Recent commentaries on the expected performance, advantages and applications of sodium-ion batteries
Keywords
Sodium-ion (Na-ion) batteries represent an interesting and emerging alternative to the currently prominent lithium-ion (Li-ion) batteries. They operate on the same “rocking chair” principle, in which shuttle ions (sodium ions, in this instance) move reversibly between the positive and negative electrodes, inserting themselves into one of these and de-inserting themselves from the counter electrode. Historically, Na-ion and Li-ion batteries emerged in parallel in the 1970s; however, the spotlight shifted to lithium considerably in the 1980s, leading to the commercialization of Li-ion batteries by Sony in 1991 and the subsequent emergence of a number of Li-ion battery subclasses [including the lithium cobalt oxide (LCO), lithium managanese oxide (LMO), nickel cobalt aluminum (NCA), nickel manganese cobalt (NMC) and lithium iron phosphate (LFP) battery chemistries]. Sodium-ion batteries returned to the research community’s attention in the 2000s and 2010s decades due to concerns about the limited reserves of lithium and, subsequently, cobalt precursors. The research in this area flourished and a significant number of papers (hundreds) were published per year[1,2]. The research in this area generated significant excitement, with some contributors expecting sodium-ion batteries to be an equal or even superior alternative to Li-ion technology.
A significant body of research and a degree of excitement is often followed by commercial efforts. Indeed, a number of emerging industry players can be identified at the moment in the sodium-ion battery space, including Faradion, Tiamat and Novosis, to name a few. Practical prototypes of the batteries appear, and their metrics and the expected performance may be quantified with reasonable certainty levels. Experts in the field can then analyze the available information and provide analytical commentaries able to define the expected performance envelopes, strengths and limitations of the emerging technology. Two such contributions have been recently published in the peer-reviewed literature, in the form of a viewpoint from Prof. Abraham[3] and a commentary from Prof. Tarascon[4]. Both contributors are well-known expert researchers in the field. Prof. Abraham has contributed to the development of lithium, Li-ion and Li-air batteries, and served on the editorial board of Journal of Power Sources and as a Chair of the Battery Division of the Electrochemical Society. Prof. Tarascon[4] is a creator of the European Network of Excellence ALISTORE-ERI and the French Network on Electrochemical Energy Storage (RS2E), and a strong research participant in the field of Li-ion and Na-ion batteries. It is interesting to compare and discuss their two opinions in the context of Na-ion batteries, as one of the expert commentators (Prof. Abraham) can be regarded as an outsider in the field, while the second expert commentator (Prof. Tarascon) is a clear insider, who significantly contributed to the sodium-based technology research and is also a shareholder and development committee member in one of the emerging Na-ion battery companies, Faradion.
Both commentators comment on the choice of materials that enable Na-ion batteries. Hard carbon is highlighted as the preferred material for negative electrodes (anodes) of these batteries. This type of carbon is made up of disordered graphene layers and nanopores and has a typical sloping voltage region followed by a plateau. Usually, optimal samples of hard carbon have reversible capacities of ~250 mAh/g, which corresponds to the formation of Na0.67C6[3]. The incorporation of sodium into hard carbon is usually described in terms of a combination of its insertion between the disordered carbon layers and the filling of nanopores present in the carbon host. Other negative electrode materials, such as Na2Ti3O7, Na3Ti2(PO4)3 and materials that alloy with Na, have also been described; however, hard carbon remains the truly dominant negative electrode material and is used in the vast majority of practical prototypes[3,4].
The choice of cathode materials is less singular and well defined. During the research activities on Na-ion batteries, three main cathode material types have emerged, including layered sodium transition metal oxides, typical for batteries produced by one of the industry pioneers Faradion, sodium vanadium fluorophosphate Na3V2(PO4)2F3 used in practical battery prototypes by Tiamat and Prussian blue analogues such as Na2-δMnFe(CN)6•yH2O, adopted in batteries developed by Novosis[3,4]. These positive electrode materials have distinctly different capacities and average voltages (up to 150 mAh/g with varied average voltages, depending on the materials, for sodium layered oxides; 128-135 mAh/g and 3.8-3.9 V for
Electrolytes in Na-ion batteries are a variation of an appropriate salt [NaPF6, NaN(SO2CF3)2 or NaClO4)] and mixed organic carbonate solvents chosen from ethylene carbonate (EC), ethyl methyl carbonate (EMC), propylene carbonate (PC), diethyl carbonate (DEC) and dimethyl carbonate (DMC). In order to enable better stability in the cell, electrolyte additives may be used, which include, most commonly, fluoroethylene carbonate (FEC) and sometimes other additives such as bis(2,2,2-trifluoroethyl) ether (BTFE). An important point is that an electrolyte formulation cannot be simply copied from somewhat analogous Li-ion batteries and need to be tailored for a particular combination of electrodes in a Na-ion cell[3,4].
An important consideration in Na-ion batteries is the possibility of avoiding the use of copper current collectors in the negative electrodes and aluminium can be used instead as a cheaper alternative. This is related to the lack of parasitic alloying of sodium with aluminum, which is, unfortunately, a problem that exists for lithium in Li-ion cells.
In his viewpoint published in ACS Energy Letters, Abraham raises a question on how comparable Na-ion batteries are to their Li-ion counterparts[3]. This is discussed through the consideration of actual and hypothetical batteries assembled in the format of 18650 cells and the analysis of their specific energies
Considering the above values, it is concluded in Abraham’s viewpoint that Na-ion cells are not comparable to high-energy Li-ion batteries such as those with LiCoO2 (LCO), LiNi0.33Mn0.33Co0.33O2 (NMC) or
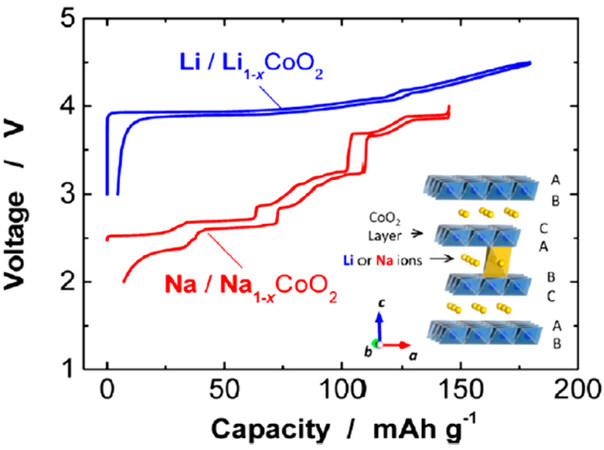
Figure 1. Comparison of charge-discharge curves of Li/LiCoO2 and Na/NaCoO2 half-cells. Schematic illustration of Li(Na)CoO2 crystal is also shown. Reprinted with permission from[1]. Copyright 2014 American Chemical Society.
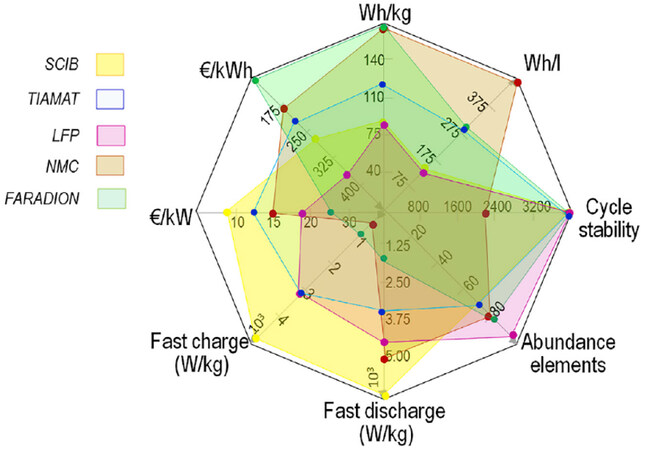
Similarly, the commentary by Tarascon is concerned with distinguishing between the hype associated with the earlier development of Na-ion batteries and reality[4]. His discussion is based on the cells publicized by three start-up companies in this space, Faradion (layered oxide cathode chemistry) Tiamat (Na3V2(PO4)2F3 cathode chemistry) and Novosis (using Prussian blue analogue as a cathode). These cells are compared with graphite - LiFePO4 batteries and, importantly, with Toshiba’s Super Charge Ion Battery (SCIB) cells. The latter benchmark is new in our discussion (not considered previously in Abraham’s viewpoint[3]) and is very interesting as these batteries represent another category of Li-ion cells - specialised low energy, high power batteries. A spider diagram [Figure 2] represents a comparison of various parameters of the cells under consideration[4]. One minor criticism addressed to Figure 2 may be the specific energy and energy density of LFP cells, which seems lower than the usually publicized data. In the opinion of the author of this Research Highlight, higher values should have been used for the LFP cells in the benchmarking exercise.
Figure 2. Tentative comparison between high power cells relying on Na-ion technology either in 18650 format from TIAMAT
It is highlighted that polyanionic materials may have a higher importance in Na-ion batteries[4]. In Li-ion batteries, the layered oxides are usually dominant, except for the specific cases of LiFePO4 or spinel
Another possible Na-ion battery chemistry based on the use of Prussian blue analogues (technology trialed commercially, for example, by Novosis) is also mentioned briefly in the assessment by Tarascon[4]. However, the cell-level metrics are not provided, and the discussion is brief. It is mentioned that the morphology and moisture content control may represent challenges for this cathode chemistry, and the energy density (in Wh/L) may be on the lower side for such cells due to the low density of the cathode material.
As a researcher with first-hand knowledge of the commercialisation effort in Na-ion batteries, Tarascon provides extra remarks on the specific advantages of these new cells not directly considered in the viewpoint by Abraham[4]. One of such remarks concerns the extra safety of Na-ion batteries. This characteristic originates from the fact that, unlike Li-ion batteries, their sodium counterparts can be shorted by connecting the opposite battery terminals without an irreversible consequence for the battery performance. As a result of this very practical property, the transportation and storage of Na-ion batteries is much easier. In the case of Li-ion batteries, they need to be treated as dangerous goods, with special safety measures linked to their inherent flammability and ability to explode. In contrast, for the batteries that can be temporarily shorted, the requirements for transportation and storage in the discharged state are much softer, favouring the adaptation of Na-ion cells in practice[4].
One of the considerably advantageous attributes of Na-ion cells that Tarascon envisages in his commentary is their capability of fast charge and performing as high power cells, particularly in the Tiamat’s cells with vanadium fluorophosphate chemistry. Their specific power compares favourably with LFP-type Li-ion batteries while offering, according to the author, better cost in terms of €/kWh or €/kW. The fast charge ability is also not dissimilar to Toshiba’s SCIB cells. While this property is somewhat inferior in Tiamat’s Na-ion cells, they offer an advantage of a higher voltage (3.7 V vs. 2.7 V) and better specific energy and energy density parameters than those of SCIB batteries. The commentator envisages that sodium-based cells may find applications in “power-hungry functions”, including regenerative braking and fast-charging public transport[4].
In conclusion, both expert commentators assessed the expected performance, advantages and applications of Na-ion batteries. It is highlighted that Na-ion cells are unlikely to be a true replacement for Li-ion batteries in all applications. This is linked to their relatively limited energy density with respect to that achievable by Li-ion batteries with layered cathodes (in LCO, NMC and NCA chemistries). Instead, Na-ion batteries (pioneered commercially by Faradion and Tiamat and also those assessed by the researchers from Washington State University and Pacific Northwest National Laboratory) with specific energies at the level of 120-150 Wh/kg are more comparable to LFP Li-ion batteries or specialised fast-charge SCIB batteries marketed by Toshiba. Specific advantages of Na-ion batteries include their excellent sustainability (the lack of critical elements such as lithium and cobalt), increased safety (an ability to be shorted during storage and transportation) and excellent fast charge capabilities and power densities for the Na3V2(PO4)2F3-based cells. Possible applications for Na-ion batteries include regenerative breaking and other “power-hungry functions” such as fast-charging e-buses, and the uses where LFP Li-ion batteries could have been otherwise considered (electric vehicles with short range, power backup or energy storage systems used with localised renewable energy generators).
This Research Highlight lags behind the two original commentaries by approximately two years. Therefore, it is interesting to reflect on what has changed since those were published. The notable developments include a larger number of start-ups and established commercial companies venturing in the space of
DECLARATIONS
Author’ contributionsThe author contributed solely to the article.
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestThe author declared that there were no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Yabuuchi N, Kubota K, Dahbi M, Komaba S. Research development on sodium-ion batteries. Chem Rev 2014;114:11636-82.
2. Hwang JY, Myung ST, Sun YK. Sodium-ion batteries: present and future. Chem Soc Rev 2017;46:3529-614.
3. Abraham KM. How comparable are sodium-ion batteries to lithium-ion counterparts? ACS Energy Lett 2020;5:3544-7.
4. Tarascon JM. Na-ion versus Li-ion batteries: complementarity rather than competitiveness. Joule 2020;4:1616-20.
5. Faradion. Available from: https://faradion.co.uk/ [Last accessed on 28 Feb 2023].
6. Tiamat. Available from: http://www.tiamat-energy.com/ [Last accessed on 28 Feb 2023].
7. HiNa battery. Available from: https://www.hinabattery.com/en/ [Last accessed on 28 Feb 2023].
8. Altris. Available from: https://www.altris.se/ [Last accessed on 28 Feb 2023].
9. Natron energy. Available from: https://natron.energy/ [Last accessed on 28 Feb 2023].
10. CATL unveils its latest breakthrough technology by releasing its first generation of sodium-ion batteries. Available from: https://www.catl.com/en/news/665.html [Last accessed on 28 Feb 2023].
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Glushenkov AM. Recent commentaries on the expected performance, advantages and applications of sodium-ion batteries . Energy Mater 2023;3:300010. http://dx.doi.org/10.20517/energymater.2022.70
AMA Style
Glushenkov AM. Recent commentaries on the expected performance, advantages and applications of sodium-ion batteries . Energy Materials. 2023; 3(2): 300010. http://dx.doi.org/10.20517/energymater.2022.70
Chicago/Turabian Style
Glushenkov, Alexey M.. 2023. "Recent commentaries on the expected performance, advantages and applications of sodium-ion batteries " Energy Materials. 3, no.2: 300010. http://dx.doi.org/10.20517/energymater.2022.70
ACS Style
Glushenkov, AM. Recent commentaries on the expected performance, advantages and applications of sodium-ion batteries . Energy Mater. 2023, 3, 300010. http://dx.doi.org/10.20517/energymater.2022.70
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 35 clicks
Cite This Article 35 clicks














Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.