Zn-based batteries for energy storage
Abstract
Zn-based electrochemistry is considered to be the most promising alternative to Li-ion batteries due to its abundant reserves and cost-effectiveness. In addition, aqueous electrolytes are more convenient to be used in
Keywords
INTRODUCTION
Since the first commercial lithium-ion battery (LIBs) developed by Yoshio Nishi in 1991, LIBs have dominated the market for portable electronic devices and electric vehicles[1-3]. However, for LIBs, limited lithium resources, soaring costs and safety hazards still inevitably hinder their development. Therefore, some alternative energy storage battery systems with lower cost, such as sodium-ion batteries (SIBs) and potassium-ion batteries (PIBs), are put on the agenda for replacing LIBs[4,5]. At the same time, some metal-based batteries with high theoretical energy density, such as lithium-oxygen batteries and lithium-sulfur batteries, have also been proposed to cope with the increasing demand for high-energy-density batteries[6,7]. Nonetheless, the electrolytes used in those metal-based batteries are usually both water and air sensitive, posing safety and environmental concerns. In contrast, aqueous batteries (Zn2+, Fe2+, Mg2+, Al3+, and so forth) are considered promising next-generation battery systems due to their safety and environmental friendliness[8,9]. Among them, rechargeable Zn-based batteries are gaining increasing attention for replacing Li-ion batteries due to their high theoretical energy density, good stability, low cost and environmental friendliness[10].
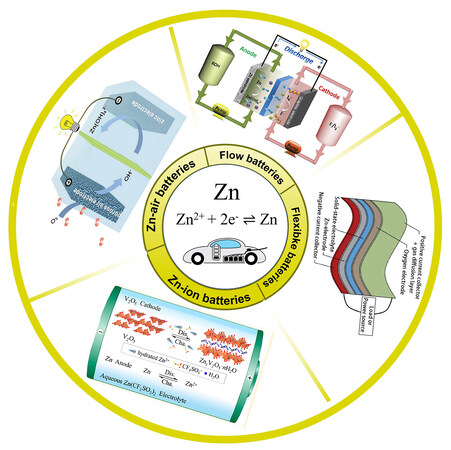
Aqueous Zn-based batteries are built on reversible Zn2+/Zn dissolution/deposition reactions with a redox potential of 0.76 V vs. standard hydrogen electrode (SHE)[11-16]. Depending on the battery system, the electrolyte can be neutral, acidic or alkaline solution. According to the electrochemistry of cathode materials, Zn-based batteries mainly consist of the following battery systems: Zn-ion batteries, Zn-based redox flow batteries and Zn-air batteries. Besides, some modified batteries, such as flexible devices, have also been proposed [Figure 1]. Despite the rapid development of Zn-based batteries in recent years, more efforts are still needed to drive them toward commercialization. In this focused review, recent progress on aqueous Zn-based battery systems is outlined. The operating mechanisms of each battery system are briefly introduced, followed by their existing challenges and research directions. Perspectives are also provided for the future development of Zn-based battery systems.
ZN BASED BATTERIES
Zn anode
As an important part of Zn-based battery systems, Zn anodes usually exist in the form of Zn foils in the battery system. When discharging, Zn loses two electrons to form Zn2+ and dissolves into the electrolyte; when charging, it regains two electrons and is plated onto the Zn flakes[17,18]. Zn anodes undergo side reactions during electrochemical processes, especially the hydrogen evolution reaction (HER) due to its poor thermodynamic stability in aqueous electrolytes, leading to severe self-discharge reactions[19-23]. There are two main solutions to this problem: surface engineering and electrolyte additives. Surface engineering strategies usually employ a layer of artificial SEI, such as inorganic passivation layers, carbon coating layers or polymer membranes, to prevent the electrolyte from directly contacting the Zn metal anode[24,25]. For example, Li et al. adopted graphite-modified Zn anode, a mitigated corrosion reaction was obtained and a high Coulombic efficiency was achieved[26]. Additionally, some organic additives, such as thiourea, diethyl ether (Et2O), sodium dodecyl sulfate (SDS), or cetyltrimethylammonium bromide (CTAB), contribute to alleviating the corrosion of Zn metal anode[27].
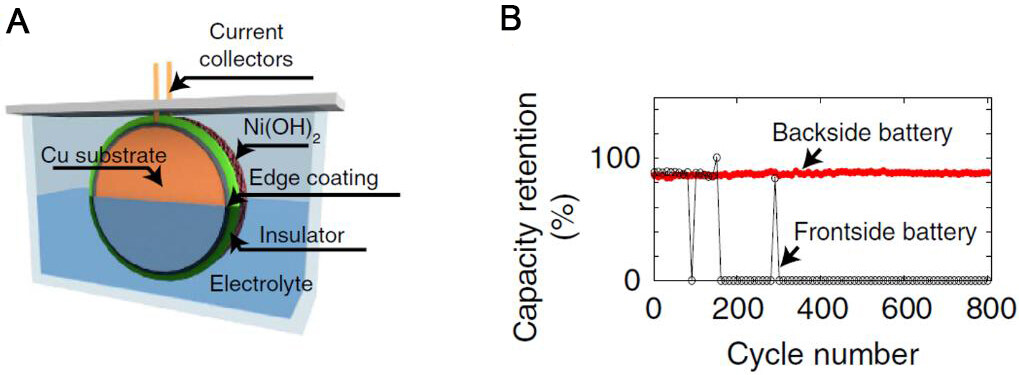
Another major problem is the growth of dendrites in developing alkaline rechargeable batteries. The growth of dendrites will not only lead to the growth of “dead Zn” which degrades the coulombic efficiency of the battery, but also may pierce the separator and cause the battery to short circuit. One of the most direct solutions is to modify the surface of the Zn anode to form a stable solid-electrolyte interface (SEI).
Zn-ion batteries
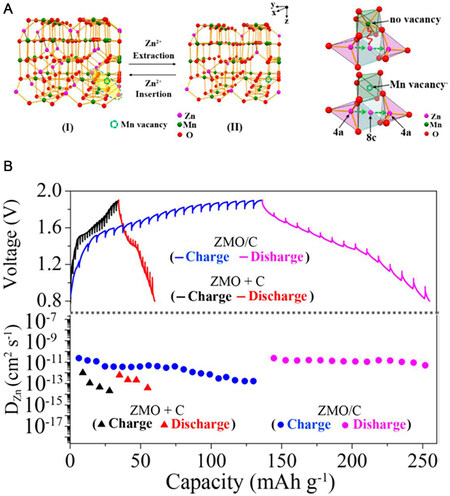
A Zn-ion battery consists of four components, a Zn metal anode, an metal oxides cathode, a separator, and an electrolyte. Generally, metal oxides are used as cathode materials in Zn-ion batteries, including manganese-based, vanadium-based, and Prussian blue analogs and organic cathode materials[12,31]. The characteristics of some cathode materials are listed in Table 1 below[32-37]. The characteristics of some cathode materials are listed in Table 1 below. Depending on how they store Zn ions, these materials can be broadly divided into two categories: intercalation chemistry or conversion chemistry. For intercalation-type materials, Zn2+ undergoes reversible intercalation/deintercalation reactions between the electrolyte and the cathode material during the charge-discharge process. One of the most serious problems in those cathode materials is structural collapse during charge and discharge. Defect engineering (cation vacancy or oxygen vacancy) is widely adopted to deal with this problem. A cation-deficient ZnMn2O4 spinel structure was proposed as a cathode material by Cai et al., demonstrating the positive effect of defects on the structural stability of the material [Figure 3A and B][38]. They believed that the migration of Zn2+ in this special structure was not affected by the large electrostatic repulsion, thus contributing to the improvement of the electrode kinetics. In addition, an oxygen-deficient β-MnO2 structure was introduced as a cathode by
The characteristics of some cathode materials in the Zn-ion batteries
| Cathode materials | Output voltage [V] | Capacity [mAh g-1]/energy Density [Wh kg-1] | Capacity retention/cycle numbers/current density | Mechanism | Ref. |
| Ni-doped Mn2O3 | 1.2 | 252 (0.1 A g-1)/327.6 | 85.6%/2500/1.0 A g-1 | H+ and Zn2+ coinsertion | [32] |
| MnO2 | 1.35 | 365 (0.5 A g-1)/486 | 93.3%/4000/4 A g-1 | H+ and NH4+ coinsertion | [33] |
| V4+-doped V2O5 | 0.6 | 430 (0.5 A g-1)/258 | 86%/1000/10 A g-1 | Zn2+ insertion | [34] |
| K2MnFe(CN)6 | 1.6 | 138 (0.2 A g-1)/221 | 72.4%/400/0.2 A g-1 | Zn2+ insertion | [35] |
| m-TAPA | 1.1 V | 210.7 (0.5 A g-1)/236 | 87.6%/1000/6 A g-1 | Cl- coordination | [36] |
| π-PMC | 0.4 | 122.5 (0.2 A g-1)/49 | 68.2%/1000/8 A g-1 | Zn2+ coordination | [37] |
As for the cathode materials based on the conversion reaction, they are usually based on the redox conversion between metal oxides and metal hydroxides, which can also be accompanied by the co-intercalation reaction of hydrogen ions. Zhang et al. found that α-MnO2 would bind a H+ during charging, and would further react with ZnSO4 and H2O to form ZnSO4[Zn(OH)2]3 in order to achieve charge balance[38]. In addition, some researchers found that the intercalation reaction of Zn2+ also triggers the structural transformation of the cathode material into layered ZnxMnO2 and/or ZnMn2O4 with the depth of discharge[28,40,41]. Besides inorganic materials, organic materials, such as quinone, have also been proposed as conversion reaction-based cathode materials, which can reversibly bind and release Zn ions. Organic cathode materials are getting more and more attention, and some compounds are gradually being reported, such as poly(pyrene-4,5,9,10-tetraone) (PPTO), quinone (C4Q), and polyaniline (PANI)[30,42]. A crystalline 3,4,9,10-perylenetetracarboxylic dianhydride (PTCDA) was introduced by Rodriguez-Perez et al. to adhere dimethyl metal ions in aqueous electrolytes, demonstrating an internal structural stability and superior electrochemical performance[43].
Neither of the above two methodes can effectively solve the inherent limitations regarding the voltage window and energy density of Zn-ion batteries. Obviously, the low electrochemical potential window of aqueous electrolytes with a redox potential of 1.23 V vs. SHE, severely limits the choice of high-voltage electrode materials. Therefore, widening the electrochemical cell window of aqueous electrolytes is crucial for high-pressure aqueous Zn-ion batteries. An effective method is to effectively prevent the water molecules of the electrolyte from contacting the Zn anode. For example, the NaCl/sodium alginate (SA) gel electrolyte exhibited an electrochemical window of 2.72 V due to the confinement of water molecules in the gel electrolyte through hydrogen bonding[44]. Consequently, the direct contact between the water molecules and the electrode material is largely alleviated. Another effective way is to form a salt concentration electrolyte. In the electrolyte with ultra-high salt concentration, the free water molecules will disappear due to the lack of water solvent, avoiding direct contact between the water molecules and electrodes. At the same time, the anions will enter into the solvated structure and induce the generation of anion-derived SEI, improving the stability of the electrode interface[45-47]. Therefore, some typical salt concentration electrolytes for high voltage batteries have been reported, including 21 m LiTFSI + 0.5 m ZnSO4[48],
Zn-air batteries
Generally speaking, a Zn-air battery consists of four components, a Zn metal anode, an air cathode, a separator, and an electrolyte. Generally, a high-concentration aqueous alkaline solution (NaOH or KOH) is used as the electrolyte, which can not only provide an alkaline environment, but also sufficient ionic conductivity[10,11,55-58]. The membrane is usually an anion exchange membrane, which allows the free movement of the anion OH- and prevents the passage of the cation Zn2+. The redox reactions occurring at the air cathode and the Zn anode are shown below[59-61]:
Air cathode reaction:
O2 + 2H2O + 4e- → 4OH- (1)
Zn anode reaction:
Zn + 2OH- → ZnO + H2O + 2e- (2)
Overall reaction:
O2 + 2Zn → 2ZnO (3)
As the core part of the air electrode, the activity and durability of the catalyst are the most important considerations in determining the performance of ZABs[62,63]. Although platinum (Pt) is considered to be one of the best catalysts for ORR, its scarcity and high price hinder its commercial application. Similarly, other noble metal catalysts such as iridium oxide (IrO2) and ruthenium oxide (RuO2) also face the same dilemma as Pt-based catalysts[64-66]. To reduce cost, transition metal compounds have been reported as promising ORR and OER electrocatalysts[65,67-69]. Although most of them do not perform as well as Pt-based catalysts for ORR, their acceptable OER activity makes them better choices for ZABs.
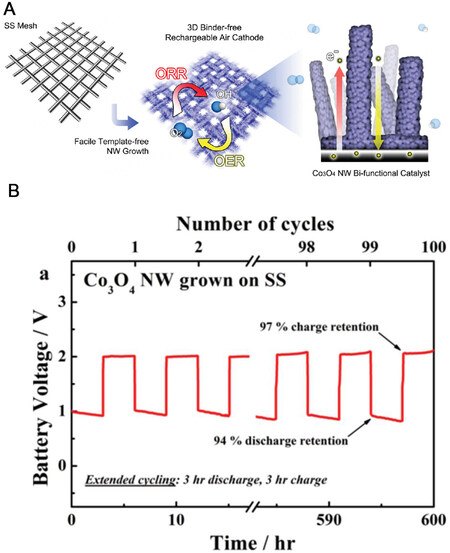
Besides metal-based catalysts, carbon-based materials are also widely used as catalysts in ZABs. They generally exhibit good chemical stability and electrical conductivity, which facilitates long-term electron transport during electrochemical processes, while the porous structure with high specific surface area facilitates the exposure of catalyst active sites and efficient mass transport[70-72]. Therefore, carbon-based catalysts have gained a lot of attention for developing reversible ZABs. Unfortunately, carbon corrosion inevitably occurs in alkaline electrolytes, and those catalysts are often prone to loss of structural stability, leading to a widening of the voltage gap[72,73]. In contrast, metal oxide-based catalysts usually have good durability, such as excellent durability of 600 h when using Co3O4 nanowire arrays as catalysts
Flow batteries
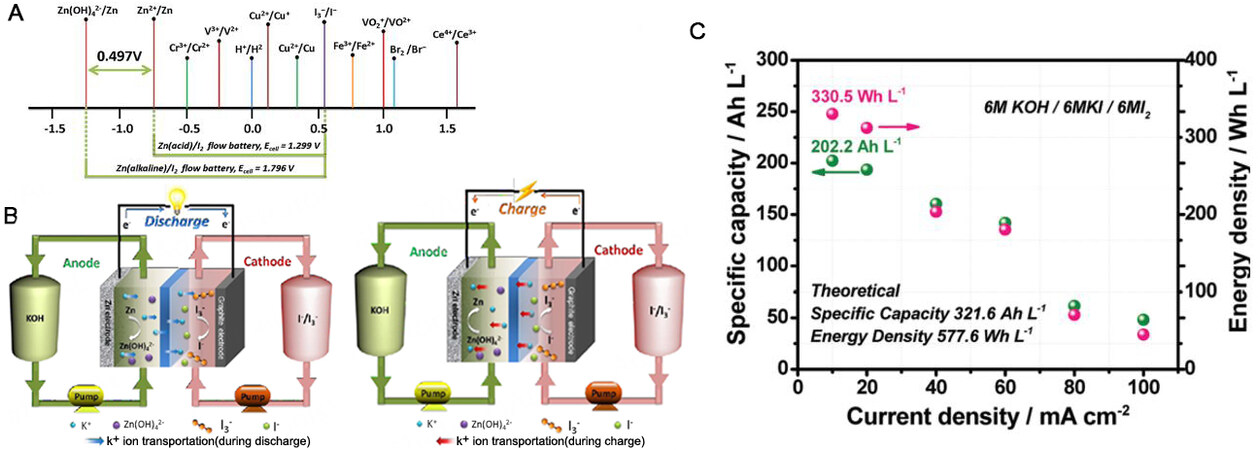
Zn-based redox flow batteries usually use Zn as the anode and redox pairs as electrodes, including Zn-Fe, Zn-Ce, Zn-halogen (Cl2, Br2, and I2), and Zn-organic couples [Figure 5][78-86]. Although substantial progress has been made in Zn-based flow batteries, more efforts are still needed to improve some important parameters to achieve the goal of commercialization. Current efforts mainly focus on optimization of the electrolyte, membrane and electrode[87]. This review focuses on the Zn-I2 flow battery as an example.
Figure 5. (A) Redox couples commonly adapted in flow batteries and corresponding standard reduction potentials; (B) schematic illustration of the charging and discharging mechanisms in alkaline Zn-I2 flow batteries; (C) specific capacity and energy density at different current densities of alkaline Zn-I2 flow batteries[85]. Reproduced with permission from Ref.[85]. Copyright 2018 Royal Society of Chemistry.
The typical structure of Zn-I2 is shown in Figure 5B[85]. Basically, repeated Zn2+/Zn deposition/dissolution reactions occur on the negative side of Zn in acidic or alkaline electrolytes, while conversion reactions between iodide anions (I-) and triiodide anions (I3-) occur on the positive side. Despite the advantages of
Flexible batteries
The adoption of flexible Zn-based batteries for wearable devices has gained increased attention due to their environmental friendliness and cost-effectiveness[18]. Flexible Zn-based batteries usually consist of flexible electrode materials and polymer electrolytes. Flexible cathode materials are usually integrated active electrode materials on flexible substrates, such as carbon cloth, nickel foam, stainless-steel mesh, etc.[93-95]. As shown in Figure 6, a typical fabrication process of flexible Zn-air battery cathodes was presented
Figure 6. (A) Schematic illustration of ordered arrays on nickel foam as self-supported for flexible Zn-air battery[96] Reproduced with permission from Ref.[96]. Copyright 2019 John Wiley & Sons, Inc.; (B) Schematic presentation of nanocellulose membrane functionalization and demonstration of flexible Zn-air battery[98]. Reproduced with permission from Ref.[98]. Copyright 2015 Royal Society of Chemistry.
Although the Zn-air battery has an ultra-high specific energy density, 6070 Wh L-1, it is not a suitable flexible battery system because its semi-open structure that ensures easy access of atmospheric oxygen to the battery system would lead to continuous water loss in the electrolyte and then causes the battery to fail[11]. Therefore, seeking a new cathode material that also has the characteristics of high specific energy density, but rarely suffers from the adverse effects of half-cells, may be the future direction. For example, Wang et al. developed a Zn-Co3O4 flexible battery with an energy density of 2807 Wh L-1[99]. In addition, Zn-ion batteries have also been adopted as flexible batteries due to their better rechargeability. For example, a Zn-MnO2 battery, with α-MnO2 nanofiber as the positive electrode, Zn sheets as the negative electrode and
PERSPECTIVES
This review describes the overall landscape of aqueous Zn-based batteries, including Zn-ion batteries, Zn-air batteries, redox flow batteries, and flexible batteries. Within each classification, the basic working principle and recent research progress are summarized. Currently, there are still many obstacles that need to be removed on the road to the commercialization of Zn-based batteries. Therefore, two main perspectives related to battery material design and intrinsic mechanism exploration are given as follows:
(1) Material design and optimization to improve electrochemical performance is still a challenge for various types of Zn-based battery configurations. For Zn-ion batteries, the search for materials that can withstand sustained multi-electron transfer while maintaining structural stability is strongly needed for both the intercalation and conversion cathodes. Electrocatalysts, with sufficient active sites, high activity and sufficient durability, are required in Zn-air batteries. The development of highly active cathode materials and cost-effective, stable and ion-conducting membranes is crucial for redox flow batteries. For flexible batteries, the mechanical flexibility and structural stability of battery materials are prerequisites for flexible devices, while the hydroxyl conductivity, water-holding ability, and alkali tolerance of gel electrolytes determine the performance of Zn-air batteries.
(2) Apart from material design, the electrochemical behavior of electrode materials is still not well understood. Deeper mechanistic studies are desperately needed to further improve the electrochemical performance of Zn-based batteries. Taking Zn-air batteries as an example, although metal-based materials are widely used as bifunctional catalysts in Zn-air batteries, it was only recently revealed that their derived metal hydroxides are the real active sites for OER. A metal hydroxide layer was found on the
DECLARATIONS
Authors’ contributionsContributed to this review: Xiao D, Lv X, Fan J
Revised this review: Li Q, Chen Z
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis research was financially supported by the National Natural Science Foundation of China (22209071), Natural Science Foundation of Jiangsu Province (BK20220339) and Natural Science Research in Colleges and universities of Jiangsu Province (22KJB150006, 22KJB430005).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Ding Y, Cano ZP, Yu A, Lu J, Chen Z. Automotive li-ion batteries: current status and future perspectives. Electrochem Energ Rev 2019;2:1-28.
3. Park OK, Cho Y, Lee S, Yoo H, Song H, Cho J. Who will drive electric vehicles, olivine or spinel? Energy Environ Sci 2011;4:1621.
5. Eftekhari A, Jian Z, Ji X. Potassium secondary batteries. ACS Appl Mater Interfaces 2017;9:4404-19.
7. Bruce PG, Freunberger SA, Hardwick LJ, Tarascon JM. Li-O2 and Li-S batteries with high energy storage. Nat Mater 2011;11:19-29.
8. Zhang T, Tao Z, Chen J. Magnesium-air batteries: from principle to application. Mater Horiz 2014;1:196-206.
9. Li M, Lu J, Ji X, et al. Design strategies for nonaqueous multivalent-ion and monovalent-ion battery anodes. Nat Rev Mater 2020;5:276-94.
10. Li Y, Lu J. Metal-air batteries: will they be the future electrochemical energy storage device of choice? ACS Energy Lett 2017;2:1370-7.
12. Ming J, Guo J, Xia C, Wang W, Alshareef HN. Zinc-ion batteries: materials, mechanisms, and applications. Mater Sci Eng R Rep 2019;135:58-84.
13. Hao J, Yuan L, Zhu Y, Jaroniec M, Qiao SZ. Triple-function electrolyte regulation toward advanced aqueous Zn-Ion batteries. Adv Mater 2022;34:e2206963.
14. Hao J, Yuan L, Johannessen B, et al. Studying the conversion mechanism to broaden cathode options in aqueous zinc-ion batteries. Angew Chem Int Ed 2021;60:25114-21.
15. Zampardi G, La Mantia F. Open challenges and good experimental practices in the research field of aqueous Zn-ion batteries. Nat Commun 2022;13:687.
16. Wang N, Wan H, Duan J, et al. A review of zinc-based battery from alkaline to acid. Mater Today Adv 2021;11:100149.
17. Deng Y, Liang R, Jiang G, Jiang Y, Yu A, Chen Z. The current state of aqueous Zn-based rechargeable batteries. ACS Energy Lett 2020;5:1665-75.
18. Li Y, Fu J, Zhong C, et al. Recent advances in flexible zinc-based rechargeable batteries. Adv Energy Mater 2019;9:1802605.
19. Liu Y, Li L, Ji X, Cheng S. Scientific challenges and improvement strategies of zn-based anodes for aqueous Zn-ion batteries. Chem Rec 2022;22:e202200114.
20. Wu M, Zhang G, Yang H, et al. Aqueous Zn-based rechargeable batteries: recent progress and future perspectives. InfoMat 2022;4.
21. Liu Y, Lu X, Lai F, et al. Rechargeable aqueous Zn-based energy storage devices. Joule 2021;5:2845-903.
22. Hao J, Li X, Zeng X, Li D, Mao J, Guo Z. Deeply understanding the Zn anode behaviour and corresponding improvement strategies in different aqueous Zn-based batteries. Energy Environ Sci 2020;13:3917-49.
23. Blanc LE, Kundu D, Nazar LF. Scientific challenges for the implementation of Zn-ion batteries. Joule 2020;4:771-99.
24. Jo YN, Prasanna K, Kang SH, et al. The effects of mechanical alloying on the self-discharge and corrosion behavior in Zn-air batteries. J Ind Eng Chem 2017;53:247-52.
25. Lee S, Kim Y, Eom S, Choi N, Kim K, Cho S. Improvement in self-discharge of Zn anode by applying surface modification for Zn-air batteries with high energy density. J Power Sources 2013;227:177-84.
26. Li Z, Wu L, Dong S, et al. Pencil drawing stable interface for reversible and durable aqueous zinc-ion batteries. Adv Function Mat 2021;31.
27. Liang M, Zhou H, Huang Q, Hu S, Li W. Synergistic effect of polyethylene glycol 600 and polysorbate 20 on corrosion inhibition of zinc anode in alkaline batteries. J Appl Electrochem 2011;41:991-7.
28. Higashi S, Lee SW, Lee JS, Takechi K, Cui Y. Avoiding short circuits from zinc metal dendrites in anode by backside-plating configuration. Nat Commun 2016;7:11801.
29. Yu Z, Wang H, Kong X, et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat Energy 2020;5:526-33.
30. Wang F, Borodin O, Gao T, et al. Highly reversible zinc metal anode for aqueous batteries. Nat Mater 2018;17:543-9.
31. Wan F, Niu Z. Design strategies for vanadium-based aqueous zinc-ion batteries. Angew Chem Int Ed 2019;131:16508-17.
32. Zhang D, Cao J, Zhang X, et al. Inhibition of manganese dissolution in Mn2O3 cathode with controllable Ni2+ incorporation for high-performance zinc ion battery. Adv Funct Mater 2021;31:2009412.
33. Zhu C, Fang G, Liang S, et al. Electrochemically induced cationic defect in MnO intercalation cathode for aqueous zinc-ion battery. Energy Stor Mater 2020;24:394-401.
34. Tamilselvan M, Madhukar Sreekanth TV, Yoo K, Kim J. Self-doped 2D-V2O5 nanoflakes - a high electrochemical performance cathode in rechargeable zinc ion batteries. Ceram Int 2021;47:29832-9.
35. Xie X, Fang G, Xu W, et al. In situ defect induction in close-packed lattice plane for the efficient zinc ion storage. Small 2021;17:e2101944.
36. Zhang H, Fang Y, Yang F, Liu X, Lu X. Aromatic organic molecular crystal with enhanced π-π stacking interaction for ultrafast Zn-ion storage. Energy Environ Sci 2020;13:2515-23.
37. Yang W, Yang W, Dong L, Shao G, Wang G, Peng X. Hierarchical ZnO nanorod arrays grown on copper foam as an advanced three-dimensional skeleton for dendrite-free sodium metal anodes. Nano Energy 2021;80:105563.
38. Zhang N, Cheng F, Liu Y, et al. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J Am Chem Soc 2016;138:12894-901.
39. Cai W, Yao YX, Zhu GL, et al. A review on energy chemistry of fast-charging anodes. Chem Soc Rev 2020;49:3806-33.
40. Hu P, Wang T, Zhao J, et al. Ultrafast alkaline Ni/Zn battery based on Ni-foam-supported Ni3S2 nanosheets. ACS Appl Mater Interfaces 2015;7:26396-9.
41. Selvakumaran D, Pan A, Liang S, Cao G. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J Mater Chem A 2019;7:18209-36.
42. Huang J, Wang Z, Hou M, et al. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat Commun 2018;9:2906.
43. Rodríguez-Pérez IA, Yuan Y, Bommier C, et al. Mg-ion battery electrode: an organic solid’s herringbone structure squeezed upon Mg-ion insertion. J Am Chem Soc 2017;139:13031-7.
44. Pan W, Wang Y, Zhao X, et al. High-performance aqueous Na-Zn hybrid ion battery boosted by “water-in-gel” electrolyte. Adv Funct Mater 2021;31:2008783.
45. Suo L, Borodin O, Wang Y, et al. “Water-in-Salt” electrolyte makes aqueous sodium-ion battery safe, green, and long-lasting. Adv Energy Mater 2017;7:1701189.
46. Shi P, Zheng H, Liang X, et al. A highly concentrated phosphate-based electrolyte for high-safety rechargeable lithium batteries. Chem Commun 2018;54:4453-6.
47. Yamada Y, Yamada A. Superconcentrated electrolytes to create new interfacial chemistry in non-aqueous and aqueous rechargeable batteries. Chem Lett 2017;46:1056-64.
48. Zhao J, Li Y, Peng X, et al. High-voltage Zn/LiMn0.8Fe0.2PO4 aqueous rechargeable battery by virtue of “water-in-salt” electrolyte. Electrochem Commun 2016;69:6-10.
49. Chen S, Lan R, Humphreys J, Tao S. Salt-concentrated acetate electrolytes for a high voltage aqueous Zn/MnO2 battery. Energy Stor Mater 2020;28:205-15.
50. Zhang L, Rodríguez-pérez IA, Jiang H, et al. ZnCl2 “Water-in-Salt” electrolyte transforms the performance of vanadium oxide as a Zn battery cathode. Adv Funct Mater 2019;29:1902653.
51. Sha M, Dong H, Luo F, Tang Z, Zhu G, Wu G. Dilute or concentrated electrolyte solutions? J Phys Chem Lett 2015;6:3713-20.
52. Yu L, Chen S, Lee H, et al. A localized high-concentration electrolyte with optimized solvents and lithium difluoro(oxalate)borate additive for stable lithium metal batteries. ACS Energy Lett 2018;3:2059-67.
53. Chen S, Zheng J, Mei D, et al. High-voltage lithium-metal batteries enabled by localized high-concentration electrolytes. Adv Mater 2018;30:1706102.
54. Ren X, Chen S, Lee H, et al. Localized high-concentration sulfone electrolytes for high-efficiency lithium-metal batteries. Chem 2018;4:1877-92.
55. Cao R, Lee J, Liu M, Cho J. Recent progress in non-precious catalysts for metal-air batteries. Adv Energy Mater 2012;2:816-29.
56. Logeshwaran N, Ramakrishnan S, Chandrasekaran SS, et al. An efficient and durable trifunctional electrocatalyst for zinc-air batteries driven overall water splitting. Appl Catal B Environ 2021;297:120405.
57. Eckert M, Peters W, Drillet JF. Fast Microwave-assisted hydrothermal synthesis of pure layered delta-MnO2 for multivalent ion intercalation. Materials 2018;11:2399.
58. Ramakrishnan S, Velusamy DB, Sengodan S, et al. Rational design of multifunctional electrocatalyst: An approach towards efficient overall water splitting and rechargeable flexible solid-state zinc-air battery. Appl Catal B Environ 2022;300:120752.
59. Fu J, Cano ZP, Park MG, Yu A, Fowler M, Chen Z. Electrically rechargeable zinc-air batteries: progress, challenges, and perspectives. Adv Mater 2017;29:1604685.
60. Cheng F, Chen J. Metal-air batteries: from oxygen reduction electrochemistry to cathode catalysts. Chem Soc Rev 2012;41:2172-92.
61. Lee J, Tai Kim S, Cao R, et al. Metal-air batteries with high energy density: Li-air versus Zn-air. Adv Energy Mater 2011;1:34-50.
62. Liang HW, Zhuang X, Brüller S, Feng X, Müllen K. Hierarchically porous carbons with optimized nitrogen doping as highly active electrocatalysts for oxygen reduction. Nat Commun 2014;5:4973.
63. Tang C, Wang HF, Zhang Q. Multiscale principles to boost reactivity in gas-involving energy electrocatalysis. ACC Chem Res 2018;51:881-9.
64. Guo S, Zhang S, Su D, Sun S. Seed-mediated synthesis of core/shell FePtM/FePt (M = Pd, Au) nanowires and their electrocatalysis for oxygen reduction reaction. J Am Chem Soc 2013;135:13879-84.
65. Chung DY, Jun SW, Yoon G, et al. Highly durable and active ptfe nanocatalyst for electrochemical oxygen reduction reaction. J Am Chem Soc 2015;137:15478-85.
66. Bu L, Guo S, Zhang X, et al. Surface engineering of hierarchical platinum-cobalt nanowires for efficient electrocatalysis. Nat Commun 2016;7:11850.
67. Xu N, Nie Q, Wei Y, et al. Bi-functional composite electrocatalysts consisting of nanoscale (La, Ca) oxides and carbon nanotubes for long-term zinc-air fuel cells and rechargeable batteries. Sustain Energy Fuels 2018;2:91-5.
68. Wang Y, Fu J, Zhang Y, et al. Continuous fabrication of a MnS/Co nanofibrous air electrode for wide integration of rechargeable zinc-air batteries. Nanoscale 2017;9:15865-72.
69. Niu W, Li Z, Marcus K, et al. Surface-modified porous carbon nitride composites as highly efficient electrocatalyst for Zn-air batteries. Adv Energy Mater 2018;8:1701642.
70. Hu S, Han T, Lin C, et al. Enhanced electrocatalysis via 3D graphene aerogel engineered with a silver nanowire network for ultrahigh-rate zinc-air batteries. Adv Funct Mater 2017;27:1700041.
71. Li B, Geng D, Lee XS, et al. Eggplant-derived microporous carbon sheets: towards mass production of efficient bifunctional oxygen electrocatalysts at low cost for rechargeable Zn-air batteries. Chem Commun 2015;51:8841-4.
72. Lu X, Yim WL, Suryanto BH, Zhao C. Electrocatalytic oxygen evolution at surface-oxidized multiwall carbon nanotubes. J Am Chem Soc 2015;137:2901-7.
73. Tang C, Wang B, Wang HF, Zhang Q. Defect engineering toward atomic Co-Nx-C in hierarchical graphene for rechargeable flexible solid Zn-air batteries. Adv Mater 2017;29:1703185.
74. Lee DU, Choi JY, Feng K, Park HW, Chen Z. Advanced extremely durable 3D bifunctional air electrodes for rechargeable zinc-air batteries. Adv Energy Mater 2014;4:1301389.
75. Liu X, Park M, Kim MG, Gupta S, Wu G, Cho J. Integrating NiCo alloys with their oxides as efficient bifunctional cathode catalysts for rechargeable zinc-air batteries. Angew Chem Int Ed 2015;54:9654-8.
76. Xu K, Chen P, Li X, et al. Metallic nickel nitride nanosheets realizing enhanced electrochemical water oxidation. J Am Chem Soc 2015;137:4119-25.
77. Lee DU, Xu P, Cano ZP, Kashkooli AG, Park MG, Chen Z. Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal-air batteries. J Mater Chem A 2016;4:7107-34.
78. Winsberg J, Janoschka T, Morgenstern S, et al. Poly(TEMPO)/Zinc hybrid-flow battery: a novel, “green”, high voltage, and safe energy storage system. Adv Mater 2016;28:2238-43.
79. Leung P, Martin T, Shah A, Mohamed M, Anderson M, Palma J. Membrane-less hybrid flow battery based on low-cost elements. J Power Sources 2017;341:36-45.
80. Gong K, Ma X, Conforti KM, et al. A zinc-iron redox-flow battery under $100 per kW h of system capital cost. Energy Environ Sci 2015;8:2941-5.
81. Li B, Nie Z, ijayakumar M, et al. Ambipolar zinc-polyiodide electrolyte for a high-energy density aqueous redox flow battery. Nat Commun 2015;6:6303.
82. Wang C, Lai Q, Feng K, Xu P, Li X, Zhang H. From zeolite-type metal organic framework to porous nano-sheet carbon: high activity positive electrode material for bromine-based flow batteries. Nano Energy 2018;44:240-7.
83. Winsberg J, Stolze C, Schwenke A, Muench S, Hager MD, Schubert US. Aqueous 2,2,6,6-tetramethylpiperidine-N-oxyl catholytes for a high-capacity and high current density oxygen-insensitive hybrid-flow battery. ACS Energy Lett 2017;2:411-6.
84. Li Y, Geysens P, Zhang X, et al. Cerium-containing complexes for low-cost, non-aqueous redox flow batteries (RFBs). J Power Sources 2020;450:227634.
85. Zhang J, Jiang G, Xu P, et al. An all-aqueous redox flow battery with unprecedented energy density. Energy Environ Sci 2018;11:2010-5.
86. Xie C, Liu Y, Lu W, Zhang H, Li X. Highly stable zinc-iodine single flow batteries with super high energy density for stationary energy storage. Energy Environ Sci 2019;12:1834-9.
87. Khor A, Leung P, Mohamed M, et al. Review of zinc-based hybrid flow batteries: from fundamentals to applications. Mater Today Energy 2018;8:80-108.
88. Wang YL, Sun QL, Zhao QQ, Cao JS, Ye SH. Rechargeable lithium/iodine battery with superior high-rate capability by using iodine-carbon composite as cathode. Energy Environ Sci 2011;4:3947.
89. Li B, Liu J, Nie Z, et al. Metal-organic frameworks as highly active electrocatalysts for high-energy density, aqueous zinc-polyiodide redox flow batteries. Nano Lett 2016;16:4335-40.
90. Weng G, Li Z, Cong G, Zhou Y, Lu Y. Unlocking the capacity of iodide for high-energy-density zinc/polyiodide and lithium/polyiodide redox flow batteries. Energy Environ Sci 2017;10:735-41.
91. Xie C, Zhang H, Xu W, Wang W, Li X. A long cycle life, self-healing zinc-iodine flow battery with high power density. Angew Chem Int Ed 2018;130:11341-6.
92. Yuan Z, Yin Y, Xie C, Zhang H, Yao Y, Li X. Advanced materials for Zinc-based flow battery: development and challenge. Adv Mater 2019;31:e1902025.
93. Ma L, Zhao Y, Ji X, et al. A usage scenario independent “air chargeable” flexible zinc ion energy storage device. Adv Energy Mater 2019;9:1900509.
94. Fu J, Lee DU, Hassan FM, et al. Flexible high-energy polymer-electrolyte-based rechargeable zinc-air batteries. Adv Mater 2015;27:5617-22.
95. Fu J, Hassan FM, Li J, et al. Flexible rechargeable zinc-air batteries through morphological emulation of human hair array. Adv Mater 2016;28:6421-8.
96. Jiang Y, Deng Y, Liang R, et al. Multidimensional ordered bifunctional air electrode enables flash reactants shuttling for high-energy flexible Zn-air batteries. Adv Energy Mater 2019;9:1900911.
97. Zhang J, Fu J, Song X, et al. Laminated cross-linked nanocellulose/graphene oxide electrolyte for flexible rechargeable zinc-air batteries. Adv Energy Mater 2016;6:1600476.
98. Fu J, Zhang J, Song X, et al. A flexible solid-state electrolyte for wide-scale integration of rechargeable zinc-air batteries. Energy Environ Sci 2016;9:663-70.
99. Wang X, Wang F, Wang L, et al. An aqueous rechargeable Zn//Co3O4 battery with high energy density and good cycling behavior. Adv Mater 2016;28:4904-11.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Xiao D, Lv X, Fan J, Li Q, Chen Z. Zn-based batteries for energy storage. Energy Mater 2023;3:300007. http://dx.doi.org/10.20517/energymater.2022.84
AMA Style
Xiao D, Lv X, Fan J, Li Q, Chen Z. Zn-based batteries for energy storage. Energy Materials. 2023; 3(1): 300007. http://dx.doi.org/10.20517/energymater.2022.84
Chicago/Turabian Style
Xiao, Dengji, Ximei Lv, Jiahong Fan, Qian Li, Zhongwei Chen. 2023. "Zn-based batteries for energy storage" Energy Materials. 3, no.1: 300007. http://dx.doi.org/10.20517/energymater.2022.84
ACS Style
Xiao, D.; Lv X.; Fan J.; Li Q.; Chen Z. Zn-based batteries for energy storage. Energy Mater. 2023, 3, 300007. http://dx.doi.org/10.20517/energymater.2022.84
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 17 clicks
Cite This Article 17 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.