Organic-inorganic hybrid quasi-2D perovskites incorporated with fluorinated additives for efficient and stable four-terminal tandem solar cells
Abstract
Quasi-two-dimensional (2D) lead halide perovskites have emerged as promising candidates for improving the environmental stability of perovskite solar cells (PSCs). Herein, we report the preparation of a new quasi-2D perovskite by introducing a fluorine-containing additive [3-(trifluoromethyl)benzylammonium iodide (3-TFMBAI)] into Cs0.17FA0.83Pb(I0.83Br0.17)3. The moderate doping of 3-TFMBAI effectively induces the formation of the Ruddlesden-Popper perovskite phase, which can passivate the trap states and restrain the ionic motion in the perovskite lattice. The constructed 3-(trifluoromethyl)benzylamine molecular planes with strong hydrophobicity favorably suppress the decomposition and collapse of the perovskite phase against humidity. Moreover, the introduction of Cs+ and Br- ions tune the bandgap and improve the absorption, crystallinity and thermal stability of the perovskite films. As a result, a champion photoelectric conversion efficiency (PCE) of 20.89% is achieved, along with an improved open-circuit voltage reaching 1.22 V. The quasi-2D PSCs without encapsulation maintain 90.7% of the initial PCE after 1000 h under continuous heating at 60 °C and simultaneous exposure to humid air with a relative humidity of 60%. Four-terminal tandem solar cells are fabricated by combining top semi-transparent quasi-2D PSCs with bottom monocrystalline silicon solar cells, achieving an overall PCE of 23.53% and favorable performance stability.
Keywords
INTRODUCTION
In the past decade, organic-inorganic hybrid metal perovskites have attracted enormous interest in photovoltaics due to their exceptional photophysical properties, such as large light absorption coefficients, tunable bandgaps, long radiative lifetimes and excellent defect tolerance[1-4]. The photoelectric conversion efficiency (PCE) of perovskite solar cells (PSCs) has rapidly increased from 3.8% to 25.8%[5,6], making these novel materials competitive candidates for solar power harvesting. However, PSCs are sensitive to heat, humidity and ultraviolet light, which seriously limits their industrial development and commercialization[7]. In order to improve the tolerance of PSCs to ambient environments, substantial techniques have been proposed, such as lowering their dimensions[8-11], incorporating stable Cs+ and Br- ions[12] and coating them with protective layers[13,14].
In recent years, two-dimensional (2D) Ruddlesden-Popper perovskites, with large hydrophobic organic spacers inserted into the perovskite crystal lattices, have exhibited unique environmental and structural stability. Layered 2D Ruddlesden-Popper perovskites have a general formula of A′2An-1PbnX3n-1X′2, where A′ is a bulky organic cation spacer, A is a small-sized cation (FA+, MA+ and Cs+), X and X′ are the halide anions and n is an integer[15,16]. Ruddlesden-Popper perovskites with one layer of A′ organic cations inserted into every n-layer of [PbX6]4- octahedra are regarded as naturally formed quantum well structures, where the semiconductor three-dimensional (3D) layers correspond to the quantum wells and the organic spacer sheets act as barriers[17]. Owing to the structural versatility of the ammonium spacers, it is feasible to improve the photoresponse performance of 2D Ruddlesden-Popper PSCs by adjusting the structures of the organic spacer cations. Recently, several spacer ammoniums have been reported for 2D perovskite solar cells, such as benzylamine[18], phenylethylamine (PEA)[19,20], naphthalenemethylammonium[21] and thiophenemethylammonium[22]. Furthermore, it is also possible to further improve the hydrophobicity of 2D halide perovskites by adopting different ammonium spacers[23-26].
Liu et al. introduced a 2D A2PbI4 perovskite layer using pentafluorophenylethylammonium as a fluorine cation and the PSC devices produced reached a champion PCE of 22.2% with an open-circuit voltage (VOC) of 1.096 V, which maintained 90% of its initial efficiency at a relative humidity (RH) of 40% after 1000 h[24]. Wang et al. reported halide substitution in phenethylammonium spacer cations, where F-PEA-based devices (n = 4) exhibited a champion PCE of 18.10% with the highest VOC of 1.21 V and 80% of the initial PCE retained under 80 °C heating for 720 h[27]. Li et al. developed a hydrophobic
Herein, we demonstrate the preparation of a thermally stable quasi-2D perovskite by utilizing
EXPERIMENTAL
Chemicals and materials. Formamidinium iodide (FAI, > 99%), 4-tert-butylpyridine (≥ 96.0%) and patterned fluorinated tin oxide (FTO) glass substrates were purchased from Advanced Election Technology Co., Ltd., China. Lead iodide (PbI2, 99.9985%), bis(trifluoromethane)sulfonimide lithium salt (Li-TFSI,
Synthesis of 3-TFMBAI. Briefly, 1520 mg (8.68 mmol) of 3-(trifluoromethyl)benzylamine were dissolved in 15.0 mL of ethanol and stirred at room temperature for 10 min. Then, 1800 µL of hydroiodic acid were added into the mixture and stirred at 50 °C for 2 h. Afterwards, the mixture was concentrated by rotary evaporation and washed with excessive diethyl ether. Finally, the precipitate was dried under vacuum to obtain a crystalline solid with a bright white color (2328 mg, 7.68 mmol and 88.5% yield).
Preparation of SnO2 layer on FTO glass substrates. The patterned FTO glass substrates were sequentially cleaned by ultrasonication in acetone, ethanol and pure water for 15 min. The substrates were then treated with ultraviolet (UV)/ozone for 1 min at 60% power to remove the last traces of organic residues. Afterwards, a thin SnO2 layer was deposited on the substrates by spin coating a diluted SnO2 colloidal solution (4 wt.% in a H2O colloidal dispersion) at 3000 rpm for 30 s, followed by annealing in air at 125 °C for 30 min. Finally, the above spin-coating and annealing procedures were repeated once more to obtain the SnO2/FTO substrates.
Preparation of (3-TFMBA)2(Cs0.17FA0.83)n-1Pbn(I0.83Br0.17)3n-1I2 perovskite layers. All the fabrication steps for the (3-TFMBA)2(Cs0.17FA0.83)n-1Pbn(I0.83Br0.17)3n-1I2 perovskite layer were performed in a N2-filled glovebox. For the sample with n = 40, a precursor mixture of 447.8 mg (0.971 mmol) of PbI2, 120.6 mg (0.329 mmol) of PbBr2, 180.9 mg (1.052 mmol) of FAI, 56.0 mg (0.215 mmol) of CsI and 19.7 mg (0.065 mmol) of
Preparation of hole transport layers and counter electrodes. The spiro-OMeTAD solution was prepared by dissolving 80 mg of spiro-OMeTAD in 1 mL of chlorobenzene, followed by the addition of 17.5 μL of
Fabrication of single-crystalline silicon solar cells. 170 μm-thick p-type monocrystalline silicon solar cells with the structure of a passivated emitter and rear cell (PERC) were fabricated via a standard industrial process. In this kind of cell, the front surface of c-Si was texturized with randomly distributed pyramids by a standard industrial wet etching method. The front antireflection coating of the standard PERC cell adopted a single-layer SiNx thin film. Here, a thickness of
Materials characterization. The XRD patterns of the perovskite films were obtained using a Bruker D-8 Advance diffractometer with a Cu Kα X-ray source. XPS analyses were carried out with a PHI-5000 VersaProbe X-ray photoelectron spectrometer with an Al Kα X-ray source. UV-vis absorbance spectra were measured by a Shimadzu
Photovoltaic measurements. Before the photovoltaic tests, the PSCs were illuminated under simulated AM 1.5 G solar light using a solar simulator (100 mW/cm2, NOWDATA SXDN-150E) for 15 min in advance for activation. The J-V curves were measured with a Keithley 2400 Source Meter under a simulated
RESULTS AND DISCUSSION
The 3-TFMBAI additive in the form of a crystalline bright white powder was synthesized by the reaction of 3-TFMBA and hydroiodic acid in ethanol, as presented in the Experimental section and Supplementary Figure 1.
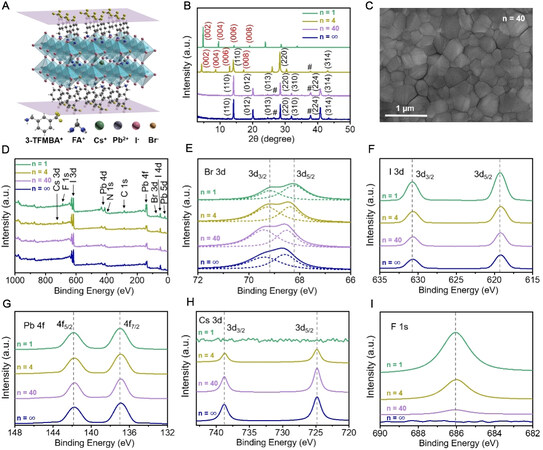
Figure 1A shows the crystalline structure of (3-TFMBA)2(Cs0.17FA0.83)n-1Pbn(I0.83Br0.17)3n-1I2 perovskites, where the perovskite layers are sandwiched between the molecular planes composed of 3-TFMBA. Figure 1B shows the XRD characterization of the perovskite films. When n = 1, the perovskite film is a pure 2D phase with the XRD peaks indexed to the planes of (002), (004), (006), (008) and so on. In addition to the characteristic peaks of the 2D phase, the peaks of the (110), (220) and (314) planes, which belong to the 3D phase, appear in the XRD pattern of the perovskite with n = 4. As the value of n increases to 40 and then ∞, the XRD patterns are dominated by the peaks of the 3D phase, which are located at 14.16°, 20.11°, 24.62°, 28.50°, 31.98°, 40.83° and 43.36°, corresponding to the (110), (012), (013), (220), (310), (224) and (314) planes of the perovskite phase, respectively.
Figure 1. Structural and compositional analyzes of as-prepared perovskite films. (A) Crystalline structure of (3-TFMBA)2(Cs0.17FA0.83)n-1Pbn(I0.83Br0.17)3n-1I2 perovskite. (B) XRD patterns (peaks labeled with “#” are derived from FTO). (C) Top-view SEM image of quasi-2D perovskite film with n = 40. (D) Survey XPS spectra and (E-I) corresponding high-resolution XPS spectra at (E) Br 3d, (F) I 3d, (G) Pb 4f, (H) Cs 3d and (I) F 1s regions of quasi-2D perovskite films with different n values.
After normalizing the XRD patterns of the perovskite samples with n = 40 and ∞ according to the strongest peak of the (110) planes, it is found that the full width at half maximum (FWHM) of the sample with n = 40 (0.145°) is smaller than that of the sample with n = ∞ (0.162°). This confirms that introducing a small amount of 3-TFMBAI into the perovskite bulk phase to form a molecular plane could indeed increase the overall crystallinity of the perovskite film due to the orientation effects of the amino hydrogen bonds
XPS analysis was performed to identify the chemical compositions of the prepared perovskite films with different n values [Figure 1D-I]. After setting the C 1s peak of adventitious carbon to 284.8 eV, the high-resolution XPS spectra of the Br 3d, I 3d, Pb 4f, Cs 3d and F 1s bands were collected [Figure 1E-I] and the characteristic binding energies of these elements are summarized in Supplementary Table 1.
Furthermore, the ammonium cation in 3-TFMBA+ can form a hydrogen bond with the Br- ion (N-H··· Br) in the [PbI5Br]4- octahedral framework in the perovskite lattice[29], which weakens the originally strong chemical interaction between Pb2+ and Br-, resulting in the shift of the Br 3d band position to lower binding energy with increasing 3-TFMBAI concentration. Due to the small electronegativity of I (ENI = 2.5, similar to carbon), the hydrogen bond between an I- ion and an ammonium cation is very weak, so the doping of
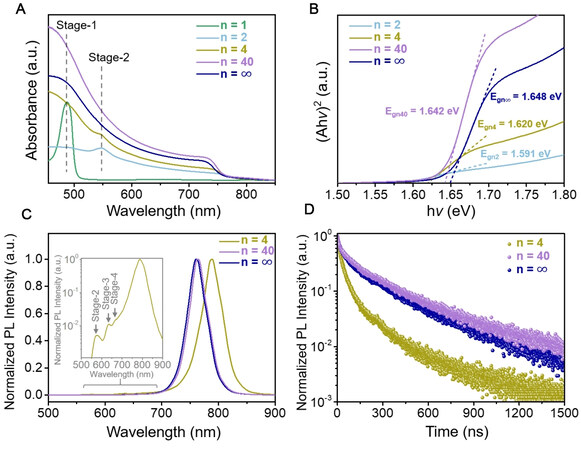
To verify the presence of 2D and quasi-2D structures, the ultraviolet–visible spectroscopy (UV-Vis) absorption spectra of the perovskite films with different n values were measured, as shown in Figure 2A. The perovskite films with n = 1 and 2 clearly exhibit strong absorption peaks at 488 and 549 nm derived from the Stage-1 and Stage-2 intercalations of
Figure 2. Photoresponse properties of as-prepared perovskite films. (A) UV-Vis absorption spectra (in which Stage-x represents x layers of [PbI5Br]4- octahedra sandwiched between each two 3-TFMBA molecular planes), (B) corresponding (Ahν)2vs.hν curves, (C) steady PL spectra and (D) TRPL spectra of (3-TFMBA)2(Cs0.17FA0.83)n-1Pbn(I0.83Br0.17)3n-1I2 perovskite films.
Similar to the UV-Vis absorption spectra, the perovskite films with n = 4, 40 and ∞ exhibited strong 3D phase PL emission peaks at 789, 763 and 761 nm, respectively, as shown in Figure 2C. By comparing the FWHM of these peaks (45.3 nm for n = 4, 40.7 nm for n = 40 and 41.6 nm for n = ∞), it can be found that the perovskite film with n = 40 has the smallest FWHM value, indicating the highest crystal quality, as also indicated by the previous XRD patterns and SEM results. After logarithmic transformation of the ordinate, small PL emission peaks of low-n phases can be clearly observed, as shown in the inset of Figure 2C. Time-resolved PL (TRPL) spectroscopy was carried out to explore the charge-recombination kinetics in perovskite films with different n values [Figure 2D]. The curves were fitted by the following bi-exponential decay function[32]:
f(t) = A1e-t/τ1 + A2e-t/τ2 (1)
where the measured parameters A1, A2, τ1 and τ2 of different n values are summarized in
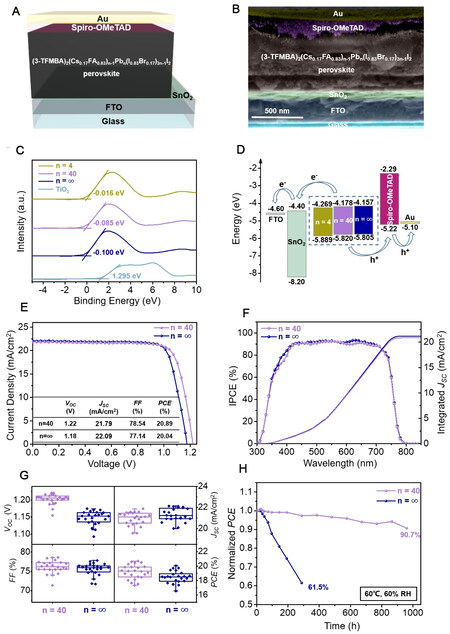
The perovskite films were then applied in PSCs with the device configuration of glass/FTO/SnO2/perovskite/spiro-OMeTAD/80-nm-Au [Figure 3A], corresponding to the layers exhibited in the cross-sectional SEM image of Figure 3B. Valence-band XPS tests were carried out to determine the energy band levels of perovskites with different n values [Figure 3C]. As the valence band maximum (VBM) of the standard TiO2 sample can be determined to -7.2 eV[33], the VBM of the perovskites with n = 4, 40 and ∞ can be calculated as -5.889, -5.820 and -5.805 eV, respectively. Furthermore, the UV-Vis absorption spectra
Figure 3. Device configuration and photovoltaic performance of PSCs. (A) Schematic structure and (B) cross-sectional SEM image of PSCs with glass/FTO/SnO2/perovskite/spiro-OMeTAD/80-nm-Au configuration. (C) Valence-band XPS spectra and (D) corresponding energy level alignment diagrams. (E) Typical J-V plots, (F) IPCE spectra and (G) box diagrams of VOC, JSC, FF and PCE distributions of 24 individual PSCs with different n values. (H) Normalized PCE retentions of different PSCs without encapsulation under continuous heating at 60 °C and simultaneous exposure to humid air with a RH of 60%.
Figure 3E and Supplementary Figure 4 show the J-V plots of PSCs with different n values and the corresponding photovoltaic parameters. The short-circuit current density (JSC) of the PSC with
Incident photon-to-electron conversion efficiency (IPCE) measurements were carried out to validate the current densities of the PSCs with n = 40 and ∞ [Figure 3F]. Similar to the UV-Vis absorption and PL spectra, the IPCE onset wavelengths are ~780 nm and the quantum efficiency values from 400 to 740 nm are over 80%. The integrated JSC values of the PSCs with n = 40 and ∞ are calculated to be 20.87 and
The long-term stability tests of PSCs with n = 40 and ∞ free of any encapsulation were carried out by heating at 60 °C and exposure to humid air with a RH of 60% [Figure 3H]. After being continuously heated at 60 °C for 960 h, the PSCs with n = 40 still maintained 90.7% of their initial PCE, which was significantly better than that of the PSCs with n = ∞ (61.5% after 288 h). To further illustrate the effect of 3-TFMBAI doping on the thermal and moisture stability of the PSCs, XRD analysis of the perovskite films
Contact angle measurements were carried out to further investigate the improvement in the humidity resistance of the perovskite films with the introduction of 3-TFMBAI, as shown in Supplementary Figure 9. The perovskite film with n = 40 exhibits a larger contact angle (62.2°) than the perovskite film with n = ∞ (56.8°). With the further increase in 3-TFMBAI doping concentration (n = 4), the contact angle continued to become larger as well (71.1°). These results could be attributed to the strong hydrophobicity of the
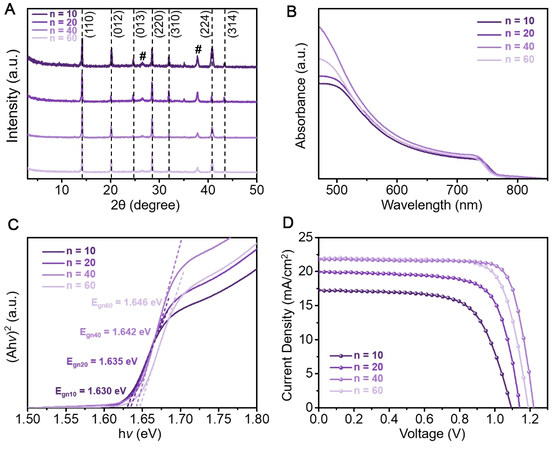
To study the effects of the 3-TFMBAI doping concentration on the crystalline structure and photoresponse properties, (3-TFMBA)2(Cs0.17FA0.83)n-1Pbn(I0.83Br0.17)3n-1I2 perovskite films with different n values (n = 10, 20, 40 and 60) were prepared [Figure 4]. The XRD patterns [Figure 4A] of the perovskites are all dominated by the peaks of the 3D perovskite phase. After normalizing the XRD patterns according to the strongest peaks of the (110) planes, it is found that the FWHM of the perovskite film with n = 40 is the smallest (0.164° for
Figure 4. Structural and photoresponse influences of 3-TFMBAI doping ratios. (A) XRD patterns (peaks labeled with “#” are derived from FTO), (B) UV-vis spectra, (C) (Ahv)2vs.hv curves and (D) typical J-V plots of quasi-2D perovskites with different 3-TFMBAI doping concentrations (n = 10, 20, 40 and 60).
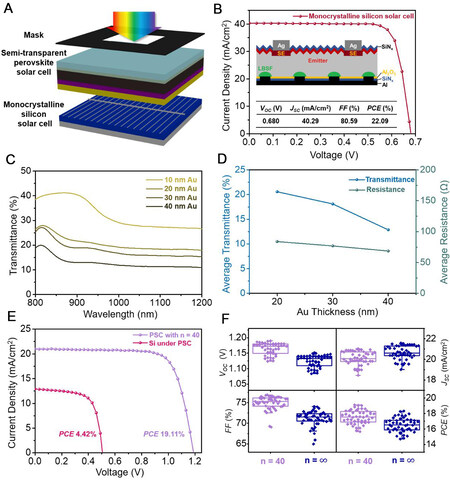
For the assembly of four-terminal tandem solar cells, semi-transparent PSCs were stacked with a p-type monocrystalline silicon solar cell, as displayed in Figure 5A. The bottom silicon solar cell with the PERC structure was fabricated via a standard industrial process, as detailed in the Experimental section. The insets of Figure 5B exhibit the cross-sectional structure and photovoltaic parameters of the bottom silicon solar cell. The J-V response of this silicon PERC solar cell was tested and the JSC, VOC and FF values were measured to be 40.29 mA/cm2, 0.680 V and 80.59%, respectively, corresponding to a photoelectric conversion efficiency of 22.09%.
Figure 5. Device configuration and performance of silicon solar cells and four-terminal tandem solar cells. (A) Device configuration of a four-terminal tandem solar cell stacked by a top PSC and a bottom silicon solar cell. (B) J-V plots of monocrystalline silicon solar cells, where the insets are the cross-sectional structure and corresponding photovoltaic parameter values. (C) Near-infrared transmittance and (D) average transmittance and internal resistance of PSCs with different thicknesses of Au counter electrodes. (E) J-V plots of top PSC with n = 40 and bottom silicon solar cell. (F) Box diagrams of VOC, JSC, FF and PCE distributions of 48 PSCs with different n values based on 20 nm-thick Au counter electrodes.
In the four-terminal tandem solar cell, the top semi-transparent PSC absorbs most of the sunlight in the visible region, with most of the near-infrared light not absorbed. The near-infrared light passes through the PSC and illuminates the bottom silicon solar cell. Therefore, it is important to improve the transmittance of the top PSC in the near-infrared region (800-1200 nm), which is the main light absorption region of silicon solar cells. Notably, the perovskite layer has little absorption in the near-infrared region
When assembled in four-terminal tandem solar cells, the top PSC (with n = 40 and a 20 nm-thick Au electrode) exhibited a champion PCE of 19.11% while the bottom silicon solar cell exhibited a champion PCE of 4.42%. The J-V curves and detailed photovoltaic parameters of these two parts are shown in Figure 5E and Supplementary Table 7, respectively. As a result, the total champion PCE of the whole four-terminal tandem solar cells reached 23.53%. For comparison, Figure 5F shows the distributions of the VOC, JSC, FF and PCE values of the top PSCs with n = 40 and ∞ based on 20 nm-thick Au electrodes, verifying the high repeatability of the fabrication process. The average PCE of 48 individual PSCs with n = 40 (17.54%) was higher than that of the PSCs with n = ∞ (16.52%) and the detailed data are summarized in Supplementary Tables 8 and 9, further confirming that n = 40 is the most favorable doping ratio of the
CONCLUSIONS
In summary, we have reported the preparation of a novel quasi-2D perovskite phase with improved absorbance, crystallinity and moisture stability via a one-step anti-solvent process by introducing a novel fluorinated additive (3-TFMBAI) into Cs0.17FA0.83Pb(I0.83Br0.17)3. This small fluorinated organic ammonium molecule could compensate for the pinholes and cracks in the perovskite during the annealing process and thus achieve a high-quality perovskite film with larger grain sizes and reduced crystalline defect densities. Furthermore, the moderate doping of 3-TFMBAI efficiently tuned the bandgap of the perovskite film, so that the VOC of the fabricated PSC increased to 1.22 V with a champion PCE of 20.89%. Moreover, the strongly hydrophobic 3-TFMBAI additive could help block water molecules and suppress the moisture/heat decomposition of the perovskite, resulting in an excellent performance stability of PSCs without encapsulation under long-term high-temperature heating in humid air. Finally, the semi-transparent quasi-2D PSCs were coupled with monocrystalline silicon solar cells to construct four-terminal tandem solar cells, achieving an overall PCE of 23.53% and favorable device stability. This work helps to tackle the intractable issue regarding the intrinsic thermal and moisture instability of organic-inorganic hybrid perovskite materials through the doping of small fluorinated organic molecules and provides a novel prototype design for future tandem solar cells for all seasons.
DECLARATIONS
Authors’ contributionsConceived the idea of this study and designed the experiments: Jin Z, Xia Y, Zhu M
Performed the material synthesis: Xia Y, Zhu M
Contributed to the XRD, SEM, IPCE, UV and XPS characterization: Xia Y, Zhu M, Qin L
Fabricated the solar cells and tested the photovoltaic performances: Xia Y, Zhu M, Zhao C
Carried out the PL characterizations: Hong D, Tian Y
Analyzed the data and wrote the paper: Jin Z, Xia Y, Zhu M
Supervised this project: Jin Z, Yan W
Availability of data and materialsThe data that support the findings of this study are available from the corresponding author upon reasonable request.
Financial support and sponsorshipThe authors acknowledge the grant supports from the National Key R&D Program of China (2017YFA0208200), the National Natural Science Foundation of China (22022505 and 21872069), the Fundamental Research Funds for the Central Universities of China (020514380266, 020514380272 and 020514380274), the Scientific and Technological Innovation Special Fund for Carbon Peak and Carbon Neutrality of Jiangsu Province (BK20220008), the Nanjing International Collaboration Research Program (202201007 and 2022SX00000955), and the Suzhou Gusu Leading Talent Program of Science and Technology Innovation and Entrepreneurship in Wujiang District (ZXL2021273). Wensheng Yan acknowledges the support from the German Research Foundation (DFG) (Ref. YA516/1-1).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
1. De Wolf S, Holovsky J, Moon SJ, et al. Organometallic halide perovskites: sharp optical absorption edge and its relation to photovoltaic performance. J Phys Chem Lett 2014;5:1035-9.
2. Kim G, Petrozza A. Defect tolerance and intolerance in metal-halide perovskites. Adv Energy Mater 2020;10:2001959.
3. McMeekin DP, Sadoughi G, Rehman W, et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 2016;351:151-5.
4. Stranks SD, Eperon GE, Grancini G, et al. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013;342:341-4.
5. Kojima A, Teshima K, Shirai Y, Miyasaka T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J Am Chem Soc 2009;131:6050-1.
6. Min H, Lee DY, Kim J, et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021;598:444-50.
7. Zhang X, Ren X, Liu B, et al. Stable high efficiency two-dimensional perovskite solar cells via cesium doping. Energy Environ Sci 2017;10:2095-102.
8. Cao DH, Stoumpos CC, Farha OK, Hupp JT, Kanatzidis MG. 2D homologous perovskites as light-absorbing materials for solar cell applications. J Am Chem Soc 2015;137:7843-50.
9. Quan LN, Yuan M, Comin R, et al. Ligand-stabilized reduced-dimensionality perovskites. J Am Chem Soc 2016;138:2649-55.
10. Stoumpos CC, Cao DH, Clark DJ, et al. Ruddlesden-popper hybrid lead iodide perovskite 2D homologous semiconductors. Chem Mater 2016;28:2852-67.
11. Tsai H, Nie W, Blancon JC, et al. High-efficiency two-dimensional Ruddlesden-Popper perovskite solar cells. Nature 2016;536:312-6.
12. Liang J, Wang C, Wang Y, et al. All-inorganic perovskite solar cells. J Am Chem Soc 2016;138:15829-32.
13. Bella F, Griffini G, Correa-Baena JP, et al. Improving efficiency and stability of perovskite solar cells with photocurable fluoropolymers. Science 2016;354:203-6.
14. Zhu H, Ren Y, Pan L, et al. Synergistic effect of fluorinated passivator and hole transport dopant enables stable perovskite solar cells with an efficiency near 24%. J Am Chem Soc 2021;143:3231-7.
15. Leng K, Abdelwahab I, Verzhbitskiy I, et al. Molecularly thin two-dimensional hybrid perovskites with tunable optoelectronic properties due to reversible surface relaxation. Nat Mater 2018;17:908-14.
16. Mao LL, Stoumpos CC, Kanatzidis MG. Two-dimensional hybrid halide perovskites: principles and promises. J Am Chem Soc 2019;141:1171-90.
17. Xi J, Spanopoulos I, Bang K, et al. Alternative organic spacers for more efficient perovskite solar cells containing ruddlesden-popper phases. J Am Chem Soc 2020;142:19705-14.
18. Zheng H, Liu G, Zhu L, et al. The effect of hydrophobicity of ammonium salts on stability of quasi-2D perovskite materials in moist condition. Adv Energy Mater 2018;8:1800051.
19. Zhang J, Qin J, Wang M, et al. Uniform permutation of quasi-2D perovskites by vacuum poling for efficient, high-fill-factor solar cells. Joule 2019;3:3061-71.
20. Zhao X, Liu T, Kaplan AB, Yao C, Loo YL. Accessing highly oriented two-dimensional perovskite films via solvent-vapor annealing for efficient and stable solar cells. Nano Lett 2020;20:8880-9.
21. Xu Z, Lu D, Liu F, et al. Phase distribution and carrier dynamics in multiple-ring aromatic spacer-based two-dimensional ruddlesden-popper perovskite solar cells. ACS Nano 2020;14:4871-81.
22. Lai H, Kan B, Liu T, et al. Two-dimensional ruddlesden-popper perovskite with nanorod-like morphology for solar cells with efficiency exceeding 15%. J Am Chem Soc 2018;140:11639-46.
23. Lai H, Lu D, Xu Z, Zheng N, Xie Z, Liu Y. Organic-salt-assisted crystal growth and orientation of quasi-2D ruddlesden-popper perovskites for solar cells with efficiency over 19%. Adv Mater 2020;32:e2001470.
24. Liu Y, Akin S, Pan L, et al. Ultrahydrophobic 3D/2D fluoroarene bilayer-based water-resistant perovskite solar cells with efficiencies exceeding 22%. Sci Adv 2019;5:eaaw2543.
25. Pan H, Zhao X, Gong X, Shen Y, Wang M. Atomic-scale tailoring of organic cation of layered ruddlesden-popper perovskite compounds. J Phys Chem Lett 2019;10:1813-9.
26. Wei Y, Chen B, Zhang F, et al. Compositionally designed 2D ruddlesden-popper perovskites for efficient and stable solar cells. Solar RRL 2021;5:2000661.
27. Wang Z, Wei Q, Liu X, et al. Spacer cation tuning enables vertically oriented and graded quasi-2D perovskites for efficient solar cells. Adv Funct Mater 2021;31:2008404.
28. Li D, Xing Z, Huang L, et al. Spontaneous formation of upper gradient 2D structure for efficient and stable quasi-2D perovskites. Adv Mater 2021;33:e2101823.
29. Zhou X, Wang Y, Li C, Wu T. Doping amino-functionalized ionic liquid in perovskite crystal for enhancing performances of hole-conductor free solar cells with carbon electrode. Chem Eng J 2019;372:46-52.
30. Zhao H, Han Y, Xu Z, et al. A Novel anion doping for stable CsPbI2Br perovskite solar cells with an efficiency of 15.56% and an open circuit voltage of 1.30 V. Adv Energy Mater 2019;9:1902279.
31. Shao M, Bie T, Yang L, et al. Over 21% efficiency stable 2D perovskite solar cells. Adv Mater 2022;34:e2107211.
32. Zhu X, Du M, Feng J, et al. High-efficiency perovskite solar cells with imidazolium-based ionic liquid for surface passivation and charge transport. Angew Chem Int Ed 2021;60:4238-44.
33. Liang J, Zhao P, Wang C, et al. CsPb0.9Sn0.1IBr2 based all-inorganic perovskite solar cells with exceptional efficiency and stability. J Am Chem Soc 2017;139:14009-12.
34. Thiesbrummel J, Le Corre VM, Peña-camargo F, et al. Universal current losses in perovskite solar cells due to mobile ions. Adv Energy Mater 2021;11:2101447.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Xia Y, Zhu M, Qin L, Zhao C, Hong D, Tian Y, Yan W, Jin Z. Organic-inorganic hybrid quasi-2D perovskites incorporated with fluorinated additives for efficient and stable four-terminal tandem solar cells. Energy Mater 2023;3:300004. http://dx.doi.org/10.20517/energymater.2022.55
AMA Style
Xia Y, Zhu M, Qin L, Zhao C, Hong D, Tian Y, Yan W, Jin Z. Organic-inorganic hybrid quasi-2D perovskites incorporated with fluorinated additives for efficient and stable four-terminal tandem solar cells. Energy Materials. 2023; 3(1): 300004. http://dx.doi.org/10.20517/energymater.2022.55
Chicago/Turabian Style
Xia, Yuren, Mengfei Zhu, Lina Qin, Cheng Zhao, Daocheng Hong, Yuxi Tian, Wensheng Yan, Zhong Jin. 2023. "Organic-inorganic hybrid quasi-2D perovskites incorporated with fluorinated additives for efficient and stable four-terminal tandem solar cells" Energy Materials. 3, no.1: 300004. http://dx.doi.org/10.20517/energymater.2022.55
ACS Style
Xia, Y.; Zhu M.; Qin L.; Zhao C.; Hong D.; Tian Y.; Yan W.; Jin Z. Organic-inorganic hybrid quasi-2D perovskites incorporated with fluorinated additives for efficient and stable four-terminal tandem solar cells. Energy Mater. 2023, 3, 300004. http://dx.doi.org/10.20517/energymater.2022.55
About This Article
Copyright
Data & Comments
Data
 Cite This Article 26 clicks
Cite This Article 26 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.