Enhanced all-climate sodium-ion batteries performance in a low-defect and Na-enriched Prussian blue analogue cathode by nickel substitution
Abstract
Cobalt hexacyanoferrate (CoHCF) is one of the most promising cathode materials for all-climate sodium-ion batteries (SIBs) due to its open three-dimensional (3D) framework structures, high theoretical specific capacity, good voltage platform and almost no Jahn-Teller effects. However, CoHCF still suffers from poor cycling stability and bad rate capability, which is closely related to the huge distortion of frame structure and poor conductivity. In this study, by choosing nickel (Ni) to partially replace cobalt (Co) in the CoHCF lattice, we successfully prepared low-defect and Na-enriched Na2Co0.7Ni0.3[Fe(CN)6] (Co0.7Ni0.3HCF) in chelate and sodium salt-assisted coprecipitation method. Both experiments and first-principles calculations demonstrate that Ni substitution can effectively suppress the lattice distortion during the charging and discharging process of CoHCF. Furthermore, the introduction of Ni increases ion mobility by reducing the ion migration barrier (0.31 eV versus 0.17 eV) and improves the electronic conductivity by reducing the bandgap. It is found that Co0.7Ni0.3HCF exhibits superior electrochemical performance compared with that of CoHCF in a wide temperature range (-30 to 60 °C). At 25 °C, Co0.7Ni0.3HCF delivers a high specific capacity of 142.2 mAh g-1 at 0.2 C, an ultrahigh rate capability with
Keywords
INTRODUCTION
The application of Lithium-ion batteries (LIBs) in the field of energy storage, especially at low temperatures, has been limited due to the shortage of lithium resources, high raw material prices, and unsatisfactory low-temperature performances. Having similar physical and chemical properties to lithium, low-cost sodium-ion batteries (SIBs) are one of the potential options for large-scale energy storage[1-3]. Meanwhile, the Stokes radius of sodium-ion is smaller than that of lithium-ion, which is more conducive to kinetic transmission and has better performance at subzero temperatures[4,5]. In addition, in the cathode materials of SIBs, Prussian blue (PB) and its analogues (PBAs, Na2M[Fe(CN)6], M = Fe, Co, Mn, Ni, Cu, etc.) have an open 3D framework structure, which can further improve the migration rate of Na+-ions[6-8]. However, the PBAs synthesized via the conventional rapid precipitation process are always Na+-deficient phase with large amounts of water and Fe(CN)64- vacancies, which leads to low capacity and poor cycling and rate stability[9-11]. The use of chelates including sodium citrate[12], ethylenediaminetetraacetic acid (EDTA)[13], sodium carboxymethylcellulose (CMC)[14] and additives such as surfactants[15] and sodium salts[16] has improved the control of crystallization, which are propitious to increase sodium content and reduce defects of PBAs.
Among the PBAs, nickel hexacyanoferrate (NiHCF) has only one redox center, severely limiting the capacity, iron hexacyanoferrate (FeHCF) with low average voltage will also reduce the energy density, and manganese hexacyanoferrate (MnHCF) is affected by Jahn-Teller effect, resulting in poor cycling performance. Whereas, CoHCF shows attractive advantages such as high theoretical capacity, good voltage platform and almost no Jahn-Teller effects[17-19]. However, even in the PB with perfect lattice, once the material reaches its theoretical specific capacity (fully desodiated state), the material will undergo huge volume change after almost all sodium ions are removed from the framework, resulting in stress and strain. The accumulation of stress and strain causes large lattice distortion and even collapse of the lattice structure, resulting in poor cycling stability[20]. Therefore, many strategies have been proposed to stabilize the lattice structures, including surface protective layers coating[21-23], transition metal elements doping[24-26], and regulation of lattice water[27,28]. Qiao et al. constructed a semiconductor ZnO protective layer outside FeHCF to form a physical barrier, which inhibited the decomposition of PB during the charging and discharging process and improved the electrochemical performance[23]. Wang et al. achieved Ni gradient doping from surface to inside of FeHCF, in which the nickel-rich outer layer provided a stable framework structure and the nickel-poor inner layer partly activated the electrochemical activity of Fe to increase the capacity[25].
In this study, to balance the high specific capacity and good cycle stability of PBAs, in the synthesis strategy of Co0.7Ni0.3HCF, we used chelate and sodium salt-assisted coprecipitation crystallization method to obtain the low-defect and Na-enriched PBA to ensure its high specific capacity. Moreover, we chose Ni to replace Co partially to further stabilize the lattice structure, improving the cycling stability. The results of inductively coupled plasma-optical emission spectrometer (ICP-OES) and thermogravimetric analysis (TGA) indicate that
EXPERIMENTAL
Materials synthesis
A series of Na2CoxNi1-x[Fe(CN)6]•yH2O (CoxNi1-xHCF) (x = 0, 0.1, …, 1.0) PBAs were synthesized by coprecipitation method. Solution A was formed by dissolving 2x mmol CoCl2·6H2O, 2(1-x) mmol
Materials characterization
XRD measurement was performed by Cu Kα radiation (λ = 1.541874 Å) in a scan range (2θ) of 10-80° on Panalytical X’pert PRO MRD. The scanning electron microscope (SEM, Japan) was used to collect FESEM images to display the morphology of the samples at an acceleration voltage of 30 kV. The transmission electron microscopy (TEM, Tecnai G2 F30 S-TWIN, FEI) was used to obtain TEM images of the cathode. The thermogravimetry measurements were performed using a NETZSCH-STA449c/3/G analyzer from 30 to 500 °C at a heating rate of 10 °C/min in N2. The Fourier transform infrared (FTIR) spectra were obtained by A VERTEX 70 FT-IR spectrometer (4000-500 cm-1 region). The composition of PBAs was measured by ICP-OES (IRIS Intrepid IIXSP, Thermo Elemental, USA). X-ray photoelectron spectroscopy (XPS) analysis was conducted through a Thermo ESCALAB 250XI instrument.
Electrochemical measurement
For the electrochemical performance tests of PBAs, CR2025 coin cells were assembled in a glove box filled with pure Ar gas. The working electrodes were made by painting the uniform slurry which contained the active material (70%), Ketjen black (conductive agent, 10%), Super P (conductive agent, 10%), sodium carboxymethylcellulose (NaCMC, binder, 5%) and styrene butadiene rubber (SBR, binder, 5%)[31,32] onto an aluminum foil and then drying in a vacuum oven at 105 °C for 12 h. The mass loading of active materials was about 1.5-3 mg cm-1 for the electrodes. The metallic sodium anode is used as the counter electrode. The composition of the electrolyte was 1 mol L-1 NaClO4 in ethylene carbonate (EC)/ propylene carbonate (PC) (1:1 v/v) with 2% fluoroethylene carbonate (FEC) as an additive (by volume). The separator was glass fibers (GF/D) from Whatman. Cyclic voltammetry (CV) was conducted on an electrochemical workstation (CHI760E) between 2.0 and 4.2 V versus Na+/Na at a scan rate of 0.1 mV s-1. Galvanostatic intermittent titration technique (GITT) testing of the charging and discharging process was performed on a Land battery system at a current density of 15 mA g-1 from 2.0-4.2 V, in which the coin cell was alternately charged for
Calculation method
The first-principles calculations were performed within the density functional theory framework by using the projector augmented wave (PAW) method, as implemented in the Vienna ab initio simulation package (VASP)[33,34]. The generalized gradient approximation (GGA) with Perdew-Burke-Ernzerhof (PBE) was used to treat the exchange-correlation interactions[35]. The energy and force convergence values were chosen as 10-5 eV and 0.01 eV Å-1, respectively. The Kohn-Sham orbitals were expanded in plane waves with a kinetic energy cut-off of 500 eV. The Brillouin zone integration and k-point sampling were performed with a Monkhorst-Pack scheme of 3 × 3 × 3 grid for all calculations[36]. The GGA + U correlation method was used and the U value of Fe, Co and Ni atoms were set as 4, 3.4 and 6 eV, respectively[37]. The diffusion barrier energies of sodium ions were explored using the climbing image nudged elastic band (CI-NEB) method[38].
RESULTS AND DISCUSSION
Structure analysis
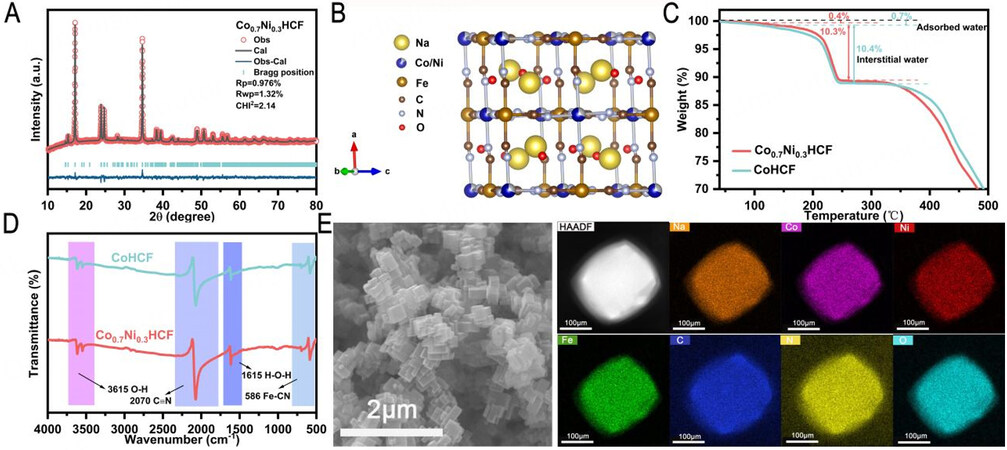
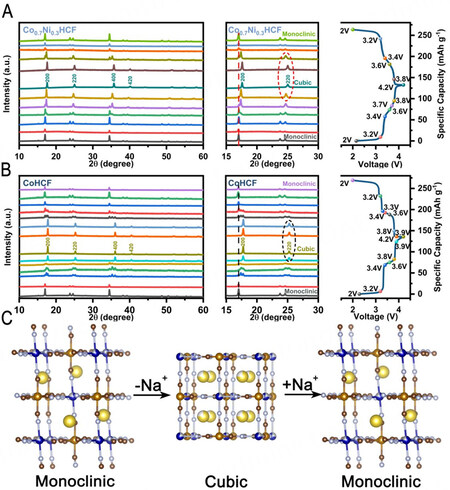
XRD patterns of a series of CoxNi1-x[Fe(CN)6] PBAs with different ratios of Co and Ni (x = 0, 0.1, …, 1.0) show monoclinic phase with a space group of P21/n [Supplementary Figure 1A], suggesting high Na content of series of samples[15,39]. The refined results for Co0.7Ni0.3HCF [Figure 1A] and CoHCF  Fe-C-N and
Fe-C-N and  C-N-Co (Ni)
C-N-Co (Ni)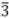 m in the cubic lattice of PBAs was detected by neutron diffraction and extended X-ray absorption fine structure (EXAFS). The lattice water can occupy sites of 8 c, 32 f and 48 g[30]. It is not difficult to find that the occupied sites of water molecules coincide with the occupied sites of sodium ions. As shown in the TGA curves [Figure 1C], the content of water of Co0.7Ni0.3HCF and CoHCF is very low due to very small amounts of vacancies formed inside the crystal structure during the effectively controlled coprecipitation process. From room temperature to 105 °C, the weight loss of Co0.7Ni0.3HCF and CoHCF is about 0.4% and 0.7%, respectively, which can be ascribed to the loss of the adsorbed water on the surface. From 150 °C to 250 °C, the weight loss of interstitial water in the open frameworks of Co0.7Ni0.3HCF and CoHCF is 10.3% and 10.4%, respectively. Combined with ICP-OES and TGA, the chemical compositions of two PBAs can be determined as Na2.008Ni0.269Co0.731[Fe(CN)6]0.997•2.09H2O and Na2.047Co[Fe(CN)6]0.997•2.19H2O, respectively. It demonstrates the two PBAs samples show similar high Na and low vacancy contents. According to FTIR spectra [Figure 1D], the peaks at ~3615 cm-1 and 1615 cm-1 are attributed to the O-H and H-O-H bending modes of the water molecules, the absorption peaks at ~2070 cm-1 and 586 cm-1 are assigned to the stretching vibration of C≡N and Fe-CN bonds, showing the characteristic functional groups of PBAs[28]. The SEM images of Co0.7Ni0.3HCF [Figure 1E] exhibit cubic morphologies with diameters ranging from 100 to 400 nm, which have sharp edges and corners. From the EDS images mapping, all elements of Co0.7Ni0.3HCF are uniformly distributed throughout the particles.
m in the cubic lattice of PBAs was detected by neutron diffraction and extended X-ray absorption fine structure (EXAFS). The lattice water can occupy sites of 8 c, 32 f and 48 g[30]. It is not difficult to find that the occupied sites of water molecules coincide with the occupied sites of sodium ions. As shown in the TGA curves [Figure 1C], the content of water of Co0.7Ni0.3HCF and CoHCF is very low due to very small amounts of vacancies formed inside the crystal structure during the effectively controlled coprecipitation process. From room temperature to 105 °C, the weight loss of Co0.7Ni0.3HCF and CoHCF is about 0.4% and 0.7%, respectively, which can be ascribed to the loss of the adsorbed water on the surface. From 150 °C to 250 °C, the weight loss of interstitial water in the open frameworks of Co0.7Ni0.3HCF and CoHCF is 10.3% and 10.4%, respectively. Combined with ICP-OES and TGA, the chemical compositions of two PBAs can be determined as Na2.008Ni0.269Co0.731[Fe(CN)6]0.997•2.09H2O and Na2.047Co[Fe(CN)6]0.997•2.19H2O, respectively. It demonstrates the two PBAs samples show similar high Na and low vacancy contents. According to FTIR spectra [Figure 1D], the peaks at ~3615 cm-1 and 1615 cm-1 are attributed to the O-H and H-O-H bending modes of the water molecules, the absorption peaks at ~2070 cm-1 and 586 cm-1 are assigned to the stretching vibration of C≡N and Fe-CN bonds, showing the characteristic functional groups of PBAs[28]. The SEM images of Co0.7Ni0.3HCF [Figure 1E] exhibit cubic morphologies with diameters ranging from 100 to 400 nm, which have sharp edges and corners. From the EDS images mapping, all elements of Co0.7Ni0.3HCF are uniformly distributed throughout the particles.
Figure 1. Analysis of the structure and morphology of the PBA samples. (A and B) Rietveld refinements and schematic illustrations of the structures of the Co0.7Ni0.3HCF. (C) TGA curves and (D) FTIR spectra of Co0.7Ni0.3HCF and CoHCF. (E) SEM and Energy dispersive spectroscopy (EDS) images mapping of Co0.7Ni0.3HCF.
Electrochemical performance at various temperatures
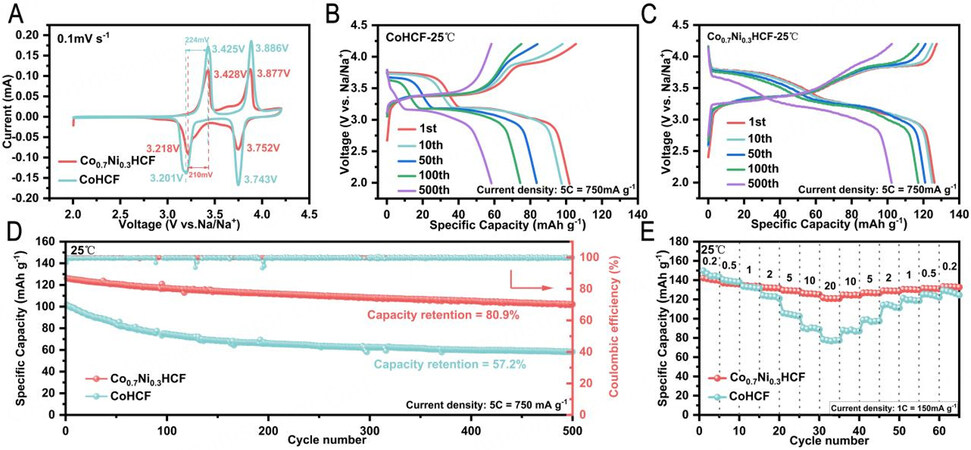
Figure 2A-E compares the electrochemical performance of Co0.7Ni0.3HCF and CoHCF at 25 °C. In the electrochemical window of 2.0-4.2 V, nickel is generally considered inactive and unable to provide capacity in PBAs[46]. Actually, the charge/discharge curves of CoxNi1-xHCF [Supplementary Figure 2A and B] clearly demonstrate that the specific capacities gradually decrease with the increasing content of Ni in CoxNi1-xHCF (x = 1.0, 0.9, …, 0), while the cycling stability is improved remarkably, as shown in
Figure 2. Electrochemical properties of Co0.7Ni0.3HCF and CoHCF at 25 °C (1 C = 150 mA g -1). (A) Typical CV curves measured at a scan rate of 0.1 mV s-1. Charge/discharge voltage profiles of (B) Co0.7Ni0.3HCF and (C) CoHCF at 5 C at different cycles. (D) Long-cycle performance at 5 C. (E) Rate performance at 0.2-20 C.
The cyclic voltammetry (CV) curve [Figure 2A] of CoHCF displays two pairs of well-defined and symmetric oxidation/redox peaks at 3.425 V/3.201 V and 3.886 V/3.743 V, corresponding to the redox reactions of N-coordinated Co2+/Co3+ and C-coordinated Fe2+/Fe3+ during the reversible sodium-ion insertion/extraction process respectively[47], which agrees with the two charge/discharge voltage plateaus. Co0.7Ni0.3HCF displays two pairs of oxidation/redox peaks at 3.428 V/3.218 V and 3.877 V/3.752 V, showing smaller voltage hysteresis of 0.210 V than CoHCF of 0.224 V. As shown in the Figure 2B and C, with the increasing of cycle numbers, the higher voltage plateau of CoHCF gradually vanishes, suggesting a decrease in the capacity contribution from the C-coordinated Fe2+/Fe3+ couple. Whereas for the Co0.7Ni0.3HCF, there is no obvious change in the charge/discharge profiles within the first 100 cycles, which exhibits much higher capacity retention of Co0.7Ni0.3HCF. It demonstrates the introduction of Ni can retain the redox activity of N-coordinated Co2+/Co3+ and C-coordinated Fe2+/Fe3+ for long cycles.
Figure 2D compares the capacity retention of Co0.7Ni0.3HCF and CoHCF at 5 C. Co0.7Ni0.3HCF delivers a specific discharge capacity of 126.2 mAh g-1 and a high capacity retention of 80.9% at 5 C after 500 cycles at 25 °C. In contrast, CoHCF only delivers a discharge capacity of 102.2 mAh g-1 and a lower capacity retention of 57.2% under similar charge and discharge conditions. Moreover, Co0.7Ni0.3HCF displays an outstanding rate (0.2-20 C) capability, which is much superior to that of the CoHCF [Figure 2E]. In particular,
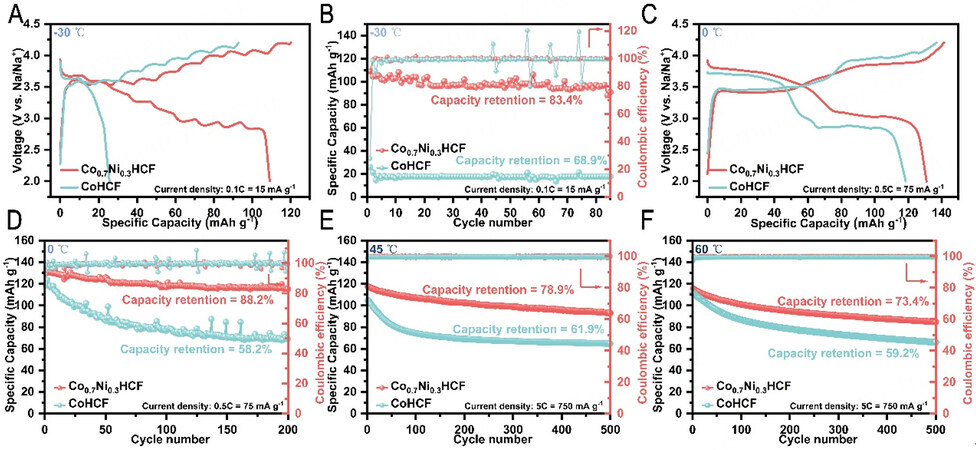
Figure 3 presents the electrochemical performance of Co0.7Ni0.3HCF and CoHCF from -30 °C to 60 °C. As shown in Figure 3A and B, CoHCF only delivers 25.7 mAh g-1 at the rate of 0.1 C at -30 °C. It is remarkable that Co0.7Ni0.3HCF can be charged at such a low temperature of -30 °C, while most secondary batteries cannot be charged at low temperatures. Moreover, it exhibits a high specific capacity of 109 mAh g-1 at 0.1 C and 83.4% capacity retention over 80 cycles at -30 °C. Figure 3C shows the charge/discharge voltage profiles of Co0.7Ni0.3HCF and CoHCF at 0 °C. Compared to CoHCF, Co0.7Ni0.3HCF shows two obvious charge/discharge platforms with much less polarization. At 0.5 C, the Co0.7Ni0.3HCF shows an initial discharge capacity of 130.9 mA h g-1 and maintains the capacity up to 115.5 mAh g-1 after 200 cycles at 0 °C [Figure 3D]. As shown in Figure 3E and F, Co0.7Ni0.3HCF also exhibits great cyclability at high temperatures. It delivers an initial capacity of about 120 mA h g-1 at a high rate of 5 C with capacity retention of 78.9% and 73.4% at 45 °C and 60 °C, respectively. As a comparison, CoHCF only exhibits about 110 mA h g-1 and maintains 61.9% and 59.2% of its capacity at the same temperature and current density, respectively. In summary, Co0.7Ni0.3HCF shows improved electrochemical performance than CoHCF over a wide temperature range. Additionally, we have made a comparison between the outstanding all-climate electrochemical performance of Co0.7Ni0.3HCF in this work and the existing literature
Figure 3. Electrochemical properties of Co0.7Ni0.3HCF and CoHCF at different temperatures (-30/0/45/60 °C). (A and C) Charge/discharge voltage profiles of Co0.7Ni0.3HCF and CoHCF at different rates (0.1/0.5 C) and temperatures (-30/0 °C). (B and D-F) Long-cycle performance of Co0.7Ni0.3HCF and CoHCF at different rates (0.1/0.5/5/5 C) and temperatures (-30/0/45/60 °C) from 2 V to 4.2 V.
Sodium-ion storage mechanism investigation
Figure 4A and B display ex-situ XRD patterns of Co0.7Ni0.3HCF and CoHCF at various charge/discharge depth. The crystalline structures of Co0.7Ni0.3HCF and CoHCF electrodes in the initial state are both monoclinic, indicating the Na content in Co0.7Ni0.3HCF and CoHCF frameworks is high. During the charging process from 2 V to 4.2 V , sodium ions are gradually extracted from the lattice, and peaks of 200 plane at around 17° and 400 plane at around 35° shift to a higher angle gradually, showing that the lattice size decreases gradually during a continuous solid-solution reaction mechanism. As the sodium ions further de-intercalated, XRD results show a merging of multi-peak from 23° to 25° indexed as -211, 020, 002 and 211 of monoclinic structure into a single sharp peak of 220 of cubic phase around 25°, indicating a two-phase transition from monoclinic phase to cubic phase. This is consistent with the previous reports[39] that when Na content is relatively low, the system energy tends to be reduced by correcting the distorted monoclinic framework skeleton, and it is the opposite when Na content is high. The lattice with very low Na content tends to exhibit cubic phase with lower energy rather than monoclinic phase to maintain stability. When charged to 4.2 V, Co0.7Ni0.3HCF and CoHCF have completely transformed into the cubic phase, indicating that large amounts of sodium ions are released, which corresponds to the high charge specific capacity. The Rietveld refinements and schematic illustrations of the cubic phase (Charged) of Co0.7Ni0.3HCF and CoHCF are shown in Supplementary Figure 7, the structural parameters of which are shown in Supplementary Table 2. During the discharging process from 4.2 V to 2 V, as sodium ions insert into the lattice, the energy of the system tends to decrease by twisting the skeleton, so the crystalline structure transitions to a more stable monoclinic phase. During the charging and discharging process, the lattice shows a reversible two-phase transition process from monoclinic to cubic phase, as shown in Figure 4C.
Figure 4. Sodium storage mechanism at 25 °C. Ex-situ XRD patterns of the (A) Co0.7Ni0.3HCF and (B) CoHCF electrodes at different states during the first cycle. The dots in different colors in the charge-discharge curves (right) represent the states tested by ex-situ XRD. (C) Schematic phase evolution as a result of Na+ extraction/insertion from/into the Co0.7Ni0.3HCF.
Even though the specific capacity of CoHCF at a low rate is high, the cycle stability is poor for long-term cycling because of incompletely reversible lattice distortion. According to the much smaller peaks shift for Co0.7Ni0.3HCF in the ex-situ XRD patterns during the charging and discharging process, the volume change of Co0.7Ni0.3HCF lattice during cycling is significantly lower than that of CoHCF. The volume change rate during the discharge process can be calculated according to the Rietveld refinements of fully charged and discharged electrodes. The volume change rate of Co0.7Ni0.3HCF and CoHCF during the two-phase transition is about 10.78% and 14.27%, respectively. Detailed lattice parameters are provided in Supplementary Figure 8 and Supplementary Table 3. The result indicates that the better cycling stability of Co0.7Ni0.3HCF than CoHCF is due to the suppressed structural distortion and the reversibility of volume change determines the cycling stability. Since the two-phase transition is caused by sufficient sodium ions de-/insertion, phase transition always relates to high capacity. Therefore, considering both the capacity and cycling stability, it is of particular significance to control the lattice distortion within a small range while ensuring more sodium ions are de-/intercalated. Here, Ni substitution in CoHCF has been successfully achieved.
Furthermore, XRD patterns and FTIR spectra of Co0.7Ni0.3HCF and CoHCF electrodes collected before and after 500 cycles are shown in Supplementary Figure 9. After 500 cycles, the peak corresponding to the 200 plane of CoHCF shifts from 16.897° to 16.940° (0.043° offset), indicating that the Na content of CoHCF electrode is reduced after 500 cycles, while the peak of Co0.7Ni0.3HCF mainly does not shift, suggesting robust structural stability during long-term cycling. The Rietveld refinements of Co0.7Ni0.3HCF and CoHCF after 500 cycles are shown in Supplementary Figure 10. According to the lattice parameters of Co0.7Ni0.3HCF and CoHCF before and after 500 cycles [Supplementary Table 4], the volume change rates before and after 500 cycles of Co0.7Ni0.3HCF and CoHCF are 0.04 % and 1.12 %, respectively. According to the occupancy of sodium ions in the lattice, reduced percentages of sodium ions of Co0.7Ni0.3HCF and CoHCF before and after 500 cycles are 0.79% and 4.01%, respectively. Therefore, it can be preliminarily judged that
It is also confirmed by the results of FTIR tests at different cycles. The absorption peaks at ~2075 cm-1 corresponding to the stretching vibration of C≡N of Co0.7Ni0.3HCF and CoHCF shift to higher wavenumbers (4 cm-1 and 9 cm-1 offset, respectively) after 500 cycles, indicating that the average valence state of transition metals in CoHCF is higher than that in Co0.7Ni0.3HCF[48,49]. This result indicates that the percentage of sodium retained in Co0.7Ni0.3HCF after long-term cycling is higher than that in CoHCF compared with pristine electrodes.
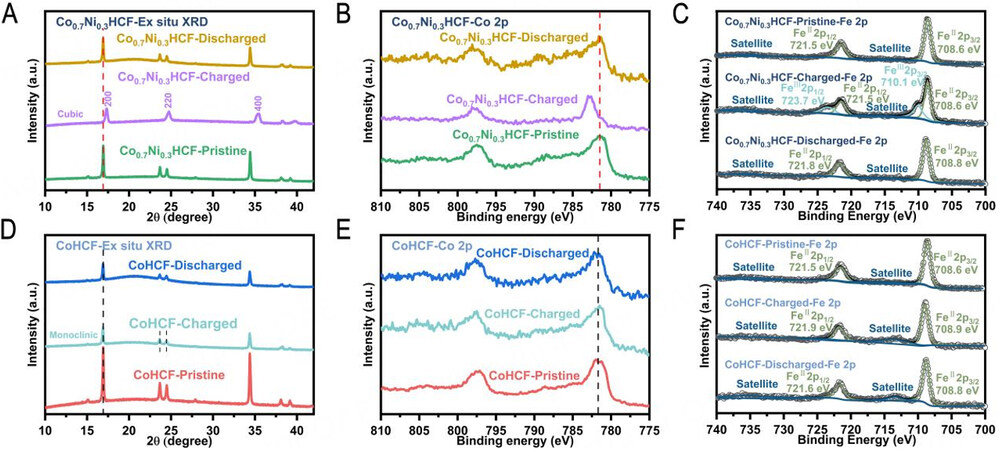
As shown in Figure 5A, the multi-peak from 23° to 25° indexed as -211, 020, 002 and 211 of monoclinic structure transform into a single peak corresponding to the 220 plane of cubic phase around 25°, indicating the phase transition from monoclinic to cubic has occurred at -30 °C in charged Co0.7Ni0.3HCF, which is owing to the extraction of enough sodium ions from the lattice. While discharge from 4.2 to 2 V at -30 °C, the phase transition occurs reversibly from cubic to monoclinic phase, indicating the extracted sodium ions can reversibly insert into the lattice even at -30 °C. This reversible two-phase transition is similar to that occurring at room temperature of Co0.7Ni0.3HCF, demonstrating a fast ion transfer kinetics in Co0.7Ni0.3HCF. In contrast, Figure 5D presents no phase transition for charged CoHCF, which maintains the monoclinic phase during the charging and discharging process at -30 °C, suggesting that too few sodium ions are extracted from the framework of CoHCF to induce a phase transition. This result can be ascribed to the fact that sodium ions in CoHCF are difficult to migrate at -30 °C and most sodium ions are still preserved in the lattice. The XPS[50] test results of Co 2p at -30 °C in Figure 5B and E show that the binding energy of Co 2p peak in CoHCF almost does not change during cycling at -30 °C, while the peak of Co 2p exhibits a significant peak shift towards higher bind energy from Co0.7Ni0.3HCF-Pristine to Co0.7Ni0.3HCF-Charged, revealing obvious electronic migration has occurred in the cobalt atoms of Co0.7Ni0.3HCF-Charged. The XPS data of Fe 2p at -30 °C are shown in Figure 5C and F for Co0.7Ni0.3HCF and CoHCF samples. Co0.7Ni0.3HCF-Charged can be fitted with an obvious FeIII 2p peak located at 710.1 eV and 723.7 eV, which is not found in CoHCF-Charged. It is clear that the electrochemical activity of transition metals in CoHCF is severely inhibited. The valence change of transition metals shows that Co0.7Ni0.3HCF has higher redox activity at low temperatures.
Figure 5. Sodium storage mechanism at -30 °C. (A and D) Ex-situ XRD patterns. (B and E) Ex-situ XPS Co 2p spectra and (C and F) Ex-situ XPS Fe 2p of Co0.7Ni0.3HCF and CoHCF at different redox states including as-prepared electrodes (Co0.7Ni0.3HCF/CoHCF-Pristine), electrodes charged to 4.2 V (Co0.7Ni0.3HCF/CoHCF-Charged) and electrodes charged to 4.2 V and then discharged to 2.0 V (Co0.7Ni0.3HCF/
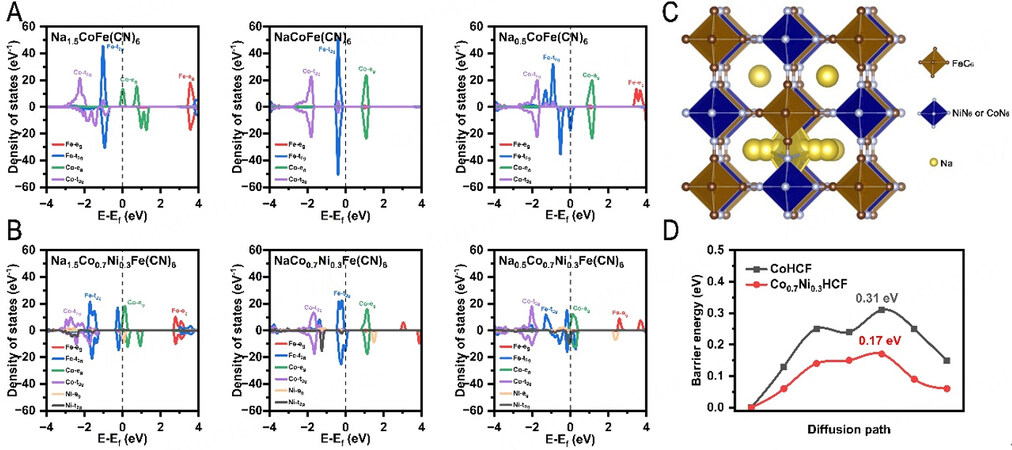
According to the partial density of states (PDOS) of Co0.7Ni0.3HCF and CoHCF, the bandgap of
Figure 6. First-principles calculations. Electronic density of states of (A) CoHCF and (B) Co0.7Ni0.3HCF at different Na concentrations, including Na-1.5, Na-1, and Na-0.5. (C) The schematic of the calculated Na+ migration paths within the lattice of the Co0.7Ni0.3HCF and CoHCF model structures. (D) Migration energy barriers of the Na+-ion diffusion within the lattice of the Co0.7Ni0.3HCF and CoHCF.
CONCLUSIONS
In conclusion, to address the low actual specific capacity, poor cycling and poor rate capability of CoHCF as the cathode material of SIBs, partial substitution of Co by Ni is proposed in low-defect and Na-enriched CoHCF sample. The introduction of Ni inhibits large lattice distortion during cycling, improves the electronic conductivity and reduces the migration barrier of sodium ions. Benefiting from the structural effect of Ni substitution, Co0.7Ni0.3HCF not only exhibits high capacity up to 142.2 mAh g-1, but also achieves high capacity retention of more than 80% after 500 cycles at room temperature. Even at such a low temperature of -30 °C, a reversible two-phase transition between monoclinic phase and cubic phase is occurred due to adequate sodium-ions insertion and extraction, which provides a high specific capacity of 109 mAh g-1. Moreover, Co0.7Ni0.3HCF also shows stable electrochemical performance at high temperatures of 45 °C and 60 °C owing to suppressed lattice variation and stable framework. It demonstrates that Ni-substituted double redox active PBAs are potential candidates for cathode material in all-climate sodium-ion batteries due to their unique structural characteristics. However, Co and Ni are too expensive to apply on a large scale, so the exploration of the next generation of low-cost all-climate sodium-ion batteries is worth studying.
DECLARATIONS
Authors’ contributionsMethodology, formal analysis and writing manuscript: Zhang J, Wan J
Data analysis and technical support: Ou M, Liu S, Huang B
Data acquisition: Xu J, Lin Y
Supervision, writing - review and editing: Sun S, Xu Y, Fang C, Han J
Availability of data and materialsThe data supporting our findings can be found in the Supplementary Material.
Financial support and sponsorshipThis work was supported by National Natural Science Foundation of China (Grant No. 52172201, 51732005, 51902118, 52102249), China Postdoctoral Science Foundation (Grant Nos. 2019M662609 and 2020T130217), and the international postdoctoral exchange fellowship program No. PC2021026 for financial support. We thank the Analytical and Testing Centre and the State Key Laboratory of Materials Processing and Die & Mould Technology and the Experiment Center for Advanced Manufacturing and Technology in School of Mechanical Science & Engineering of HUST for the material characterization.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
2. Larcher D, Tarascon JM. Towards greener and more sustainable batteries for electrical energy storage. Nat Chem 2015;7:19-29.
3. Qian J, Wu C, Cao Y, et al. Prussian blue cathode materials for sodium-ion batteries and other ion batteries. Adv Energy Mater 2018;8:1702619.
4. Fang Y, Luan D, Lou XW. Recent advances on mixed metal sulfides for advanced sodium-ion batteries. Adv Mater 2020;32:e2002976.
5. Li Y, Yang Y, Lu Y, et al. Ultralow-concentration electrolyte for na-ion batteries. ACS Energy Lett 2020;5:1156-8.
6. Piernas Muñoz MJ, Castillo Martínez E. Electrochemical performance of prussian blue and analogues in aqueous rechargeable batteries. In Prussian Blue Based Batteries; 2018, pp. 23-44.
7. Wessells CD, Peddada SV, Huggins RA, et al. Nickel hexacyanoferrate nanoparticle electrodes for aqueous sodium and potassium ion batteries. Nano Lett 2011;11:5421-5.
8. Peng J, Gao Y, Zhang H, et al. Ball milling solid-state synthesis of highly crystalline prussian blue analogue Na2-xMnFe(CN)6 cathodes for all-climate sodium-ion batteries. Angew Chem Int Ed 2022;61:e202205867.
9. Imanishi N, Morikawa T, Kondo J, et al. Lithium intercalation behavior into iron cyanide complex as positive electrode of lithium secondary battery. J Power Sources 1999;79:215-9.
10. Pramudita JC, Schmid S, Godfrey T, et al. Sodium uptake in cell construction and subsequent in operando electrode behaviour of Prussian blue analogues, Fe[Fe(CN)6](1-x)·yH2O and FeCo(CN)6. Phys Chem Chem Phys 2014;16:24178-87.
11. Peng J, Zhang W, Liu Q, et al. Prussian blue analogues for sodium-ion batteries: past, present, and future. Adv Mater 2022;34:2108384.
12. Wu X, Wu C, Wei C, et al. Highly crystallized Na2CoFe(CN)6 with suppressed lattice defects as superior cathode material for sodium-ion batteries. ACS Appl Mater Interfaces 2016;8:5393-9.
13. Shang Y, Li X, Song J, et al. Unconventional Mn vacancies in Mn-Fe prussian blue analogs: suppressing jahn-teller distortion for ultrastable sodium storage. Chem 2020;6:1804-18.
14. Jiang M, Hou Z, Wang J, et al. Balanced coordination enables low-defect Prussian blue for superfast and ultrastable sodium energy storage. Nano Energy 2022;102:107708.
15. Peng J, Wang J, Yi H, et al. A Dual-insertion type sodium-ion full cell based on high-quality ternary-metal prussian blue analogs. Adv Energy Mater 2018;8:1702856.
16. Wang W, Gang Y, Hu Z, et al. Reversible structural evolution of sodium-rich rhombohedral Prussian blue for sodium-ion batteries. Nat Commun 2020;11:980.
17. Gong W, Wan M, Zeng R, et al. Ultrafine prussian blue as a high-rate and long-life sodium-ion battery cathode. Energy Technol 2019;7:1900108.
18. Han B, Zhang D, Liu X, et al. Ordered assembly of potassium cobalt hexacyanoferrate hollow multivoid nanocuboid arrays for high-performance aqueous K-ion batteries towards all-climate energy storage. J Mater Chem A 2022;10:13508-18.
19. Tang Y, Li W, Feng P, et al. Investigation of alkali-ion (Li, Na and K) intercalation in manganese hexacyanoferrate KxMnFe(CN)6 as cathode material. Chem Eng J 2020;396:125269.
20. Shao T, Li C, Liu C, et al. Electrolyte regulation enhances the stability of Prussian blue analogues in aqueous Na-ion storage. J Mater Chem A 2019;7:1749-55.
21. Feng F, Chen S, Zhao S, et al. Enhanced electrochemical performance of MnFe@NiFe Prussian blue analogue benefited from the inhibition of Mn ions dissolution for sodium-ion batteries. Chem Eng J 2021;411:128518.
22. Gebert F, Cortie DL, Bouwer JC, et al. Epitaxial nickel ferrocyanide stabilizes jahn-teller distortions of manganese ferrocyanide for sodium-ion batteries. Angew Chem Int Ed 2021;60:18519-26.
23. Qiao Y, Wei G, Cui J, et al. Prussian blue coupling with zinc oxide as a protective layer: an efficient cathode for high-rate sodium-ion batteries. Chem Commun 2019;55:549-52.
24. Kim J, Yi SH, Li L, et al. Enhanced stability and rate performance of zinc-doped cobalt hexacyanoferrate (CoZnHCF) by the limited crystal growth and reduced distortion. J Energy Chem 2022;69:649-58.
25. Wang B, Han Y, Chen Y, et al. Gradient substitution: an intrinsic strategy towards high performance sodium storage in Prussian blue-based cathodes. J Mater Chem A 2018;6:8947-54.
26. Peng J, Zhang B, Hua W, et al. A disordered Rubik’s cube-inspired framework for sodium-ion batteries with ultralong cycle lifespan. Angew Chem Int Ed 2023;62:e202215865.
27. Hu J, Tao H, Chen M, et al. Interstitial water improves structural stability of iron hexacyanoferrate for high-performance sodium-ion batteries. ACS Appl Mater Interfaces 2022;14:12234-42.
28. Wang W, Gang Y, Peng J, et al. Effect of eliminating water in prussian blue cathode for sodium-ion batteries. Adv Funct Mater 2022;32:2111727.
29. Bhatt P, Thakur N, Mukadam MD, et al. Evidence for the existence of oxygen clustering and understanding of structural disorder in prussian blue analogues molecular magnet M1.5[Cr(CN)6]·zH2O (M = Fe and Co): reverse monte carlo simulation and neutron diffraction study. J Phys Chem C 2013;117:2676-87.
30. Wardecki D, Ojwang DO, Grins J, et al. Neutron diffraction and EXAFS studies of K2x/3Cu[Fe(CN)6]2/3·nH2O. Cryst Growth Des 2017;17:1285-92.
31. Oumellal Y, Delpuech N, Mazouzi D, et al. The failure mechanism of nano-sized Si-based negative electrodes for lithium ion batteries. J Mater Chem 2011;21:6201-8.
32. Liu W, Yang M, Wu H, et al. Enhanced cycle life of Si anode for Li-ion batteries by using modified elastomeric binder. Electrochem Solid-State Lett 2005;8:A100-3.
34. Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 1999;59:1758-75.
35. Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett 1996;77:3865-68.
37. Jain A, Hautier G, Ong SP, et al. Formation enthalpies by mixing GGA and GGA +
38. Smidstrup S, Pedersen A, Stokbro K, et al. Improved initial guess for minimum energy path calculations. J Chem Phys 2014;140:214106.
39. Xu Y, Wan J, Huang L, et al. Structure distortion induced monoclinic nickel hexacyanoferrate as high-performance cathode for na-ion batteries. Adv Energy Mater 2019;9:1803158.
40. Rietveld HM. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Cryst 1967;22:151-2.
41. Loopstra BO, Rietveld HM. Further refinement of the structure of WO. Acta Cryst 1969;B25:1420-1.
42. Feng Z, Hou Q, Zheng Y, et al. Method of artificial intelligence algorithm to improve the automation level of Rietveld refinement. Comp Mater Sci 2019;156:310-4.
43. Cui X, Feng Z, Jin Y, et al. AutoFP: a GUI for highly automated Rietveld refinement using an expert system algorithm based on FullProf. J Appl Cryst 2015;48:1581-6.
44. McCusker LB, Von Dreele RB, Cox DE, et al. Rietveld refinement guidelines. J Appl Cryst 1999;32:36-50.
46. Peng J, Ou M, Yi H, et al. Defect-free-induced Na+ disordering in electrode materials. Energy Environ Sci 2021;14:3130-40.
47. Takachi M, Matsuda T, Moritomo Y. Cobalt hexacyanoferrate as cathode material for Na+secondary battery. Appl Phys Express 2013;6:025802.
48. Li W, Zhang F, Xiang X, et al. Electrochemical properties and redox mechanism of Na2Ni0.4Co0.6[Fe(CN)6] Nanocrystallites as high-capacity cathode for aqueous sodium-ion batteries. J Phys Chem C 2017;121:27805-12.
49. Luo D, Lei P, Tian G, et al. Insight into electrochemical properties and reaction mechanism of a cobalt-rich prussian blue analogue cathode in a NaSO3CF3 electrolyte for aqueous sodium-ion batteries. J Phys Chem C 2020;124:5958-65.
50. Fang D, He F, Xie J, et al. Calibration of binding energy positions with C1s for XPS results. J Wuhan Univ Technol-Mat Sci Ed 2020;35:711-8.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zhang J, Wan J, Ou M, Liu S, Huang B, Xu J, Sun S, Xu Y, Lin Y, Fang C, Han J. Enhanced all-climate sodium-ion batteries performance in a low-defect and Na-enriched Prussian blue analogue cathode by nickel substitution. Energy Mater 2023;3:300008. http://dx.doi.org/10.20517/energymater.2022.71
AMA Style
Zhang J, Wan J, Ou M, Liu S, Huang B, Xu J, Sun S, Xu Y, Lin Y, Fang C, Han J. Enhanced all-climate sodium-ion batteries performance in a low-defect and Na-enriched Prussian blue analogue cathode by nickel substitution. Energy Materials. 2023; 3(1): 300008. http://dx.doi.org/10.20517/energymater.2022.71
Chicago/Turabian Style
Zhang, Jingwen, Jing Wan, Mingyang Ou, Siying Liu, Bicheng Huang, Jia Xu, Shixiong Sun, Yue Xu, Yaqing Lin, Chun Fang, Jiantao Han. 2023. "Enhanced all-climate sodium-ion batteries performance in a low-defect and Na-enriched Prussian blue analogue cathode by nickel substitution" Energy Materials. 3, no.1: 300008. http://dx.doi.org/10.20517/energymater.2022.71
ACS Style
Zhang, J.; Wan J.; Ou M.; Liu S.; Huang B.; Xu J.; Sun S.; Xu Y.; Lin Y.; Fang C.; Han J. Enhanced all-climate sodium-ion batteries performance in a low-defect and Na-enriched Prussian blue analogue cathode by nickel substitution. Energy Mater. 2023, 3, 300008. http://dx.doi.org/10.20517/energymater.2022.71
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 19 clicks
Cite This Article 19 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.