Progress in the high-temperature synthesis of atomically dispersed metal on carbon and understanding of their formation mechanism
Abstract
The development of various high-performance electrochemical devices is crucial for mitigating the global climate crisis, and thus the design and fabrication of advanced electrode materials is highly significant. Currently, atomically dispersed metal on catalysts (ADMCs) have shown great potential in boosting the performance of various energy storage/conversion devices involving aqueous and aprotic catalytic processes, including fuel cells, water electrolyzers, CO2 electrolyzers, metal-air batteries, and metal-sulfur batteries, as well as systems involving noncatalytic deposition/adsorption of metals. To date, several reliable fabrication methodologies that can ensure the formation of ADMCs have been demonstrated, and continuous optimization is still being performed. To further reinforce the basic scientific research and promote possible practical applications of these materials, we have analyzed, compared, and summarized progress in the fabrication methodology and formation mechanism of ADMCs in this review. This review aims to draw a comprehensive picture of the current methodology and underlying mechanism in the field of material fabrication to serve as guidance for future material design.
Keywords
INTRODUCTION
The rapid development witnessed in the past several centuries has brought very severe global climate change, the consequent food crisis, social unrest, poverty, and human health challenges, that are affecting us all in one way or another[1,2]. Such an urgent situation has triggered rapidly increasing research worldwide on various electrochemical devices for energy conversion and storage, such as batteries, fuel cells, supercapacitors, and electrolyzers. It should be noted that the performance of these devices depends largely on the nature of the electrode materials, and therefore, designing and fabricating novel materials with the desired performance, low cost, durability, and easy manufacturability has been the center topic for the development of advanced electrochemical devices[3-8].
Atomically dispersed metals on catalysts (ADMCs) have been the focus of attention in the field of aqueous catalysis. The major advantage of ADMCs benefiting their utilization in catalytic systems is the combination of the intrinsic advantages of carbon materials, high electric conductivity, large surface area, and tunable porosity, with the maximized atomic utilization of metal centers. As a result, the specific activity of ADMCs with rational design can be remarkably greater than that of conventional metal catalysts. For example, when catalyzing the electrochemical oxygen reduction reaction (ORR), some atomically dispersed metals (ADMs) with various active metal centers, even nonprecious metals, are more active than benchmark Pt/C catalysts[9-12]. Similar phenomena can also be observed in hydrogen evolution reaction (HER)[13,14]. In addition, because metal centers in ADMCs are stabilized by surrounding nonmetallic atoms, it is possible to tune their electronic structure and adsorption properties by adjusting the coordination environment of metal atoms. As a result, the regulation of reaction selectivity on ADMCs can be achieved, benefiting their application in electrolyzers aimed at producing certain chemicals[15-18]. It was reported that the reaction pathway of oxygen reduction reaction can be tuned by adjusting the chemical nature of N in CoN4 moieties. When coordinated by pyridinic N, Co atoms with high spin enable full reduction of O2 to H2O, and the reaction pathway can be altered to H2O2-selective in pyrrolic N bonded CoN4[19].
Very recently, ADMCs have extended their effectiveness in various battery systems[20-22]. The possible application of ADMCs as the cathode material for Li-air batteries is based on the catalytic nature of the charge/discharge process in the cathode. For example, Ru ADMs are believed to be effective in accelerating sluggish ORR and oxygen evolution reaction (OER), decreasing the polarization of the Li-air battery[23]. Our group also found that Cu ADMs are able to regulate the morphology of Li2O2 during discharge and further accelerate its decomposition kinetics via a one-electron transfer mechanism during the subsequent charging process[24]. In addition, the performance of Li-S batteries can also be promoted by ADMCs. Besides, some ADMCs are capable of regulating the morphology of metal clusters during electroreduction and facilitating their uniform deposition, which is important to prevent dendrites and reinforce the safety of metal electrodes. As a result, ADMCs are showing potential application as anode materials in various metal-ion batteries, including traditional Li batteries and newly emerged Na-ion and Zn-ion batteries[25,26].
In addition to the remarkable performance enhancement brought in by ADMCs, the relatively uniform structure of ADMCs has provided a perfect platform for research on reaction pathways and detailed mechanisms[27].
Generally, isolated ADMs are extremely unstable due to the significantly increased surface free energy when downsizing bulk metals[28]. Thus, the successful fabrication of ADM-containing materials is the perfect example of precisely manipulating material chemistry to satisfy the needs of human beings. The present results strongly indicate that achieving a strong chemical interaction between ADMs and the support is critical for the successful fabrication of ADM-based materials[29]. This brief review focuses on research updates on the formation mechanisms of ADMCs. In addition, the fabrication methodologies, together with their correlation with the obtained coordination structure, are also reviewed and summarized. In the following sections, the correlation between the ADM structure and fabrication methods is first discussed, followed by a summary of the formation mechanism of ADMCs by high-temperature thermal-chemical reactions, trying to summarize the commonality among different procedures. The formation mechanisms are roughly subclassified into three types based on the reaction characteristics, including the transformation of pre-existing coordination structure, fixation via ligand replacement, and direct anchoring of metal atoms, as illustrated in Figure 1. The review ends with outlooks highlighting the future directions and challenges for scientific research on the fabrication methods of ADMCs and their formation mechanism.
GENERAL DISCUSSION
The formation process of functional materials has always attracted the interest of scientists. Behind the various chemical reactions that lead to the formation of the desired chemical composition or the required spatial structure are the reaction mechanisms. Uncovering the formation mechanism is important to provide useful methodologies for the development of suitable materials and valuable guidance for the further rational optimization of the process.
Commonalities and specialties of ADMCs
It has been found that ADMs can be stabilized in a wide range of substances through favored metal-metal or metal-nonmetal interactions between metal atoms and the support. For example, a small number of foreign ADMs can be stabilized within the metal bulk in the form of single-atom alloys [Figure 2A][30], and several metal oxides/hydroxides are capable of bonding foreign ADMs [Figure 2B-D] by oxygen species on the surface or coordination bonds into the lattice[31,32]. Similarly, there are reports on the utilization of metal heteropolymetalates/carbides as stabilizers [Figure 2E and F][33-37]. For carbon-based substrates, strong metal-substrate interactions via coordination bonds are also necessary.
Figure 2. Characteristic results and illustration of ADMs on non-carbon substrates and illustration of ADMCs. (A) STM image of Pd/Cu single atom alloy with 0.01 ML Pd[30], reproduced from Ref.[30] with permission. Copyright 2013 Royal Society of Chemistry. (B and C) Aberration-corrected HAADF-STEM images of Pt1/Fe2O3 and Pt1/γ-Al2O3, respectively[31], reproduced from Ref.[31] with permission. Copyright 2017 American Chemical Society. (D) Aberration-corrected HAADF-STEM image of sAu/NiFe LDH[32], reproduced from Ref.[32] with permission. Copyright 2018 American Chemical Society. (E) Pt L3-edge EXAFS of Pt1/Ti3-xC2Ty and Ref.[33], reproduced from Ref.[33] with permission. Copyright 2019 American Chemical Society. (F) Illustration of the most stable configuration of Pt atom on phosphomolybdic acid/graphene[34], reproduced from Ref.[34] with permission. Copyright 2016 Wiley-VCH. (G and H) Illustration of Cu ADMCs formed by adsorption by surface -CN[38] and embedding into the carbon framework[40], reproduced from Ref.[38] (Copyright 2019 Wiley-VCH) and Ref.[40] (Copyright 2021 Springer Nature) with permission.
However, unlike the abovementioned systems, the coordination structure of carbon-based ADMCs is more complicated. Judging from the local chemical environment, ADMCs can be roughly divided into two categories. The first one has metal centers coordinated by no more than two heteroatoms with a three-dimensional bonding geometry[38], as illustrated in Figure 2G. The second form of ADMCs, as reported by the majority of related work, has metal centers located at the vacancies of the carbon framework and coordinated by more than two nonmetal atoms from the carbon framework in a planar/quasi-planar position[39,40], as shown in Figure 2H.
Correlation between the ADMCs structure and the synthesis process
The formation of both forms of ADMCs is highly related to the nonmetal atoms as anchoring points, especially heteroatoms introduced into the carbon substrate. These dopants can be introduced by various doping agents either simultaneously with or before metal precursors, depending on the detailed fabrication process. To date, several methods have been proven to be effective in producing ADMCs. They can be roughly divided into three categories based on the reaction parameters critical for the formation of ADMs: thermal-chemical reactions, atomic layer deposition[41,42], and wet-chemical reactions[38,43]. Thermal reactions involve high-temperature treatment of a mixture of carbon sources, metal sources, and heteroatom doping agents, during which ADMs are formed. The reaction temperature varies from below 300 °C to above
The embedded structure is generally constructed by simultaneously forming the central metal atoms and their surrounding coordination environment in-situ during a high-temperature reaction. Taking the most common MN4 moieties as an example, there is evidence that during high-temperature pyrolysis processes, the formation of N4 defects and their bonding with metal atoms occur at the same time[44-46], and the formation energy of MN4 is smaller than that of N4 defects on carbon frameworks[47]. A recent study has even shown that the vacancies that host metal atoms for carbon frameworks are unlikely to be formed in the absence of metal[48]. The formation of the second form of ADMs is usually related to wet-chemical reactions, and the following high-temperature treatment is avoided. In solution, metal ions can be spontaneously adsorbed by N or other nonmetal elements from the carbon framework, and the key to such a simple adsorption pattern is the separation of metal adsorption with substrate formation, i.e., the carbon framework as a substrate is already fully formed and does not undergo structural change during later metal adsorption[38]. Inferring from the current experimental evidence, the embedded form of ADMs is more energetically favored at high temperatures[49]. Thus, the transformation of the coordination environment can occur during the fabrication process when the metal ions adsorbed by heteroatoms convert into embedded ADMs.
It should be noted that the discussion here is only brief, and it is not sufficient to establish a certain correlation between the geometry of ADMCs and the synthesis process. In some cases, ADMCs with embedded MN4 moieties can also be formed by wet-chemical reactions. For some, materials with isolated metal macrocyclic molecules adsorbed on carbon without heat treatment can sometimes be recognized as ADMCs as well, and the embedded nature of metal atoms in macrocyclic molecules remained after adsorption[50-52]. Another example is that metal atoms in solutions can be trapped by pre-existing N4 structures from the carbon framework[39,43,53].
Techniques for studying formation mechanisms
To decode the formation process, knowing the change in substances during high-temperature treatment is necessary. The most common procedure to obtain such information is to perform temperature-dependent ex-situ characterization by stopping the thermal treatment at different temperatures and characterizing the as-prepared samples. By various phase, spectroscopic, and microscopic characterization techniques, the evolution of metal species can be reproduced, provided that no additional changes occur during the cooling process. A more accurate method is to perform in-situ characterization, during which the evolution of ADMs can be directly observed. Such techniques cannot be realized until recent years. The development of modern characterization techniques has granted us an unprecedented opportunity to look into the detailed structure of the materials of interest on the nanometer- and even sub-nanometer-scales. In the research of ADMCs, both spherical aberration-corrected transmission electron microscopy and X-ray absorption spectroscopy (XAS) have become irreplaceable in identifying ADMs as well as in revealing their detailed chemical composition and coordination structures. The high sensitivity of XAS to the local environment and the relatively fast data collection process make it possible to decode even the tiny temperature-dependent or time-dependent structural evolution of ADMs, opening the gate to unraveling the thermal-chemical reaction process.
Target of this review
The formation mechanism of ADMs seems direct and apparent in the early days when researchers began to treat ORR-active macrocyclic compounds with carbon support at high temperatures, hoping to stabilize these less stable compounds. It was expected that due to the similar chemical structure of the six-membered ring building block of carbon with macrocyclic compounds, the adsorption becomes stronger as the temperature rises. As proven by modern characterization techniques, ADMs can be fixed in situ into the conjugated carbon framework. However, the situation became more complicated when it was found that similar materials with ADMs can also be obtained by pyrolyzing a mixture of a proper carbon source with nitrogen- and metal-containing compounds. The production of ADMCs has been a “black box” for a long time, as we only know that ADMCs can be obtained by adding the right starting materials to the box and that the composition can be regulated by starting materials, temperatures, and pyrolysis processes, but the reaction mechanism remains unknown due to the complexity of the thermal reaction system and the interference of high temperatures.
This review focuses on high-temperature-based thermal reactions for fabricating ADMCs. One reason is that among all the methodologies, the high-temperature thermal-chemical reaction is the most widely adopted, especially for application in electrochemical devices, due to its enhanced electron conductivity by thermal treatment and relatively low cost (cheap precursors and needless high-end equipment). Another reason is that the formation mechanism of ADMCs through wet-chemical and ALD methods are much more straightforward to understand, and as a result, research on the formation mechanism through high-temperature-based thermal reactions, i.e., the changes during the transformation from metal precursors to ADMs, is at the center of scientific research. Such a mechanism is at the base of understanding the formation of ADMs, including hotspot materials with dual or multiple metal atoms, whose formation is even more complicated. In addition, it plays an important part in rationally choosing precursors as well as designing reaction routes to obtain targeting materials with desired coordination structures. However, to date, the understanding of the formation mechanism of ADMCs via thermal reactions remains quite primary. Considering that most ADMCs are developed mimicking macrocyclic compounds with MN4 structures for electrochemical catalysis in the first place and have been mainstream catalysts until today, the following parts of this review will mainly discuss ADMCs fully or partially stabilized by N atoms, as they are the most commonly seen and studied.
Before moving into the detailed discussion on the fabrication mechanism of ADMCs, it is noteworthy that despite similar fabrication processes based on thermal reactions, the detailed emergence mechanisms of ADMs may vary from one system to another depending on the available observational evidence, suggesting that there is no universal pattern describing every scenario. However, on a relatively narrow scale, limited to the specific starting materials or reaction conditions, the similarities become clearer and can be summarized.
TRANSFORMATION VIA COORDINATION INHERITANCE
Evidence on the formation mechanism
The phrase “coordination inheritance” describes the situation where the major coordination environment of the metal atoms remains the same during the transformation from precursor to ADMs during thermal treatment.
The very first attempt to fabricate ADMCs was performed with macrocycle compounds. It is known that by pyrolyzing macrocycle compounds with carbon, the electrochemical stability of these molecules can be improved. Sa et al. studied the thermal evolution of Fe-based macrocycle compound, FeTMMPPCl, adsorbed on the surface of carbon nanotubes (CNTs) under a protective layer of SiO2 using in-situ XAS[54]. As suggested by the in-temperature XANES shown in Figure 3A, the original 5-coordinated structure of FeTMMPPCl becomes a square planar FeN4 configuration after the removal of axially-coordinated Cl at
Figure 3. Thermal evolution of the metal center in macrocycle compound and ZIF-8 revealed by XAS. (A) In-Temperature Fe K-edge XANES, and (B) EXAFS spectra of FeTMPPCl[54], reproduced from Ref.[54] with permission. Copyright 2016 American Chemical Society. (C) Temperature-dependent Zn K-edge XANES, and (D) EXAFS spectra of ZIF-8 treated at different temperatures after acid wash[55], reproduced from Ref.[55] with permission. Copyright 2020 Elsevier.
In addition to macrocyclic compounds, it is believed that there are MNx species within the structure of ZIF-based precursors. Using temperature-dependent XAS, Wang et al. studied the coordination nature of Zn in ZIF-8 after being pyrolyzed at different temperatures ranging from 500 to 900 °C and acid washed to remove aggregated species[55]. The crystalline structure of ZIF-8 with tetrahedral ZnN4 local coordination is not destroyed until 600 °C, as evidenced by the identical XANES and EXAFS spectra of ZIF-8 and ZIF-8-500 [Figure 3C and D]. When the temperature reaches 600 °C, the coordination configuration becomes planar similar to macrocyclic compounds with similar features in the XAS spectra. A weight ratio as high as 3 wt.% was detected for ZIF-8-600, suggesting that the stabilization of ZnN4 moieties occurs at the very early stage of ZIF-8 carbonization. Although the research is only limited to ZIF-8, it is reasonable to believe that other metal ions coordinated by the imidazole ring in the ZIF framework can undergo similar processes and become ADMS after pyrolysis.
Applicable scenario
All-solid thermal-chemical reaction
The most applicable scenario for ADMCs’ formation via coordination inheritance is the all-solid thermal-chemical reaction. It is the most traditional method for fabricating ADMCs, which can be dated back to when researchers tried to stabilize macrocyclic compounds by pyrolyzing these molecules with carbon[56]. Later, it was found that similar ADMs can be obtained by pyrolysis of simple metal salts with proper carbon and nitrogen precursors, opening the gate for the half-century-long exploration of ADMCs[57].
In all solid thermal-chemical reactions, all precursors are thoroughly mixed before undergoing simple pyrolysis in an inert or reducing atmosphere without intentionally introducing gaseous reactants from outside the system. The chemical changes can be rather complicated within the mixture at elevated temperatures, with multiple reactions occurring at the same time, including the formation of ADMs and further structure evolution[49], accompanied by[58,59]or independent from[60,61] heteroatom doping, depending on the nature of the chosen precursors. The pyrolysis parameters, pre- and post-pyrolysis treatments are also highly dependent on the type of precursors[62-64].
Organics as carbon precursors
The most commonly utilized carbon precursors are organics, which undergo carbonization during high-temperature pyrolysis, favoring the in-situ formation of ADMs. In general, metal-organic frameworks (MOFs), polymers, biomass, and simple organic molecules are the four types of most commonly seen carbon precursors for ADMCs.
In the past few years, metal-organic frameworks (MOFs) have been discovered as promising starting materials for ADMCs via high-temperature pyrolysis. To date, various MOFs, including Zn-based ZIF-8, Co-based ZIF-67[65], Zr-based MOF-545[66] and UiO-66[67], Al- and Ga-based MIL-53[68] and some other MOFs with Cd center[69], Mn center[70], Bi center[71] have been investigated as carbon precursor for ADMCs. The basic building blocks of MOFs consist of metal centers coordinated with organic linkers and are repeatedly arranged in three dimensions, forming an ordered pore structure, as illustrated by the example of ZIF in Figure 4A. Thus, most MOFs are extremely rich in micropores. The pore structure (porosity, pore size, and elemental composition) can be regulated by ligand molecular structure, providing an efficient way to construct the precursor composition and structure[72].
Figure 4. (A) Topological illustration of ZIF-67 and ZIF-8[72], reproduced from Ref.[72] with permission. Copyright 2010 American Chemical Society. (B) Illustration of the preparation of Mn-N-C via metal nods replacement and (C) HAADF-STEM image and EDS element mapping of Mn-N-C[73], reproduced from Ref.[73] with permission. Copyright 2020 American Chemical Society. (D) Illustration of the preparation of ADMCs via host-guest interaction[74], reproduced from Ref.[74] with permission. Copyright 2020 Springer Nature. (E) Pore diameter distribution of ZIF-8 and Co(acac)3@ZIF-8 and (F) Co K-edge XANES spectra of Co(acac)3@ZIF-8 and
As mentioned above, ADMs on carbon are, in most cases, stabilized by heteroatoms within the carbon framework by forming coordination bonds. It is suggested that the existence of chemical bonds between metal atoms and the ligand through desired nonmetal atoms in the precursor is beneficial for the formation of ADMs. In some cases, metal-heteroatom coordination is so abundant and stable that no additional heteroatom source is needed to stabilize ADMs during carbonization. Such systems with fewer precursors make it easier for researchers to precisely control the chemical composition and structure of the precursor and to uncover the formation reaction mechanism. Zn-based zeolitic imidazolate framework-8 (ZIF-8) may be one of those MOFs with such advantages.
ZIF-8 is composed of Zn centers and N-containing 2-methylimidazole linkers. There are two critical features in ZIF-8 that are beneficial for the formation of ADMCs. One is the porosity of the resultant carbon. During the thermal carbonization process, as the organic linkers decompose while releasing gaseous products, the microporous structure can partially maintain within the carbon residuals[73], facilitating the formation of ADMs [Figure 4B and C]. Besides, the sublimation of metallic Zn generated from the reduction of Zn(II) by carbon during thermal treatment introduces even more micropores in the resultant. Another important factor facilitating the usage of ZIF-8 as carbon precursors for ADMCs is element compatibility. Zn(II) centers in ZIF-8 can be replaced by Co(II) in all proportions and by many other transition metal cations for a small amount without compromising the crystal structure. The pre-existing metal-N bonding is considered vital for the stabilization of metal species in the form of ADMs
Although there are several ZIFs with compositions similar to that of ZIF-8, only ZIF-8 is the most utilized precursor for ADMCs. Wang et al. studied the thermal behavior of various Zn-based ZIFs with different imidazolate linkers[82]. It was found that due to various carbonization pathways, the chemical composition, surface properties, and graphitization level are highly related to the molecular structure of imidazolates. The 5-membered nature of the imidazole ring, in the absence of side chains, triggers carbonization coupled with denitrogenation, resulting in a higher graphitization level and leaving less N in the carbon framework. The methyl chain in 2-methylimidazole, however, is able to participate in the carbonization reaction, balancing the graphitization degree and N content, making ZIF-8 the most suitable precursor for ADMCs.
Thus, ZIF-8 is able to serve as a microreactor where foreign metal atoms, from either ionic salts or covalent molecules, can be stabilized within the framework while remaining spatially separate. In Table 1, we summarize some of the commonly reported fabrication methods of ADMCs based on ZIF-8 precursors. It is noteworthy that these metal introduction methods can be combined to achieve higher metal loading. More importantly, it enables the successful fabrication of several dual-metal sites, as demonstrated by
Methods utilizing ZIF-8 as carbon precursors
| Material | Metal precursor | Fabrication system | Interaction | Application | Ref |
| Fe-N-C-950 | Fe(acac)3 | Wet-chemical | host-guest | ORR | [83] |
| Mn-N-C-HCl-800/1100 | MnCl2 | Wet-chemical | Metal substitution | ORR | [84] |
| Ce SAS/HPNC | Ce(NO3)3 | Wet-chemical | Metal substitution | ORR | [85] |
| Ir1-N/C | IrCl3 | Solid-state | Metal substitution | ORR | [86] |
| Fe2-Z8-C | Fe(Ac)2 | Solid-state | Metal substitution | ORR | [87] |
| Ir-SACs | Ir(acac)3 | Wet-chemical | host-guest | ORR | [11] |
| Fe2-N-C | Fe2(CO)9 | Wet-chemical | host-guest | ORR | [88] |
| (Fe,Co)/N-C | Co(NO3)2 | Wet-chemical | Metal substitution | ORR | [83] |
| (Fe,Co)/N-C | FeCl3 | Wet-chemical | Adsorption | ORR | [83] |
| C-AFC@ZIF-8 | Ammonium ferric citrate | Wet-chemical | Adsorption | ORR | [89] |
| FeCo-DACs/NC | FeCo binuclear phthalocyanines | Wet-chemical | host-guest | ORR/ OER | [90] |
| Co-SA/P in situ | Co(NO3)2 | Wet-chemical | Metal substitution | HER | [91] |
| Y1/NC | Y2O3 | Solid-state | Metal substitution | NRR/ CO2RR | [92] |
| Ni2/NC | Ni2(dppm)2Cl3 | Wet-chemical | Post adsorption | CO2RR | [60] |
| Ni1/NC | NiCl2 | Wet-chemical | Post adsorption | CO2RR | [61] |
Polymers have a well-defined molecular structure, and a variety of polymers can transform into carbon after treatment at high temperatures in an inert atmosphere with acceptable carbon yield. Thus, polymers may be a rational choice as carbon precursors. However, not all polymers can be utilized as carbon sources for ADMCs. An ideal carbon precursor should have proper carbonization yield, as a yield that is too low will gradually increase the concentration of metal species during the carbonization, which usually leads to aggregation. In addition, the composition and structure of the resultant carbon should be suitable for anchoring ADMs. Similar to the MOF discussed above, achieving heteroatom doping is vital for the fabrication of ADMCs in the case of polymers as well. Thus, those with heteroatoms, especially N atoms, in the polymer chain are more favored, such as polyaniline, polypyrrole, polydopamine, and polyacrylonitrile.
Polyaniline (PANI) and polypyrrole (PPy) are N-rich polymers, sharing similarities in fabrication procedures and molecular structures. Both polymers can be obtained simply by oxidative polymerization of respective monomers by oxidation agents in aqueous media[96,97]. The N atoms within the polymer chain are functional in stabilizing metal species[98] [Figure 5A], and after high-temperature treatment, a considerable amount of N remains within the carbon framework. Some metal ions, such as Fe3+, are oxidative enough to trigger the polymerization of aniline and pyrrole. Li et al. demonstrated that by treating CoFe2O4 nanoparticles in an acid solution containing pyrrole, Fe3+ is leached off from CoFe2O4, starting the polymerization of pyrrole at the surface of nanoparticles[99]. As a result, a hollow PPy shell can be obtained, within which Fe3+ and Co2+ are bonded. By simple pyrolysis, ADMCs with Fe and Co sites can be obtained
Figure 5. (A) Illustration of the preparation process of CNT@Fe-N-PC via adsorption[98], reproduced from Ref. [98] with permission. Copyright 2018 Springer Nature. (B) SEM images of the CoFe2O4 sphere, and (C) SEM, (D) TEM, (E) HAADF-STEM images of FeCo-N-HCN[99], reproduced from Ref.[99]with permission. Copyright 2021 Wiley-VCH. (F and G) TEM, (H) aberration-corrected HAADF-STEM images, and (I) EDS element mapping of CoNi-SAs/NC[103], reproduced from Ref.[103] with permission. Copyright 2019 Wiley-VCH. (J) Illustration of the fabrication process of meso-Fe-N-C, and (K) TEM image of meso-Fe-N-C[104], reproduced from Ref.[104] with permission. Copyright 2021 American Chemical Society. (L) TEM image of CoSA/N,S-HCS[105], reproduced from Ref.[105] with permission. Copyright 2020 Wiley-VCH. (M) Illustration of the fabrication process of flexible electrode based on SA@NCF/CNF, (N) digital photos of Co SA@NCF/CNF flexible electrode under rolling, bending, and twisting state, and (O) SEM image of Co SA@NCF/CNF[111], reproduced from Ref.[111] with permission. Copyright 2019 Wiley-VCH. (P) HAADF-STEM image, (Q) EDS element mapping, and (R) aberration-corrected HAADF-STEM image of Co-N/CNFs[112], reproduced from Ref.[112] with permission. Copyright 2017 American Chemical Society.
Polydopamine (PDA) has attracted increasing attention as a carbon precursor for its ability to grow outside other substances, duplicating the morphology of the substrate. Zhang et al. found that PDA can grow tightly on the surface of various metal oxides/hydroxides, and after high-temperature pyrolysis, a thin layer of carbon with metal ADMs diffused from the inner core can be obtained[102]. Similarly, Han et al. demonstrated that PDA can duplicate the cubic morphology of CoNi-based Prussian blue analogs
Polyacrylonitrile (PAN) is another commonly used polymer as a carbon precursor, especially in electrospinning to fabricated ADMCs with fiber-like morphologies. Even though the cyano groups are rich in N content, the research on the thermal behavior of PAN reveals that the massive denitrogenation of PAN starts at 700 °C, making the participation of additional heteroatom precursors necessary in the formation of ADMs. Zhao et al. demonstrated the effectiveness of PMMA as an N precursor in the fabrication of Sn ADMCs using electrospinning[107]. Metal macrocyclic compounds such as cobalt porphyrins can be stabilized directly in carbon fiber without additional N sources, as demonstrated by Zhang[108]. Besides contributing to the formation of a carbon framework supporting ADMCs, the PAN-based electrospinning technique is effective in fabricating fiber-like morphologies with other carbon precursors. Yang et al. fabricated fiber-like Fe ADMCs doped with S and N. Thiourea and Fe/ZIF-8 were added into the DMF solution of PAN for electrospinning, thiourea and Fe[109]. Cheng et al. reported a porous carbon fiber derived from electrospinning of the DMF solution of PAN with SiO2, which is utilized for the growth of
The conversion of biomass into functional materials, including ADMCs, has long been of interest. This biomass contains proteins, carbohydrates, lipids, and other organic components, which release a large amount of gas when treated at high temperatures, contributing to the formation of micropores.
Simple organic molecules, such as dicyandiamide[116], melamine[117,118], glucosamine[119], amino acids[120], glucose[121,122], chitosan[123,124], EDTA[125], can also be utilized as carbon precursors. Some of the N-containing molecules can also serve as heteroatom precursors at the same time.
Carbon or its derivatives as carbon precursors
Instead of obtaining carbon from the carbonization of organics, various carbon materials or carbon derivatives have been utilized as carbon precursors for ADMCs: carbon black, graphite, graphene oxide, carbon nanotubes, carbon nanofibers, and graphene.
Different from other organics, the structure of carbon is relatively stable and does not undergo severe reconstruction in thermal treatment. Thus, it is doubtful whether the carbon structure participates in the formation of the local structure of ADMs. In some other reports, defects in carbon are able to serve as ligands and directly stabilize ADMs without heteroatom doping. Liu et al. demonstrated that by pyrolyzing platinum acetylacetonate with defective carbon black pre-treated in hydrogen peroxide, Pt atoms can be anchored at the divacancies by four carbon atoms in the form of PtC4[126]. This phenomenon further highlights a common principle in the formation of ADMs: strong chemical interaction between ADMs and the support. Should there be suitable anchoring points (such as carbon vacancies), ADMs can form on carbon even without additional heteroatom dopants[127].
The heteroatoms can be introduced via a second precursor during pyrolysis or by surface functionalization in a pre-pyrolysis step. The surface chemical composition of carbon can be easily regulated by various functionalization reactions. After surface functionalization by concentrated nitric acid, the oxygen-containing functional groups are able to bind Co atoms to form CoN2O2 ADMS[128]. These carbonaceous components not only serve directly as anchoring substrates for ADMs but can also act as diluents that increase the dispersion of other carbon precursors or as electron conductors that facilitate charge transfer.
Carbon black, including XC-72, Ketjenblack, Black Pearls and et al., have partially graphitic moieties together with defects. The pore structure varies with the type of carbon black. The microporous structure of carbon black benefits the adsorption of heteroatom/metal precursor and the formation of ADMs in later pyrolysis. As reported by Yang et al., by evaporating the dispersion of carbon black, metal chloride/nitride, and 1,10-phenanthroline monohydrate, a black powder with uniformly distributed metal ions can be obtained, which after a mild thermal treatment at 600 °C, transform into atomically dispersed metal sites (ADMSs) with well-defined single atom structure[129], shown in Figure 6A. It is suggested that such a method is feasible to fabricate Ni ADMCs with Ni loading varying from 2.5 to 5.3 wt.% [Figure 6B-E], and due to the controllable procedure and commercial availability of carbon black, it is able to produce at a large scale [Figure 6F]. Xie et al. demonstrated a post-formation procedure using carbon black as a carbon precursor. Upon adsorbing Cu species in a concentrated ammonia solution with dissolved CuCl and thermal treatment at 500 °C, Cu ADMs can be formed, shown in Figure 6G and H[130]. Wang et al. demonstrated Ni ADMCs prepared by simple pyrolyzing oxidized carbon black decorated with Ni-containing complex[131].
Figure 6. (A) Illustration of the universal procedure to produce ADMCs, (B-E) aberration-corrected HAADF-STEM image for Ni-SAC with Ni loading of 2.5, 3.4, 4.5, and 5.3 wt.%, respectively, and (F) photograph for Ni-SAC-2.5 synthesized in large scale[129], reproduced from Ref.[129] with permission. Copyright 2019 Springer Nature. Cu K-edge (G) XANES, and (H) EXAFS spectra of Cu-N-C[130], reproduced from Ref.[130] with permission. Copyright 2018 Wiley-VCH. Aberration-corrected (I) TEM, (J) Co K-edge XANES and (K) EXAFS spectra of CoN4/GN with other Ref.[52], reproduced from Ref.[52] with permission. Copyright 2016 Wiley-VCH (L) Illustration of the thermal reduction reaction of graphene oxide[132], reproduced from Ref.[132] with permission. Copyright 2010 American Chemical Society. (M) Illustration of the synthesis of Ti1/rGO, (N) TEM and (O) aberration-corrected HAADF-STEM images of
Graphene and carbon nanotubes are carbon allotropes composed of sp2 hybridized carbon with hexagonal-arranged structures. Unlike carbon black, ideal graphene and carbon nanotube are highly crystallized with few defects. Strictly speaking, graphene is a single-layer graphite sheet. However, in the booming of research since its discovery, few-layered graphite sheets are sometimes referred to as graphene as well. Graphite has a highly-ordered structure with satisfying electron conductivity. In some cases, graphite powder serves directly as a carbon precursor undergoing ball-milling with metal macrocyclic compounds when the adsorbed molecules gradually become connected to the surface of graphite particles, becoming ADMCs, as shown in Figure 6I-K[52]. However, due to the stacked layer in graphite not favoring the full exposure of the carbon surface for the anchoring of ADMs, graphene with single or few layered carbon sheets is considered more suitable and qualified as carbon precursors.
In most cases, instead of graphene itself, graphene oxide, as a carbon derivative, is utilized based on the following considerations. For one, graphene oxide comes from successive oxidation, intercalation, and exfoliation of graphite, which is rather feasible on a lab scale. For another, due to the abundant oxygen-containing functional groups on graphene, it can be well dispersed in an aqueous solution easily, making it possible to form composites with other substances or construct unique morphology. These functional groups on graphene are removed during high-temperature pyrolysis, recovering the crystalline structure and conductivity of graphene.
Despite the seeming advantages of graphene, it is not easy to construct graphene-based ADMCs using all solid thermal reactions. First, graphene sheets have a high tendency to restack at elevated temperatures, especially when surface oxygen-containing functional groups are removed. Second, the rearrangement of atoms during pyrolysis is minor, with detaching of O-containing functional groups, shown in
One effective protocol to stabilize embedded ADMs with N coordination on graphene involves highly reactive gas, which will be introduced in Section "FIXATION VIA LIGAND REPLACEMENT". The condition is similar to carbon nanotubes as well.
Heteroatom/Metal precursor and pyrolysis parameter
As stated above, most users reported ADMs to have non-carbon heteroatoms as anchoring points for metal atoms; thus, a heteroatom precursor is needed. For those MOFs or polymers with heteroatoms within the molecular structure, a second precursor can be omitted. For example, most ZIF-8 precursors with pre-existing M-N bonds for MNx ADMs and MIL-88(Al) MOF with pre-existing Al-O bonds for Al-O ADMs[68].
The N precursors can be N-rich organics, including amines, urea, or inorganics, such as ammonium salt, similar to N-doping of carbon. There is evidence that the ammonia produced by precursor decomposition at elevated temperatures is the key ingredient for the formation of ADMs. Besides N, nonmetal elements such as B, S, O, and P introduced into the carbon can participate in the coordination of metal atoms as well.
As for the metal precursors, even though simple metal salts are capable of fabricating ADMs, it is believed that the existing M-N bond in the precursors is beneficial for the formation of ADMs, probably due to simplified atomic rearrangement, making it easier and faster to inherit the original coordination in the precursors. This is exactly true for the scenarios when metal macrocyclic compounds and other metal precursors with N coordination are used.
The pyrolysis temperatures have an enormous impact on the chemical composition and structure of the products produced since not only the formation of ADMs is temperature-dependent, but also the carbonization of organics and the development of the structure and composition of the carbon skeleton are strongly dependent on temperature. Thus, the nature of precursors should be taken into consideration when deciding the pyrolysis parameters. Generally speaking, if the stabilization of metal atoms is achieved by the functional groups on carbon, a lower pyrolysis temperature should be chosen compared with those with additional heteroatom precursors. The in-situ formation of embedded ADMs out of simple metal salt requires a higher temperature than using macrocyclic compounds or biomolecules with well-defined M-N coordination. The decomposition temperature of the metal salt is also vital, as revealed by Wan et al. that the usage of metal chlorides or nitrides has brought tremendous differences in the composition of the final product[136].
The temperature chosen is determined mainly by the targeted structure of the product. Generally speaking, raising pyrolysis temperatures results in higher graphitic degrees and fewer heteroatoms within the carbon framework, which is beneficial for improving conductivity and electrochemical stability, but destabilizes some ADMs in the meantime. It is comprehensible that for purposes other than the electrochemical field, the electron conductivity need not considering, and the pyrolysis condition can be as mild as below
FIXATION VIA LIGAND REPLACEMENT
Research on the formation mechanism
With the successful demonstration of several newly emerged fabrication methodologies, evidence appeared that the formation of ADMs can be achieved via direct fixation of gas species via ligand replacement, similar to the phenomenon observed in the wet-chemical reaction[43].
Such a mechanism is typical for systems with the gas-phase introduction of key species. For instance,
Figure 7. (A) DFT-calculated energy pathway for the formation of Ni-N4 from the NiCl2, and (B) Ni K-edge EXAFS spectra of NiCl2/NC precursor, NiCl/NC intermediate, and Ni1/NC[61], reproduced from Ref.[61] with permission. Copyright 2021 Springer Nature. (C) Illustration of the formation process of Pt ADMS using thermal emitting[140], reproduced from Ref.[140] with permission. Copyright 2019 American Chemical Society. (D) Illustration of the formation process of Fe ADMS from metal center replacement, (E and F) TG-MS signals revealing the gas emission[141] during metal center replacement, reproduced from Ref.[141] with permission. Copyright 2021 Springer Nature.
Other attempts highlighted the importance of N precursors in the formation of gaseous metal species and their fixation, and it is inferred that these ADMs are formed via ligand replacement as well. Ou et al. suggested that with the help of ammonia at high temperatures, metallic Cu is able to release Cu(NH3)x species based on strong Lewis acid-base interactions, which can diffuse and then be trapped by defects of the carbon substrate, forming Cu ADMs[139]. Based on a similar process, Co and Ni can also be introduced from bulk metals. Ou et al. suggested that it is the ammonia generated by the decomposition of dicyandiamide that contributes to the emission of Pt atoms from bulk Pt [Figure 7C][140].
Different from the abovementioned mechanism, as demonstrated by Jiao et al., the gaseous metal salt can not only be captured by proper vacancies forming ADMs but also react with existing moieties and replace the metal center [Figure 7D][141]. By introducing FeCl3 into a fully carbonized ZIF-8, the original Zn-N4 moieties are transformed into Fe-N4 according to the following equation:
FeCl3 + Zn-N4 + X = Fe-N4 + ZnCl2 + XCl
Such a mechanism is supported by mass spectrometry with an intense signal assigned to HCl and ZnCl2 during thermal treatment [Figure 7E and F][141].
Applicable scenario
With the experimental observation of the traditional all-solid procedures, the nature of the complicated thermal reactions is partially uncovered, with evidence of the substance contributing the most to the formation of ADMs. With such knowledge, new procedures with stronger pertinency and higher efficiency have been developed. In this section, the thermal reaction involving active gaseous species is introduced.
Gas-phase introduction of key nonmetal species
Despite the fact that there is always gas evolution during the thermal treatment of organics in the all-solid reactions described above, some other procedures have unique features in that the key nonmetal species are introduced directly from additional cylinders or originate from the compound placed in a separate vessel in the furnace without direct contact with the carbon source. On the one hand, these procedures prove the important role played by these gaseous nonmetallic species. On the other hand, they save researchers the trouble of possible purification when the origin of these species is mixed with the main product, as is the case with all the solid reactions mentioned above.
Up to now, ammonia has been proven the most effective in promoting the atomization of metal species. The detailed mechanism is not yet understood, and it is speculated that NH3 plays an important role in the production of metal-containing gaseous species, which can easily be fixed within the carbon framework[139]. At the same time, the reaction between ammonia and carbon creates micropores and dopes N into the framework, which also benefits the fixation of ADMs[142]. Such effects are so strong that they can reverse the metal aggregation at elevated temperatures, making some of the less favored systems, GO and CNT, for example, capable of producing ADMs. Even though graphene has a huge surface area, it is not a qualified carbon substrate due to the accessible micropore structure being insufficient to host a large number of ADMs. This situation is more apparent when using graphene oxide as support. During the thermal treatment of graphene oxide, the rearrangement of carbon atoms is not thoroughly compared with the carbonization of organics, resulting in little chance of fixing ADMs. Thus, direct pyrolysis of graphene oxide with metal precursors and solid metal dopants usually ended up with metal aggregates. With the help of ammonia, various metal elements can be fixed onto the carbon framework with MNx coordination, and in those cases, a second solid N precursor is not necessary [Figure 8A and B][121,143-146]. In other procedures, it is believed that the N-containing active gas, ammonia, for example, is responsible for the formation of metal-containing species and the formation of ADMs on carbon. Our group obtained Cu ADMCs based on a high-temperature procedure where the mixture of carbon derivatives (graphene oxide, functionalized CNT, or active carbon) and dicyandiamide are placed in a capping boat wrapped tightly by Cu foil. They believe that the participation of the decomposition product of dicyandiamide in the formation of reactive Cu-containing species [Figure 8C-E][147].
Figure 8. (A) Illustration of the ammonia-assisted fabrication process of Fe/NG[146], reproduced from Ref.[146] with permission. Copyright 2018 Wiley-VCH. (B) Illustration of the ammonia-assisted fabrication process of Cu ADMCs[140], reproduced from Ref.[140] with permission. Copyright 2019 American Chemical Society. (C) TEM, (D) EDS element mapping, and (E) aberration-corrected HAADF-STEM images of Cu-G[147], reproduced from Ref.[147] with permission. Copyright 2019 Elsevier. (F) Illustration of the transformation of pyridinic FeN4 to pyrrolic FeN4 by ammonia treatment, and (G) C K-edge and N K-edge XANES spectra od HP-FeN4 and FeN4[12], reproduced from Ref.[12] with permission. Copyright 2020 Royal Society of Chemistry. (H) Illustration of the fabrication process of
In addition to promoting the atomization of metal elements, it has been reported that ammonia treatment has tremendous effects on the coordination structure of ADMs. According to Zhang et al., compared with Ar, Fe ADMCs fabricated in ammonia have a regulated second coordination shell, converting the original pyridinic N for the anchoring of Fe atoms into pyrrolic type while maintaining the original FeN4 first coordination shell [Figure 8F and G][12].
Very recently, the utilization of HCl derived from ammonia chloride has also proven effective in downsizing metal aggregates into ADMs. Li et al. reported that by thermally treating a mixture of ZIF-8 derived carbon with nanosized Fe powder and ammonia chloride at 500 °C, with the help of reactive gaseous hydrogen chloride and ammonia, metal aggregates can be converted entirely into ADMs [Figure 8H][148].
Gas-phase transfer of metal species
In addition to regular solid metal precursors, some metal precursors can be introduced via gas-phase transfer. Compared with solid mixing, an apparent advantage of doing so is the higher dispersion of the metal precursor in the gas phase, guaranteeing the uniform mixing of the metal precursor and carbon substrate. Another advantage is that only surfaces with sufficient gas-diffusion pathways can serve as anchoring points for ADMs, facilitating their utilization, especially in catalysis.
Metal precursors can be volatile metal salts, such as metal chlorides and acetylacetonates with lower boiling points, which evaporate as the temperature rises and then make contact with the carbon precursor.
Wang et al. developed a noncontact gas-migration-trapping strategy using CoCl2 as a metal precursor to form Co-based ADMs on carbon placed in a separated boat during pyrolysis[21]. Jiao et al. demonstrated a similar procedure with the gaseous introduction of FeCl3, obtaining Fe-N4 moieties within the carbon framework placed in a separate boat[141]. Hai et al. suggested that by introducing gaseous chloride molecules into the chamber where substrate materials exist and performing two thermal treatments spirited by a washing step, a high loading level of metal can be achieved on carbon [Figure 8I][61]. This procedure showed remarkable universality, with 15 metal elements being able to be fixed in the form of ADMs.
In other reports, metal is introduced from metal bulk or involatile oxide with or without the assistance of gaseous N-containing species, depending on the procedure. Han et al. developed a novel self-initiated dispersing protocol, proven effective in fabricating Cu-based ADMCs out of metallic Cu with the help of dicyandiamide within a quasi-enclosed system[147]. Because the metal source and carbon substrate were not in contact, the appearance of Cu ADMs on the carbon substrate clearly indicated the gaseous transfer of the Cu source. Ou et al. demonstrated a method using bulk metal as a source, which introduces Cu/Co/Ni ADMs into carbon via gas-phase transfer with the help of ammonia at high temperatures[139].
DIRECT ANCHORING
As the all-solid procedures are the major procedure for the fabrication of ADMCs, several mechanisms via solid-solid interactions have been proposed based on direct observation or indirect speculation. It is suggested that active metal atoms may be emitted from the bulk, which are then captured by the proper vacancies, forming ADMCs. The entire reaction can occur at the interface of the metal source and substrate or in noncontact situations.
The formation of ADMCs at the interface of the metal source and substrate requires the diffusion of metal atoms from the bulk metal source and their fixation within the substrate. In fact, the phenomenon of solid-state metal diffusion occurring in the contact area of two metals was discovered a long time ago, and the diffusion of metal atoms into the carbon was observed for graphene or carbon nanotubes fabricated by CVD under the catalysis of transition metal nanoparticles[149,150]. However, the single metal atoms within the carbon lattice without proper anchoring points were much less stable than those in the metal lattice. It is believed that creating vacancies (usually single or dual vacancies) is efficient in improving the stability of metal atoms on carbon substrates, which can be further elevated by introducing heteroatoms such as N, S, or P as anchoring points for the formation of ADMCs[127], similar to the phenomena described in the above two sections.
Zhang et al. reported that by wrapping various metal oxide/hydroxide particles with polydopamine followed by high-temperature pyrolysis, MNx moieties can be formed on the outer carbon shells[102]. Such a strategy is effective in fabricating ADMCs with Fe, Co, Ni, Mn, FeCo, FeNi, et al. It is believed that the metal oxide/hydroxide at the interface is first reduced by carbon at elevated temperature into a thin layer of metal, during which time there is a strong interaction between metal atoms and the substrate. As a result, when the inner core is removed with acid, stable ADMs are left on the surface of the carbon. It has been shown that polyaniline and polypyrrole with abundant N are also capable of stabilizing metal atoms from the inner core. In the case of ultrasmall metal aggregates, as in the case of nanoparticles, it is possible for these nanoparticles to transform entirely into ADMs. Liu et al. suggested that Fe-, Co-, Ni-, and Mn-based ADMCs can be obtained by thermal atomization of the corresponding metal oxide assisted by O-rich graphene oxide and a polymer coating layer[151]. These results highlight the importance of heteroatoms in the carbon substrate.
Such a mechanism receives strong support from the in-temperature identical location transmission electron microscopy (IL-TEM) observation on several systems containing metal nanoparticles. IL-TEM is a powerful tool for observing the changes of material in a selected area as temperature and atmosphere change.
Figure 9. (A) In-situ TEM observation, and (B) average number and diameter of Pd particles observed on Pd@ZIF-8 pyrolyzed at
It is observed that during the process, these particles moved disorderly and collided intensively within the ZIF-8 and the derived carbon particles. It is then inferred that the thermal motion of Pd within ZIF-8 is necessary for its atomization, considering the saturation of anchoring points within a small area and the suppressed emission of larger metal particles. Such a hypothesis is supported by the failure of atomization of Pd on the surface of the ZIF-8 particle, in which case Pd particles were unable to move into the ZIF-8 particle. Thus, it is believed that the evolution of Pd nanoparticle into single atoms are driven by the two competitive processes of atomization and agglomeration, represented by the competitive bonding between Pd-N and Pd-Pd. Using DFT, it is proven exothermic to move a single Pd atom from a cluster to a nearby N4 vacancy, forming Pd-N4 ADMs with a large exothermicity of 3.96 eV and an energy barrier of 1.47 eV. In comparison, the aggregation of two Pd atoms is also exothermic, with a small energy barrier of 0.58 eV, shown in Figure 9C. As a result, sintering is the major process at a lower temperature, and when the temperature is high enough, atomization is thermodynamically favored.
In another demonstration by Yang et al., Ni nanoparticles acted similarly to precious metals in the above work, i.e., aggregated and then atomized[153]. Because the loading of these particles on the surface of ZIF-8 and the N-containing defects was not sufficient, the atomization was not complete, and a certain content of particles remained. When observing Ni particles loaded on N-free XC-72 carbon black, only aggregation was witnessed, highlighting the importance of N on the atomization of metal particles. However, Qiu et al. reported that the CVD process using precursors without N on a porous Ni substrate obtained Ni-based ADMs with NiC3 configuration[154]. Thus, it is highly likely that despite similar so-called carbon substrates, the detailed structures varied depending on the precursor and thermal treatment parameters.
From the abovementioned information, the following rules relating to the atomization of metal particles into ADMs can be summarized.
1. The formation of ADMs at the interface of metal particles and carbon is thermodynamically favored, and the temperature should be high enough to provide energy to overcome the activation barrier[152].
2. The atomization of metal is achieved by N-containing defects or defects with other forms that can stabilize ADMs[127].
3. The atomization has a saturation level and once reached, the atomization is suppressed[152].
One of the very first attempts to employ the in-temperature XAS technique to investigate the changes in simple Fe salt during high-temperature pyrolysis was made by Li et al.[155]. It was proposed that single Fe atoms can be emitted from tetrahedral Fe-O4 and become Fe-N4, as illustrated in Figure 9D. The information obtained on several Fe-containing systems was critical to uncovering the evolution of the local coordination environment of Fe as the temperature rises. As demonstrated in the in-temperature Fe K-edge XANES and EXAFS spectra in Figure 9E and F evidence has pictured a clear transition pathway of Fe in the mixture of Fe(ac)2, 1,10-phenanthroline monohydrate and ZIF-8, a commonly seen all-solid system, from the original Fe(ac)2 to Fe-N4 via Fe2O3, FeO with octahedral Fe-O6 and tetrahedral Fe-O4. The carbonization process of ZIF does not affect the results, and the transition of tetrahedral Fe-O4 to in-plane Fe-N4 is irreversible, while the formation of Fe-N4 is thermodynamically favored, as shown in Figure 9G. It is then hypothesized after comparing the third systems containing FeCl2·4H2O and SiO2 that as O is gradually removed from tetrahedral Fe-O4, single Fe atoms are formed, which, if captured by nearby N, become
However, as suggested by Li et al., ADMs can be formed directly from iron oxide nanoparticles at temperatures as low as 400 °C, much lower than 600 °C for the formation of active Fe via removal of the lattice O[49]. According to their studies, most of the Fe3+ ions adsorbed on N-doped carbon derived from ZIF-8 transform into ultrafine iron oxide nanoparticles upon drying and exposure to air, as evidenced by XAS [Figure 9I and J]. Once treated at 400 °C, these nanoparticles disappeared [Figure 9K and L], and the EELS spectra with high spatial resolution in Figure 9M suggested the formation of FeN coordination, which was in line with the XAS results. DFT calculations also revealed that even though it was energetically unfavorable to remove Fe ions from bulk FeOx, once captured by pre-existing N4 vacancies, the entire process became highly exothermic.
Thus, it was concluded that the fixation of metals from simple salts such as chlorides and acetates may not be as simple as once thought. An intermediate state of unstable metal oxide and single metal atoms may be involved.
As stated above, based on in-situ XAS observations of the pyrolysis process of various Fe-containing systems, a mechanism based on active Fe atoms obtained from the removal of O from oxides has been proposed. Even though the mechanism targets solid state systems, according to a supplemented experiment by the authors, these active single Fe atoms may be in a gaseous state, without direct contact at elevated temperature, and a small portion of Fe is able to migrate from Fe2O3 into N-doped carbon in the form of Fe ADMs. It is not yet clear whether such a mechanism applies to systems containing other metal species, but there have been several studies that found similar phenomena in metals other than Fe. Yang et al. reported the thermal emission of Cu atoms from Cu2O into an N-doped carbon framework to fabricate Cu ADMCs. In their study, MoO3 and SnO2 powders were shown to be effective through thermal emission to realize corresponding ADMCs[156].
OUTLOOK AND CHALLENGES
There is no doubt that ADMCs have become the star material in various fields and are gaining more attention from researchers who are trying to widen their application. After years of exploration, several chemical procedures have been proven effective and controllable in fabricating ADMCs. For studies on the formation mechanism, despite the progress made in recent years with the aid of high-end techniques, especially in-temperature XAS and TEM, the composition evolution pathway according to which ADMCs are formed during the thermal reaction is not yet fully and completely understood. Further investigation of the common phenomena is still valuable, for example, the interaction between metal atoms and the surrounding coordination environment and the role pyrolysis parameters play in the evolution of metal moieties. Hopefully, a better understanding would grant us more controllability over the thermal-chemical reactions, thus promoting research on novel fabrication methods, especially those able to precisely manipulate the local structure of ADMs, for example, multi-atom centers with well-defined structures and second and even further coordination shells. Furthermore, methods with high atomic efficiency, low energy consumption, and large-scale production prospects should be developed for possible application at the industrial level.
Notably, despite the progress made in recent years, the formation mechanism of ADMCs is far from being uncovered. There are a few challenges for researchers to pay conscious effort to. The first is to understand the formation of ADMSs to a greater extent. In this review, the formation mechanism is limited to the changes during the transformation from metal precursors to ADMs, without considering the nearby carbon structure. It is discovered that the second or even third coordination shells of the metal center play an important part in the performance of ADMs. Thus, understanding the formation of the entire ADMSs to a greater extent is a valuable yet challenging task. Another challenge we are facing today is to uncover the formation mechanisms of newly-emerged double-atom or even triple-atom ADMs, which are less studied and remain unknown. Uncovering how two or more atoms favorably attract and coordinate on the support is critical for the controllable fabrication of such material.
DECLARATIONS
Authors’ contributionsProposed the topic of this review: Du C
Prepared the manuscript references data: Du J, Yan Y
Prepared the manuscript: Han G, Zhang W, Li L
Collectively discussed and revised the manuscript: Geng L, Tong Y
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work is financially supported by the National Natural Science Foundation of China (Grant No. 51634003) and Heilongjiang Touyan Innovation Team Program (HITTY-20190033).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. bp statistical review of world energy 2020. Available from: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf [Last accessed on 27 March 2023].
2. bp statistical review of world energy 2021. Available from: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf [Last accessed on 27 March 2023].
3. Liu Q, Ranocchiari M, van Bokhoven JA. Catalyst overcoating engineering towards high-performance electrocatalysis. Chem Soc Rev 2022;51:188-236.
4. Zhang Z, Liu J, Curcio A, et al. Atomically dispersed materials for rechargeable batteries. Nano Energy 2020;76:105085.
5. Venkateswara Raju C, Hwan Cho C, Mohana Rani G, et al. Emerging insights into the use of carbon-based nanomaterials for the electrochemical detection of heavy metal ions. Coord Chem Rev 2023;476:214920.
6. Dong F, Wu M, Chen Z, et al. Atomically dispersed transition metal-nitrogen-carbon bifunctional oxygen electrocatalysts for zinc-air batteries: recent advances and future perspectives. Nanomicro Lett 2021;14:36.
7. Li W, Guo Z, Yang J, et al. Advanced strategies for stabilizing single-atom catalysts for energy storage and conversion. Electrochem Energy Rev 2022;5:9.
8. Yang X, Priest C, Hou Y, Wu G. Atomically dispersed dual-metal-site PGM-free electrocatalysts for oxygen reduction reaction: opportunities and challenges. SusMat 2022;2:569-90.
9. Wang J, Liu W, Luo G, et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy Environ Sci 2018;11:3375-9.
10. He T, Chen Y, Liu Q, et al. Theory-guided regulation of FeN4 spin state by neighboring Cu atoms for enhanced oxygen reduction electrocatalysis in flexible metal-air batteries. Angew Chem Int Ed 2022;61:e202201007.
11. Xiao M, Zhu J, Li G, et al. A single-atom iridium heterogeneous catalyst in oxygen reduction reaction. Angew Chem Int Ed 2019;58:9640-5.
12. Zhang N, Zhou T, Chen M, et al. High-purity pyrrole-type FeN4 sites as a superior oxygen reduction electrocatalyst. Energy Environ Sci 2020;13:111-8.
13. Liu D, Li X, Chen S, et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat Energy 2019;4:512-8.
14. Mu X, Gu X, Dai S, et al. Breaking the symmetry of single-atom catalysts enables an extremely low energy barrier and high stability for large-current-density water splitting. Energy Environ Sci 2022;15:4048-57.
15. Li BQ, Zhao CX, Liu JN, Zhang Q. Electrosynthesis of hydrogen peroxide synergistically catalyzed by atomic Co-Nx-C sites and oxygen functional groups in noble-metal-free electrocatalysts. Adv Mater 2019;31:e1808173.
16. Pan F, Li B, Sarnello E, et al. Pore-edge tailoring of single-atom iron-nitrogen sites on graphene for enhanced CO2 reduction. ACS Catal 2020;10:10803-11.
17. Zang W, Yang T, Zou H, et al. Copper single atoms anchored in porous nitrogen-doped carbon as efficient pH-universal catalysts for the nitrogen reduction reaction. ACS Catal 2019;9:10166-73.
18. Gokana MR, Wu C, Motora KG, Qi JY, Yen W. Effects of patterned electrode on near infrared light-triggered cesium tungsten bronze/poly(vinylidene)fluoride nanocomposite-based pyroelectric nanogenerator for energy harvesting. J Power Sources 2022;536:231524.
19. Chen S, Luo T, Li X, et al. Identification of the highly active Co-N4 coordination motif for selective oxygen reduction to hydrogen peroxide. J Am Chem Soc 2022;144:14505-16.
20. Du Z, Chen X, Hu W, et al. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium-sulfur batteries. J Am Chem Soc 2019;141:3977-85.
21. Wang P, Ren Y, Wang R, et al. Atomically dispersed cobalt catalyst anchored on nitrogen-doped carbon nanosheets for lithium-oxygen batteries. Nat Commun 2020;11:1576.
22. Xia Q, Zhai Y, Zhao L, et al. Carbon-supported single-atom catalysts for advanced rechargeable metal-air batteries. Energy Mater 2022;2:200015.
23. Hu X, Luo G, Zhao Q, et al. Ru single atoms on N-doped carbon by spatial confinement and ionic substitution strategies for high-performance Li-O2 batteries. J Am Chem Soc 2020;142:16776-86.
24. Li X, Han G, Lou S, et al. Tailoring lithium-peroxide reaction kinetics with CuN2C2 single-atom moieties for lithium-oxygen batteries. Nano Energy 2022;93:106810.
25. Yang T, Qian T, Sun Y, Zhong J, Rosei F, Yan C. Mega high utilization of sodium metal anodes enabled by single zinc atom sites. Nano Lett 2019;19:7827-35.
26. Lu C, Fang R, Chen X. Single-atom catalytic materials for advanced battery systems. Adv Mater 2020;32:e1906548.
27. Yang X, Zheng Y, Yang J, et al. Modeling Fe/N/C catalysts in monolayer graphene. ACS Catal 2017;7:139-45.
28. Yang XF, Wang A, Qiao B, Li J, Liu J, Zhang T. Single-atom catalysts: a new frontier in heterogeneous catalysis. ACC Chem Res 2013;46:1740-8.
29. Ji S, Chen Y, Wang X, Zhang Z, Wang D, Li Y. Chemical synthesis of single atomic site catalysts. Chem Rev 2020;120:11900-55.
30. Boucher MB, Zugic B, Cladaras G, et al. Single atom alloy surface analogs in Pd0.18Cu15 nanoparticles for selective hydrogenation reactions. Phys Chem Chem Phys 2013;15:12187-96.
31. Lou Y, Liu J. CO oxidation on metal oxide supported single Pt atoms: the role of the support. Ind Eng Chem Res 2017;56:6916-25.
32. Zhang J, Liu J, Xi L, et al. Single-atom Au/NiFe layered double hydroxide electrocatalyst: probing the origin of activity for oxygen evolution reaction. J Am Chem Soc 2018;140:3876-9.
33. Zhao D, Chen Z, Yang W, et al. MXene (Ti3C2) vacancy-confined single-atom catalyst for efficient functionalization of CO2. J Am Chem Soc 2019;141:4086-93.
34. Zhang B, Asakura H, Zhang J, Zhang J, De S, Yan N. Stabilizing a platinum1 single-atom catalyst on supported phosphomolybdic acid without compromising hydrogenation activity. Angew Chem Int Ed 2016;55:8319-23.
35. Zhang B, Asakura H, Yan N. Atomically dispersed rhodium on self-assembled phosphotungstic acid: structural features and catalytic CO oxidation properties. Ind Eng Chem Res 2017;56:3578-87.
36. Sakamoto R, Toyoda R, Jingyan G, et al. Coordination chemistry for innovative carbon-related materials. Coord Chem Rev 2022;466:214577.
37. Umapathi R, Ghoreishian SM, Sonwal S, Rani GM, Huh YS. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Coord Chem Rev 2022;453:214305.
38. Bakandritsos A, Kadam RG, Kumar P, et al. Mixed-valence single-atom catalyst derived from functionalized graphene. Adv Mater 2019;31:e1900323.
39. Liu Z, Li S, Yang J, et al. Ultrafast construction of oxygen-containing scaffold over graphite for trapping Ni2+ into single atom catalysts. ACS Nano 2020;14:11662-9.
40. Han G, Zhang X, Liu W, et al. Substrate strain tunes operando geometric distortion and oxygen reduction activity of CuN2C2 single-atom sites. Nat Commun 2021;12:6335.
41. Li J, Jiang YF, Wang Q, et al. A general strategy for preparing pyrrolic-N4 type single-atom catalysts via pre-located isolated atoms. Nat Commun 2021;12:6806.
42. Yan H, Zhao X, Guo N, et al. Atomic engineering of high-density isolated Co atoms on graphene with proximal-atom controlled reaction selectivity. Nat Commun 2018;9:3197.
43. Mehmood A, Pampel J, Ali G, Ha HY, Ruiz-zepeda F, Fellinger T. Facile metal coordination of active site imprinted nitrogen doped carbons for the conservative preparation of non-noble metal oxygen reduction electrocatalysts. Adv Energy Mater 2018;8:1701771.
44. Hai X, Zhao X, Guo N, et al. Engineering local and global structures of single Co atoms for a superior oxygen reduction reaction. ACS Catal 2020;10:5862-70.
45. Wang X, Chen Z, Zhao X, et al. Regulation of coordination number over single Co sites: triggering the efficient electroreduction of CO2. Angew Chem Int Ed 2018;57:1944-8.
46. Pan Y, Chen Y, Wu K, et al. Regulating the coordination structure of single-atom Fe-NxCy catalytic sites for benzene oxidation. Nat Commun 2019;10:4290.
47. Zhai P, Wang T, Yang W, et al. Uniform lithium deposition assisted by single-atom doping toward high-performance lithium metal anodes. Adv Energy Mater 2019;9:1804019.
48. Ha M, Kim DY, Umer M, Gladkikh V, Myung CW, Kim KS. Tuning metal single atoms embedded in NxCy moieties toward high-performance electrocatalysis. Energy Environ Sci 2021;14:3455-68.
49. Li J, Zhang H, Samarakoon W, et al. Thermally driven structure and performance evolution of atomically dispersed FeN4 sites for oxygen reduction. Angew Chem Int Ed 2019;58:18971-80.
50. Chen K, Liu K, An P, et al. Iron phthalocyanine with coordination induced electronic localization to boost oxygen reduction reaction. Nat Commun 2020;11:4173.
51. Li Z, Zhuang Z, Lv F, et al. The marriage of the FeN4 moiety and MXene boosts oxygen reduction catalysis: Fe 3D electron delocalization matters. Adv Mater 2018;30:e1803220.
52. Cui X, Xiao J, Wu Y, et al. A graphene composite material with single cobalt active sites: a highly efficient counter electrode for dye-sensitized solar cells. Angew Chem Int Ed 2016;55:6708-12.
53. Marshall-Roth T, Libretto NJ, Wrobel AT, et al. A pyridinic Fe-N4 macrocycle models the active sites in Fe/N-doped carbon electrocatalysts. Nat Commun 2020;11:5283.
54. Sa YJ, Seo DJ, Woo J, et al. A general approach to preferential formation of active Fe-Nx sites in Fe-N/C electrocatalysts for efficient oxygen reduction reaction. J Am Chem Soc 2016;138:15046-56.
55. Wang Q, Ina T, Chen WT, et al. Evolution of Zn(II) single atom catalyst sites during the pyrolysis-induced transformation of ZIF-8 to N-doped carbons. Sci Bull 2020;65:1743-51.
56. Jahnke H, Schönborn M, Zimmermann G. Organic dyestuffs as catalysts for fuel cells. In: Schäfer FP, Gerischer H, Willig F, et al., editors. Physical and chemical applications of dyestuffs. Berlin/Heidelberg: Springer-Verlag; 1976. pp. 133-81.
57. Gupta S, Tryk D, Bae I, Aldred W, Yeager E. Heat-treated polyacrylonitrile-based catalysts for oxygen electroreduction. J Appl Electrochem 1989;19:19-27.
58. Li L, Wen Y, Han G, et al. Tailoring the stability of Fe-N-C via pyridinic nitrogen for acid oxygen reduction reaction. Chem Eng J 2022;437:135320.
59. Sun Y, Silvioli L, Sahraie NR, et al. Activity-selectivity trends in the electrochemical production of hydrogen peroxide over single-site metal-nitrogen-carbon catalysts. J Am Chem Soc 2019;141:12372-81.
60. Ding T, Liu X, Tao Z, et al. Atomically precise dinuclear site active toward electrocatalytic CO2 reduction. J Am Chem Soc 2021;143:11317-24.
61. Hai X, Xi S, Mitchell S, et al. Scalable two-step annealing method for preparing ultra-high-density single-atom catalyst libraries. Nat Nanotechnol 2022;17:174-81.
62. Gao J, Yang HB, Huang X, et al. Enabling direct H2O2 production in acidic media through rational design of transition metal single atom catalyst. Chem 2020;6:658-74.
63. Zhang N, Zhou T, Ge J, et al. High-density planar-like Fe2N6 structure catalyzes efficient oxygen reduction. Matter 2020;3:509-21.
64. Xiao M, Gao L, Wang Y, et al. Engineering energy level of metal center: Ru single-atom site for efficient and durable oxygen reduction catalysis. J Am Chem Soc 2019;141:19800-6.
65. Li X, Huang X, Xi S, et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient fenton-like catalysis. J Am Chem Soc 2018;140:12469-75.
66. Jiao L, Wan G, Zhang R, Zhou H, Yu S, Jiang H. From metal-organic frameworks to single-atom Fe implanted N-doped porous carbons: efficient oxygen reduction in both alkaline and acidic media. Angew Chem Int Ed 2018;130:8661-5.
67. Chen W, Pei J, He CT, et al. Single tungsten atoms supported on MOF-derived N-doped carbon for robust electrochemical hydrogen evolution. Adv Mater 2018;30:e1800396.
68. Yang Q, Xu W, Gong S, et al. Atomically dispersed lewis acid sites boost 2-electron oxygen reduction activity of carbon-based catalysts. Nat Commun 2020;11:5478.
69. Al-Zoubi T, Zhou Y, Yin X, et al. Preparation of nonprecious metal electrocatalysts for the reduction of oxygen using a low-temperature sacrificial metal. J Am Chem Soc 2020;142:5477-81.
70. Yang Y, Mao K, Gao S, et al. O-, N-atoms-coordinated Mn cofactors within a graphene framework as bioinspired oxygen reduction reaction electrocatalysts. Adv Mater 2018;30:e1801732.
71. Zhang E, Wang T, Yu K, et al. Bismuth single atoms resulting from transformation of metal-organic frameworks and their use as electrocatalysts for CO2 reduction. J Am Chem Soc 2019;141:16569-73.
72. Phan A, Doonan CJ, Uribe-Romo FJ, Knobler CB, O’Keeffe M, Yaghi OM. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. ACC Chem Res 2010;43:58-67.
73. Chen M, Li X, Yang F, et al. Atomically dispersed MnN4 catalysts via environmentally benign aqueous synthesis for oxygen reduction: mechanistic understanding of activity and stability improvements. ACS Catal 2020;10:10523-34.
74. Li Z, Chen Y, Ji S, et al. Iridium single-atom catalyst on nitrogen-doped carbon for formic acid oxidation synthesized using a general host-guest strategy. Nat Chem 2020;12:764-72.
75. Xie X, He C, Li B, et al. Performance enhancement and degradation mechanism identification of a single-atom Co-N-C catalyst for proton exchange membrane fuel cells. Nat Catal 2020;3:1044-54.
76. Wang J, Han G, Wang L, et al. ZIF-8 with ferrocene encapsulated: a promising precursor to single-atom Fe embedded nitrogen-doped carbon as highly efficient catalyst for oxygen electroreduction. Small 2018;14:e1704282.
77. Ji S, Chen Y, Zhao S, et al. Atomically dispersed ruthenium species inside metal-organic frameworks: combining the high activity of atomic sites and the molecular sieving effect of MOFs. Angew Chem Int Ed 2019;58:4271-5.
78. Jiang R, Li L, Sheng T, Hu G, Chen Y, Wang L. Edge-site engineering of atomically dispersed Fe-N4 by selective C-N bond cleavage for enhanced oxygen reduction reaction activities. J Am Chem Soc 2018;140:11594-8.
79. Wan X, Liu X, Li Y, et al. Fe-N-C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat Catal 2019;2:259-68.
80. Xiong Y, Dong J, Huang ZQ, et al. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat Nanotechnol 2020;15:390-7.
81. Chen Y, Ji S, Wang Y, et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew Chem Int Ed 2017;129:7041-5.
82. Wang H, Grabstanowicz LR, Barkholtz HM, et al. Impacts of imidazolate ligand on performance of zeolitic-imidazolate framework-derived oxygen reduction catalysts. ACS Energy Lett 2019;4:2500-7.
83. Xiao M, Zhu J, Ma L, et al. Microporous framework induced synthesis of single-atom dispersed Fe-N-C acidic ORR catalyst and its in situ reduced Fe-N4 active site identification revealed by X-ray absorption spectroscopy. ACS Catal 2018;8:2824-32.
84. Li J, Chen M, Cullen DA, et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat Catal 2018;1:935-45.
85. Zhu M, Zhao C, Liu X, et al. Single atomic cerium sites with a high coordination number for efficient oxygen reduction in proton-exchange membrane fuel cells. ACS Catal 2021;11:3923-9.
86. Liu Q, Li Y, Zheng L, et al. Sequential synthesis and active-site coordination principle of precious metal single-atom catalysts for oxygen reduction reaction and PEM fuel cells. Adv Energy Mater 2020;10:2000689.
87. Liu Q, Liu X, Zheng L, Shui J. The solid-phase synthesis of an Fe-N-C electrocatalyst for high-power proton-exchange membrane fuel cells. Angew Chem Int Ed 2018;57:1204-8.
88. Ye W, Chen S, Lin Y, et al. Precisely tuning the number of fe atoms in clusters on N-doped carbon toward acidic oxygen reduction reaction. Chem 2019;5:2865-78.
89. Ye Y, Cai F, Li H, et al. Surface functionalization of ZIF-8 with ammonium ferric citrate toward high exposure of Fe-N active sites for efficient oxygen and carbon dioxide electroreduction. Nano Energy 2017;38:281-9.
90. Liu M, Li N, Cao S, et al. A “pre-constrained metal twins” strategy to prepare efficient dual-metal-atom catalysts for cooperative oxygen electrocatalysis. Adv Mater 2022;34:e2107421.
91. Wan J, Zhao Z, Shang H, et al. In situ phosphatizing of triphenylphosphine encapsulated within metal-organic frameworks to design atomic Co1-P1N3 interfacial structure for promoting catalytic performance. J Am Chem Soc 2020;142:8431-9.
92. Liu J, Kong X, Zheng L, Guo X, Liu X, Shui J. Rare earth single-atom catalysts for nitrogen and carbon dioxide reduction. ACS Nano 2020;14:1093-101.
93. Wang J, Huang Z, Liu W, et al. Design of N-coordinated dual-metal sites: a stable and active Pt-free catalyst for acidic oxygen reduction reaction. J Am Chem Soc 2017;139:17281-4.
94. Xiao M, Chen Y, Zhu J, et al. Climbing the apex of the ORR volcano plot via binuclear site construction: electronic and geometric engineering. J Am Chem Soc 2019;141:17763-70.
95. Ren W, Tan X, Yang W, et al. Isolated diatomic Ni-Fe metal-nitrogen sites for synergistic electroreduction of CO2. Angew Chem Int Ed 2019;58:6972-6.
96. Wu J, Zhang Q, Wang J, Huang X, Bai H. A self-assembly route to porous polyaniline/reduced graphene oxide composite materials with molecular-level uniformity for high-performance supercapacitors. Energy Environ Sci 2018;11:1280-6.
97. An H, Zhang R, Li Z, Zhou L, Shao M, Wei M. Highly efficient metal-free electrocatalysts toward oxygen reduction derived from carbon nanotubes@polypyrrole core-shell hybrids. J Mater Chem A 2016;4:18008-14.
98. Li J, Yang Z, Tang D, et al. N-doped carbon nanotubes containing a high concentration of single iron atoms for efficient oxygen reduction. NPG Asia Mater 2018;10:e461-e461.
99. Li H, Wen Y, Jiang M, et al. Understanding of neighboring Fe-N4-C and Co-N4-C dual active centers for oxygen reduction reaction. Adv Funct Mater 2021;31:2011289.
100. Jin Z, Li P, Meng Y, Fang Z, Xiao D, Yu G. Understanding the inter-site distance effect in single-atom catalysts for oxygen electroreduction. Nat Catal 2021;4:615-22.
101. Weng CC, Ren JT, Zhao H, Hu ZP, Yuan ZY. Iron-salt thermally emitted strategy to prepare graphene-like carbon nanosheets with trapped Fe species for an efficient electrocatalytic oxygen reduction reaction in the all-pH range. ACS Appl Mater Interfaces 2019;11:27823-32.
102. Zhang M, Wang YG, Chen W, et al. Metal (Hydr)oxides@polymer core-shell strategy to metal single-atom materials. J Am Chem Soc 2017;139:10976-9.
103. Han X, Ling X, Yu D, et al. Atomically dispersed binary Co-Ni sites in nitrogen-doped hollow carbon nanocubes for reversible oxygen reduction and evolution. Adv Mater 2019;31:e1905622.
104. Zhou Y, Yu Y, Ma D, et al. Atomic Fe dispersed hierarchical mesoporous Fe-N-C nanostructures for an efficient oxygen reduction reaction. ACS Catal 2021;11:74-81.
105. Zhang Z, Zhao X, Xi S, et al. Atomically dispersed cobalt trifunctional electrocatalysts with tailored coordination environment for flexible rechargeable Zn-air battery and self-driven water splitting. Adv Energy Mater 2020;10:2002896.
106. Qu Y, Wang L, Li Z, et al. Ambient synthesis of single-atom catalysts from bulk metal via trapping of atoms by surface dangling bonds. Adv Mater 2019;31:e1904496.
107. Zhao Y, Liang J, Wang C, Ma J, Wallace GG. Tunable and efficient tin modified nitrogen-doped carbon nanofibers for electrochemical reduction of aqueous carbon dioxide. Adv Energy Mater 2018;8:1702524.
108. Zhang H, Zhou W, Chen T, Guan BY, Li Z, Lou XW. A modular strategy for decorating isolated cobalt atoms into multichannel carbon matrix for electrocatalytic oxygen reduction. Energy Environ Sci 2018;11:1980-4.
109. Yang L, Zhang X, Yu L, Hou J, Zhou Z, Lv R. Atomic Fe-N4/C in flexible carbon fiber membrane as binder-free air cathode for Zn-air batteries with stable cycling over 1,000 h. Adv Mater 2022;34:e2105410.
110. Cheng C, Li S, Xia Y, et al. Atomic Fe-Nx coupled open-mesoporous carbon nanofibers for efficient and bioadaptable oxygen electrode in Mg-air batteries. Adv Mater 2018:e1802669.
111. Ji D, Fan L, Li L, et al. Atomically transition metals on self-supported porous carbon flake arrays as binder-free air cathode for wearable zinc-air batteries. Adv Mater 2019;31:e1808267.
112. Cheng Q, Yang L, Zou L, et al. Single cobalt atom and N codoped carbon nanofibers as highly durable electrocatalyst for oxygen reduction reaction. ACS Catal 2017;7:6864-71.
113. Zhang Z, Sun J, Wang F, Dai L. Efficient oxygen reduction reaction (ORR) catalysts based on single iron atoms dispersed on a hierarchically structured porous carbon framework. Angew Chem Int Ed 2018;130:9176-81.
114. Zhang Z, Gao X, Dou M, Ji J, Wang F. Biomass derived N-doped porous carbon supported single Fe atoms as superior electrocatalysts for oxygen reduction. Small 2017;13:1604290.
115. Wang C, Chen W, Xia K, Xie N, Wang H, Zhang Y. Silk-derived 2D porous carbon nanosheets with atomically-dispersed Fe-Nx-C sites for highly efficient oxygen reaction catalysts. Small 2019;15:e1804966.
116. Yang G, Zhu J, Yuan P, et al. Regulating Fe-spin state by atomically dispersed Mn-N in Fe-N-C catalysts with high oxygen reduction activity. Nat Commun 2021;12:1734.
117. Zeng Z, Gan LY, Bin Yang H, et al. Orbital coupling of hetero-diatomic nickel-iron site for bifunctional electrocatalysis of CO2 reduction and oxygen evolution. Nat Commun 2021;12:4088.
118. Yang HB, Hung S, Liu S, et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat Energy 2018;3:140-7.
119. Zhu C, Fu S, Song J, et al. Self-assembled Fe-N-doped carbon nanotube aerogels with single-atom catalyst feature as high-efficiency oxygen reduction electrocatalysts. Small 2017;13:1603407.
120. Chen Y, Li Z, Zhu Y, et al. Atomic Fe dispersed on N-doped carbon hollow nanospheres for high-efficiency electrocatalytic oxygen reduction. Adv Mater 2019;31:e1806312.
121. Zhang X, Zhang S, Yang Y, et al. A general method for transition metal single atoms anchored on honeycomb-like nitrogen-doped carbon nanosheets. Adv Mater 2020;32:e1906905.
122. Li X, Bi W, Chen M, et al. Exclusive Ni-N4 sites realize near-unity CO selectivity for electrochemical CO2 reduction. J Am Chem Soc 2017;139:14889-92.
123. Lu Z, Wang B, Hu Y, et al. An isolated zinc-cobalt atomic pair for highly active and durable oxygen reduction. Angew Chem Int Ed 2019;58:2622-6.
124. Chen W, Pei J, He CT, et al. Rational design of single molybdenum atoms anchored on N-doped carbon for effective hydrogen evolution reaction. Angew Chem Int Ed 2017;56:16086-90.
125. Zhu Y, Cao T, Cao C, et al. One-pot pyrolysis to N-doped graphene with high-density Pt single atomic sites as heterogeneous catalyst for alkene hydrosilylation. ACS Catal 2018;8:10004-11.
126. Liu J, Jiao M, Mei B, et al. Carbon-supported divacancy-anchored platinum single-atom electrocatalysts with superhigh Pt utilization for the oxygen reduction reaction. Angew Chem Int Ed 2019;58:1163-7.
127. Kiciński W, Dyjak S. Transition metal impurities in carbon-based materials: Pitfalls, artifacts and deleterious effects. Carbon 2020;168:748-845.
128. Tang C, Chen L, Li H, et al. Tailoring acidic oxygen reduction selectivity on single-atom catalysts via modification of first and second coordination spheres. J Am Chem Soc 2021;143:7819-27.
129. Yang H, Shang L, Zhang Q, et al. A universal ligand mediated method for large scale synthesis of transition metal single atom catalysts. Nat Commun 2019;10:4585.
130. Xie X, Liu J, Li T, Song Y, Wang F. Post-formation copper-nitrogen species on carbon black: their chemical structures and active sites for oxygen reduction reaction. Chem Eur J 2018;24:9968-75.
131. Wang Y, Shi R, Shang L, et al. High-efficiency oxygen reduction to hydrogen peroxide catalyzed by nickel single-atom catalysts with tetradentate N2O2 coordination in a three-phase flow cell. Angew Chem Int Ed 2020;59:13057-62.
132. Gao X, Jang J, Nagase S. Hydrazine and thermal reduction of graphene oxide: reaction mechanisms, product structures, and reaction design. J Phys Chem C 2010;114:832-42.
133. Gao C, Chen S, Wang Y, et al. Heterogeneous single-atom catalyst for visible-light-driven high-turnover CO2 reduction: the role of electron transfer. Adv Mater 2018;30:e1704624.
134. Liang S, Zhu C, Zhang N, et al. A novel single-atom electrocatalyst Ti1/rGO for efficient cathodic reduction in hybrid photovoltaics. Adv Mater 2020;32:e2000478.
135. Fei H, Dong J, Wan C, et al. Microwave-assisted rapid synthesis of graphene-supported single atomic metals. Adv Mater 2018;30:e1802146.
136. Wan G, Yang C, Zhao W, et al. Anion-regulated selective generation of cobalt sites in carbon: toward superior bifunctional electrocatalysis. Adv Mater 2017;29:1703436.
137. Guo L, Hwang S, Li B, et al. Promoting atomically dispersed mnn4 sites via sulfur doping for oxygen reduction: unveiling intrinsic activity and degradation in fuel cells. ACS Nano 2021;15:6886-99.
138. Sun W, Du L, Tan Q, et al. Engineering of nitrogen coordinated single cobalt atom moieties for oxygen electroreduction. ACS Appl Mater Interfaces 2019;11:41258-66.
139. Qu Y, Li Z, Chen W, et al. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms. Nat Catal 2018;1:781-6.
140. Qu Y, Chen B, Li Z, et al. Thermal emitting strategy to synthesize atomically dispersed Pt metal sites from bulk Pt metal. J Am Chem Soc 2019;141:4505-9.
141. Jiao L, Li J, Richard LL, et al. Chemical vapour deposition of Fe-N-C oxygen reduction catalysts with full utilization of dense Fe-N4 sites. Nat Mater 2021;20:1385-91.
142. Wu M, Wang K, Yi M, Tong Y, Wang Y, Song S. A facile activation strategy for an MOF-derived metal-free oxygen reduction reaction catalyst: direct access to optimized pore structure and nitrogen species. ACS Catal 2017;7:6082-8.
143. Zhang C, Sha J, Fei H, et al. Single-atomic ruthenium catalytic sites on nitrogen-doped graphene for oxygen reduction reaction in acidic medium. ACS Nano 2017;11:6930-41.
144. Bai L, Duan Z, Wen X, Si R, Guan J. Atomically dispersed manganese-based catalysts for efficient catalysis of oxygen reduction reaction. Appl Catal B Environ 2019;257:117930.
145. Fei H, Dong J, Arellano-Jiménez MJ, et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat Commun 2015;6:8668.
146. Zhang C, Yang S, Wu J, et al. Electrochemical CO2 reduction with atomic iron-dispersed on nitrogen-doped graphene. Adv Energy Mater 2018;8:1703487.
147. Han G, Zheng Y, Zhang X, et al. High loading single-atom Cu dispersed on graphene for efficient oxygen reduction reaction. Nano Energy 2019;66:104088.
148. Li Y, Wang S, Wang XS, et al. Facile top-down strategy for direct metal atomization and coordination achieving a high turnover number in CO2 photoreduction. J Am Chem Soc 2020;142:19259-67.
149. Banhart F. Interactions between metals and carbon nanotubes: at the interface between old and new materials. Nanoscale 2009;1:201-13.
150. Banhart F, Charlier J, Ajayan PM. Dynamic behavior of nickel atoms in graphitic networks. Phys Rev Lett 2000;84:686-9.
151. Liu J, Cao C, Liu X, et al. Direct observation of metal oxide nanoparticles being transformed into metal single atoms with oxygen-coordinated structure and high-loadings. Angew Chem Int Ed 2021;60:15248-53.
152. Wei S, Li A, Liu JC, et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat Nanotechnol 2018;13:856-61.
153. Yang J, Qiu Z, Zhao C, et al. In situ thermal atomization to convert supported nickel nanoparticles into surface-bound nickel single-atom catalysts. Angew Chem Int Ed 2018;57:14095-100.
154. Qiu HJ, Ito Y, Cong W, et al. Nanoporous graphene with single-atom nickel dopants: an efficient and stable catalyst for electrochemical hydrogen production. Angew Chem Int Ed 2015;54:14031-5.
155. Li J, Jiao L, Wegener E, et al. Evolution pathway from iron compounds to Fe1(II)-N4 sites through gas-phase iron during pyrolysis. J Am Chem Soc 2020;142:1417-23.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Han G, Zhang W, Li L, Du J, Yan Y, Geng L, Tong Y, Du C. Progress in the high-temperature synthesis of atomically dispersed metal on carbon and understanding of their formation mechanism. Energy Mater 2023;3:300013. http://dx.doi.org/10.20517/energymater.2022.77
AMA Style
Han G, Zhang W, Li L, Du J, Yan Y, Geng L, Tong Y, Du C. Progress in the high-temperature synthesis of atomically dispersed metal on carbon and understanding of their formation mechanism. Energy Materials. 2023; 3(2): 300013. http://dx.doi.org/10.20517/energymater.2022.77
Chicago/Turabian Style
Han, Guokang, Wei Zhang, Lingfeng Li, Jiannan Du, Yuqi Yan, Lin Geng, Yujin Tong, Chunyu Du. 2023. "Progress in the high-temperature synthesis of atomically dispersed metal on carbon and understanding of their formation mechanism" Energy Materials. 3, no.2: 300013. http://dx.doi.org/10.20517/energymater.2022.77
ACS Style
Han, G.; Zhang W.; Li L.; Du J.; Yan Y.; Geng L.; Tong Y.; Du C. Progress in the high-temperature synthesis of atomically dispersed metal on carbon and understanding of their formation mechanism. Energy Mater. 2023, 3, 300013. http://dx.doi.org/10.20517/energymater.2022.77
About This Article
Copyright
Data & Comments
Data

 Cite This Article 28 clicks
Cite This Article 28 clicks


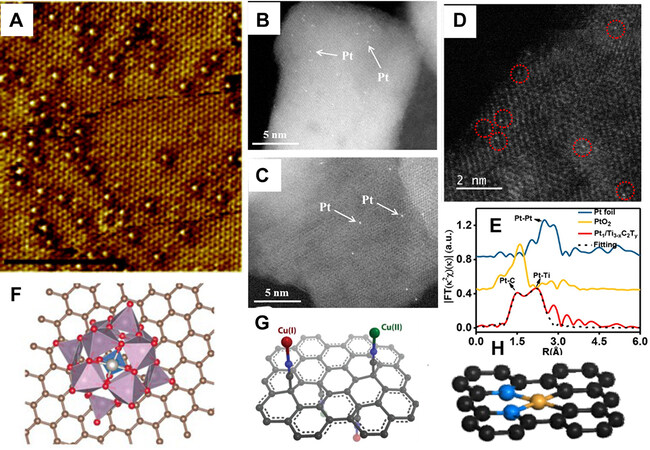
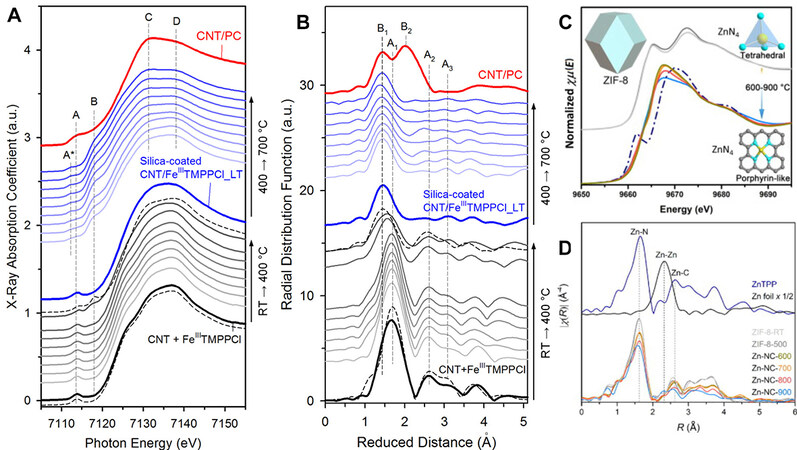
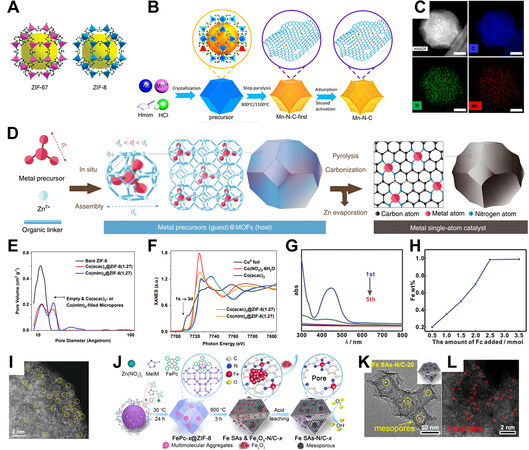
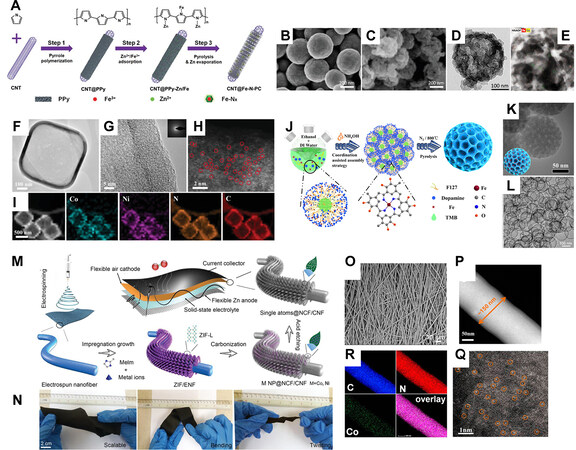
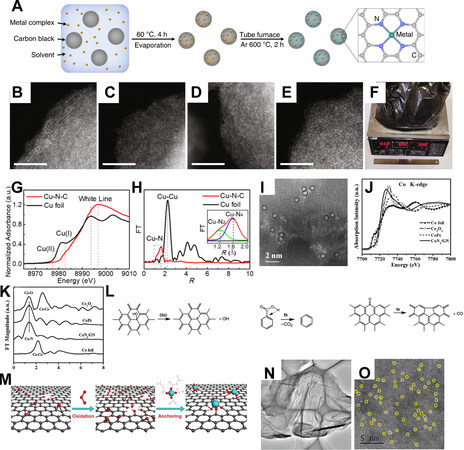
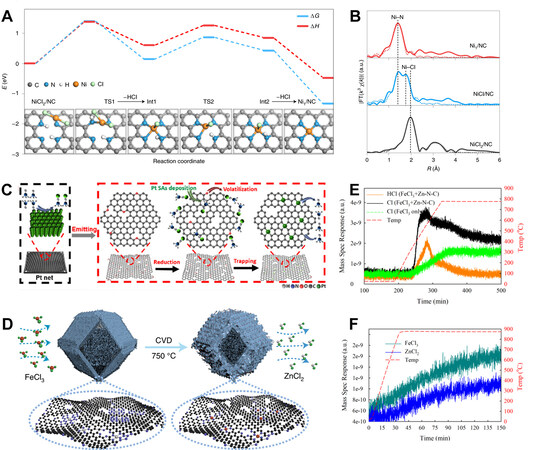
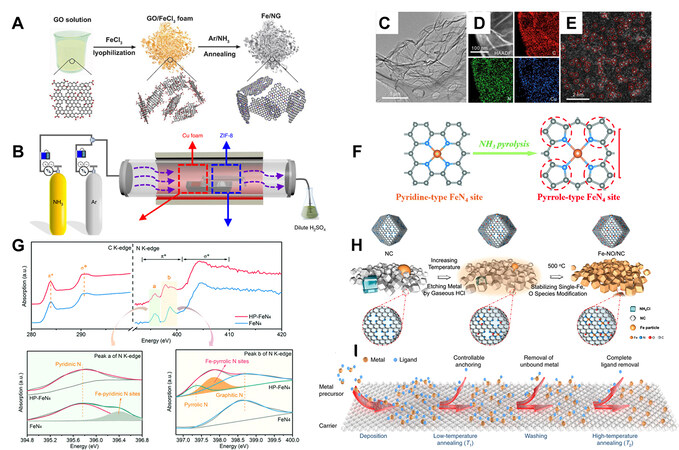
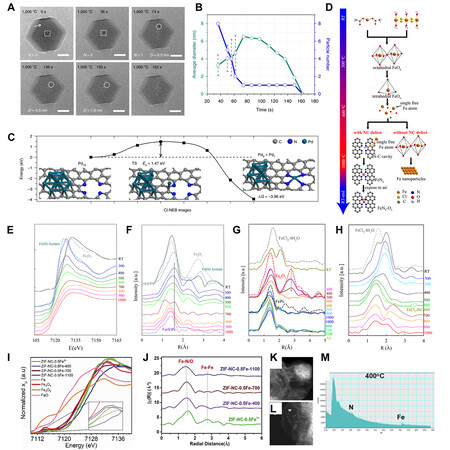










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.