 2019;16:203-11.
28. Rao M, Liu J, Li W, Liang Y, Zhou D. Preparation and performance analysis of PE-supported P(AN-co-MMA) gel polymer electrolyte for lithium ion battery application. J Membr Sci 2008;322:314-9.
29. Liang Y, Deng S, Xia Y, et al. A superior composite gel polymer electrolyte of Li7La3Zr2O12- poly(vinylidene fluoride-hexafluoropropylene) (PVDF-HFP) for rechargeable solid-state lithium ion batteries. Mater Res Bull 2018;102:412-7.
30. Jie J, Liu Y, Cong L, et al. High-performance PVDF-HFP based gel polymer electrolyte with a safe solvent in Li metal polymer battery. J Energy Chem 2020;49:80-8.
31. Wang Y, Kim D. Crystallinity, morphology, mechanical properties and conductivity study of in situ formed PVdF/LiClO4/TiO2 nanocomposite polymer electrolytes. Electrochim Acta 2007;52:3181-9.
32. Duan H, Fan M, Chen WP, et al. Extended electrochemical window of solid electrolytes via heterogeneous multilayered structure for high-voltage lithium metal batteries. Adv Mater 2019;31:e1807789.
33. Liu Y, Fu F, Sun C, et al. Enabling stable interphases via in situ two-step synthetic bilayer polymer electrolyte for solid-state lithium metal batteries. Inorganics 2022;10:42.
34. Stoševski I, Krstić J, Vokić N, Radosavljević M, Popović ZK, Miljanić Š. Improved Poly(vinyl alcohol) (PVA) based matrix as a potential solid electrolyte for electrochemical energy conversion devices, obtained by gamma irradiation. Energy 2015;90:595-604.
35. Hirankumar G, Mehta N. Effect of incorporation of different plasticizers on structural and ion transport properties of PVA-LiClO4 based electrolytes. Heliyon 2018;4:e00992.
36. York SS, Buckner M, Frech R. Ion-polymer and Ion-ion interactions in linear poly(ethylenimine) complexed with LiCF3SO3 and LiSbF6. Macromolecules 2004;37:994-9.
37. Zhang J, Zhao N, Zhang M, et al. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 2016;28:447-54.
38. Wan Z, Lei D, Yang W, et al. Low resistance-integrated all-solid-state battery achieved by Li7La3Zr2O12 nanowire upgrading polyethylene oxide (PEO) composite electrolyte and PEO cathode binder. Adv Funct Mater 2019;29:1805301.
39. Yue H, Li J, Wang Q, et al. Sandwich-like poly(propylene carbonate)-based electrolyte for ambient-temperature solid-state lithium ion batteries. ACS Sustain Chem Eng 2018;6:268-74.
40. Zhang X, Liu T, Zhang S, et al. Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and Poly(vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes. J Am Chem Soc 2017;139:13779-85.
41. Chowdhury FI, Khandaker MU, Amin YM, Kufian MZ, Woo HJ. Vibrational, electrical, and structural properties of PVDF-LiBOB solid polymer electrolyte with high electrochemical potential window. Ionics 2017;23:275-84.
42. Zhang X, Xu B, Lin Y, Shen Y, Li L, Nan C. Effects of Li6.75La3Zr1.75Ta0.25O12 on chemical and electrochemical properties of polyacrylonitrile-based solid electrolytes. Solid State Ion 2018;327:32-8.
43. Sadiq M, Khan MA, Hasan Raza MM, Aalam SM, Zulfequar M, Ali J. Enhancement of electrochemical stability window and electrical properties of CNT-based PVA-PEG polymer blend composites. ACS Omega 2022;7:40116-31.
44. Wu G, Lin S, Yang C. Preparation and characterization of PVA/PAA membranes for solid polymer electrolytes. J Membr Sci 2006;275:127-33.
45. Pan J, Zhao P, Wang N, Huang F, Dou S. Research progress in stable interfacial constructions between composite polymer electrolytes and electrodes. Energy Environ Sci 2022;15:2753-75.
46. Sengwa R, Dhatarwal P. Predominantly chain segmental relaxation dependent ionic conductivity of multiphase semicrystalline PVDF/PEO/LiClO4 solid polymer electrolytes. Electrochim Acta 2020;338:135890.
47. Li J, Lin Y, Yao H, Yuan C, Liu J. Tuning thin-film electrolyte for lithium battery by grafting cyclic carbonate and combed poly(ethylene oxide) on polysiloxane. ChemSusChem 2014;7:1901-8.
49. Hou W. The effect of different lithium salts on conductivity of comb-like polymer electrolyte with chelating functional group. Electrochim Acta 2003;48:679-90.
50. Lee MJ, Han J, Lee K, et al. Elastomeric electrolytes for high-energy solid-state lithium batteries. Nature 2022;601:217-22.
51. Huo H, Zhao N, Sun J, Du F, Li Y, Guo X. Composite electrolytes of polyethylene oxides/garnets interfacially wetted by ionic liquid for room-temperature solid-state lithium battery. J Power Sources 2017;372:1-7.
52. Chen L, Li Y, Li S, Fan L, Nan C, Goodenough JB. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018;46:176-84.
53. Ma J, Liu Z, Chen B, et al. A strategy to make high voltage LiCoO2 compatible with Polyethylene oxide electrolyte in all-solid-state lithium ion batteries. J Electrochem Soc 2017;164:A3454-61.
54. Marchiori CFN, Carvalho RP, Ebadi M, Brandell D, Araujo CM. Understanding the electrochemical stability window of polymer electrolytes in solid-state batteries from atomic-scale modeling: the role of Li-ion salts. Chem Mater 2020;32:7237-46.
55. Zhang X, Guo W, Zhou L, Xu Q, Min Y. Surface-modified boron nitride as a filler to achieve high thermal stability of polymer solid-state lithium-metal batteries. J Mater Chem A 2021;9:20530-43.
56. Pan J, Zhang Y, Wang J, et al. A quasi-double-layer solid electrolyte with adjustable interphases enabling high-voltage solid-state batteries. Adv Mater 2022;34:e2107183.
57. Pan X, Sun H, Wang Z, et al. High voltage stable polyoxalate catholyte with cathode coating for all-solid-state Li-Metal/NMC622 batteries. Adv Energy Mater 2020;10:2002416.
58. Wang P, Chai J, Zhang Z, et al. An intricately designed poly(vinylene carbonate-acrylonitrile) copolymer electrolyte enables 5 V lithium batteries. J Mater Chem A 2019;7:5295-304.
59. Zheng H, Li G, Ouyang R, et al. Origin of lithiophilicity of lithium garnets: compositing or cleaning? Adv Funct Mater 2022;32:2205778.
60. Duan H, Yin YX, Shi Y, et al. Dendrite-free Li-metal battery enabled by a thin asymmetric solid electrolyte with engineered layers. J Am Chem Soc 2018;140:82-5.
61. Monroe C, Newman J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J Electrochem Soc 2005;152:A396.
62. Zhang X, Wang A, Liu X, Luo J. Dendrites in lithium metal anodes: suppression, regulation, and elimination. ACC Chem Res 2019;52:3223-32.
63. Zhou W, Wang Z, Pu Y, et al. Double-layer polymer electrolyte for high-voltage all-solid-state rechargeable batteries. Adv Mater 2019;31:e1805574.
64. Stone GM, Mullin SA, Teran AA, et al. Resolution of the modulus versus adhesion dilemma in solid polymer electrolytes for rechargeable lithium metal batteries. J Electrochem Soc 2012;159:A222-7.
65. Sångeland C, Tjessem T, Mindemark J, Brandell D. Overcoming the obstacle of polymer-polymer resistances in double layer solid polymer electrolytes. J Phys Chem Lett 2021;12:2809-14.
66. Deng C, Chen N, Hou C, Liu H, Zhou Z, Chen R. Enhancing interfacial contact in solid-state batteries with a gradient composite solid electrolyte. Small 2021;17:e2006578.
67. Yao Z, Zhu K, Li X, et al. Double-layered multifunctional composite electrolytes for high-voltage solid-state lithium-metal batteries. ACS Appl Mater Interfaces 2021;13:11958-67.
68. Arrese-igor M, Martinez-ibañez M, López del Amo JM, et al. Enabling double layer polymer electrolyte batteries: Overcoming the Li-salt interdiffusion. Energy Stor Mater 2022;45:578-85.
69. Li F, Liu J, He J, et al. Additive-assisted hydrophobic Li+-solvated structure for stabilizing dual electrode electrolyte interphases through suppressing LiPF6 hydrolysis. Angew Chem Int Ed 2022;61:e202205091.
70. Wang C, Wang T, Wang L, et al. Differentiated lithium salt design for multilayered PEO electrolyte enables a high-voltage solid-state lithium metal battery. Adv Sci 2019;6:1901036.
71. Li L, Wang J, Zhang L, Duan H, Deng Y, Chen G. Rational design of a heterogeneous double-layered composite solid electrolyte via synergistic strategies of asymmetric polymer matrices and functional additives to enable 4.5 V all-solid-state lithium batteries with superior performance. Energy Stor Mater 2022;45:1062-73.
72. Wang C, Yang Y, Liu X, et al. Suppression of lithium dendrite formation by using LAGP-PEO (LiTFSI) composite solid electrolyte and lithium metal anode modified by PEO (LiTFSI) in all-solid-state lithium batteries. ACS Appl Mater Interfaces 2017;9:13694-702.
73. Bi Z, Mu S, Zhao N, Sun W, Huang W, Guo X. Cathode supported solid lithium batteries enabling high energy density and stable cyclability. Energy Stor Mater 2021;35:512-9.
74. Yang S, Zhang Z, Shen L, et al. Gravity-driven Poly(ethylene glycol)@Li1.5Al0.5Ge1.5(PO4)3 asymmetric solid polymer electrolytes for all-solid-state lithium batteries. J Power Sources 2022;518:230756.
75. Gai J, Ma F, Zhang Z, et al. Flexible organic-inorganic composite solid electrolyte with asymmetric structure for room temperature solid-state li-ion batteries. ACS Sustain Chem Eng 2019;7:15896-903.
76. Wang Q, Wang H, Liu Y, Wu K, Liu W, Zhou H. An asymmetric quasi-solid electrolyte for high-performance Li metal batteries. Chem Commun 2020;56:7195-8.
77. Cai D, Qi X, Xiang J, et al. A cleverly designed asymmetrical composite electrolyte via
78. Zhao C, Zhao B, Yan C, et al. Liquid phase therapy to solid electrolyte-electrode interface in solid-state Li metal batteries: a review. Energy Stor Mater 2020;24:75-84.
79. Wu N, Li Y, Dolocan A, et al. In situ formation of Li3P layer enables fast Li+ conduction across Li/solid polymer electrolyte interface. Adv Funct Mater 2020;30:2000831.
80. Luo Y, Li X, Zhang Y, Ge L, Chen H, Guo L. Electrochemical properties and structural stability of Ga- and Y- co-doping in Li7La3Zr2O12 ceramic electrolytes for lithium-ion batteries. Electrochim Acta 2019;294:217-25.
81. Yuan C, Sheldon BW, Xu J. Heterogeneous reinforcements to mitigate Li penetration through solid electrolytes in all-solid-state batteries. Adv Energy Mater 2022;12:2201804.
82. Doan Tran H, Kim C, Chen L, et al. Machine-learning predictions of polymer properties with Polymer Genome. J Appl Phys 2020;128:171104.
83. Liu M, Clement C, Liu K, Wang X, Sparks TD. A data science approach for advanced solid polymer electrolyte design. Comput Mater Sci 2021;187:110108.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zhang N, Wu S, Zheng H, Li G, Liu H, Duan H. Recent progress of multilayer polymer electrolytes for lithium batteries. Energy Mater 2023;3:300009. http://dx.doi.org/10.20517/energymater.2022.64
AMA Style
Zhang N, Wu S, Zheng H, Li G, Liu H, Duan H. Recent progress of multilayer polymer electrolytes for lithium batteries. Energy Materials. 2023; 3(1): 300009. http://dx.doi.org/10.20517/energymater.2022.64
Chicago/Turabian Style
Zhang, Nan, Shaoping Wu, Hongpeng Zheng, Guoyao Li, Hezhou Liu, Huanan Duan. 2023. "Recent progress of multilayer polymer electrolytes for lithium batteries" Energy Materials. 3, no.1: 300009. http://dx.doi.org/10.20517/energymater.2022.64
ACS Style
Zhang, N.; Wu S.; Zheng H.; Li G.; Liu H.; Duan H. Recent progress of multilayer polymer electrolytes for lithium batteries. Energy Mater. 2023, 3, 300009. http://dx.doi.org/10.20517/energymater.2022.64
About This Article
Copyright
Data & Comments
Data
 Cite This Article 27 clicks
Cite This Article 27 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.