Revealing energy storage mechanism of CsPbBr3 perovskite for ultra-stable symmetric supercapacitors
Abstract
Inorganic metal halide perovskites such as CsPbX3 (X = I, Br) have been intensively studied in optoelectrical applications such as solar cells and light-emitting diodes due to better thermal and structural stability compared to the organic-inorganic hybrid perovskite counterparts. Limited studies have shown that inorganic perovskites could potentially be promising electrode materials in energy storage devices like supercapacitors. Nevertheless, there is some controversy regarding their electrochemical properties and energy storage mechanism. Furthermore, the stability of the inorganic perovskites in electrochemical energy storage systems is a big concern. In this work, we studied the electrochemical properties of CsPbBr3 electrodes composed of pure CsPbBr3 nanocrystals without any additives to reveal their intrinsic electrochemical characteristics. We carefully selected the electrolyte solution composed of tetrabutylammonium hexafluorophosphate in dichloromethane and the electrode substrate based on FTO glass to ensure they do not cause damage to the perovskite material or introduce side reactions during the charge-discharge process. The results showed that the CsPbBr3 perovskite demonstrates electrical double-layer capacitive behaviour, and the specific capacitance of the electrode can reach 528 mF g-1. A symmetrical supercapacitor based on this perovskite demonstrated exceptional cycling stability with a capacitance retention of 90% after 10,000 charge and discharge cycles at a discharge current density of 100 mA g-1. The device also exhibited a constant power density of 25.0 mW kg-1 with increasing energy density up to 33.3 mWh kg-1. Further characterizations have revealed the important role of the large cations and anions of tetrabutylammonium hexafluorophosphate in the electrolyte in stabilizing the perovskite electrode material.
Keywords
INTRODUCTION
Metal halide perovskites that adopt the chemical formula of ABX3 (X = I, Br, Cl) where A is a monovalent cation such as MA+, FA+ or Cs+ (MA: methyl ammonium, FA: formamidinium), and B is a divalent cation such as Pb2+, Sn2+[1] have demonstrated exceptional performance in optoelectronic devices like solar cells, light emitting diodes etc.[2] owing to their strong light absorption, tuneable bandgap, unexceptional tolerance to defects and facile film processibility by cost-effective solution processing[3,4]. Exploration of metal halide perovskite materials in energy storage devices was first reported by Xia et al., who demonstrated that MAPbI3 and MAPbBr3 could be used as anode materials in lithium-ion batteries[5]. Kostopoulou et al. demonstrated that CsPbBr3 microcubes exhibit good electrochemical performance in a Li-ion battery, with a specific capacity of 549 mAh g-1[6]. Paul et al. also reported using CsPbBr3 as an active anode material in half- and full-lithium-ion batteries. The fabricated half-cell exhibited a discharge specific capacity of 376 mAh g-1 at a current density of 30 mA g-1[7]. Liu et al. used carbon nanotubes wrapped around CsPbBr3 as the anode of a Li-ion battery, which exhibited a reversible capacity of 644.6 mAh g-1 at a current density of 100 mA g-1 due to the embedded pseudocapacitive behaviour[8]. Further studies have shown that the reduction of Pb2+, generating different forms of LiPb alloys, may be responsible for the observed energy storage behaviour, although there are still many open questions regarding the operational mechanism of perovskite electrodes[8-10].
Supercapacitors, also called electrochemical capacitors, are a type of energy storage device that is complementary to batteries with distinctive advantages such as much faster charge and discharge rates (higher power density)[11], outstanding cycling capability and the ability to operate over a broader temperature range[12-14], However, compared to battery research, the study of perovskite in supercapacitors is very rare. This could be related to the notoriously poor stability of perovskites in aqueous electrolyte solutions that are used in most supercapacitors. Only a few reports on perovskite supercapacitors have been reported so far. For example, Zhou et al. in situ grown MAPbI3 (methylammonium lead iodide) on a FTO (fluorine-doped tin oxide) glass substrate by a solution method and demonstrated the potential of MAPbI3 as an electrode material and solid electrolyte[10]. Popoola et al. fabricated a symmetric MAPbI3 supercapacitor with an areal capacitance of 21.50 μF/cm2[15]. Pious et al. used (CH3NH3)3Bi2I9, a lead-free hybrid perovskite, as the electrode material of their supercapacitor[16]. Li et al. reported a new hybrid bismuth halide perovskite electrode (CN2SH5)3BiI6 for supercapacitor and got a high electrode areal capacitance of 3.32 F/cm2[17]. Besides, there are only two reports on supercapacitors using lead halide perovskites nanocrystal such as CsPbI3 microwires[18] or CsPbBr3 microrods[19]. In both cases, the electrode was composed of a perovskite and other additives including conductive carbon black and polyvinylidene fluoride (PVDF) based binder. The symmetric supercapacitor using cesium lead iodide (CsPbI3) microwires as electrodes in an electrolyte of CsI in a butanol solution showed a very low specific capacitance of only 7.23 mF cm-2 at a scan rate of 2 mV s-1[18]. The other report demonstrated a very high specific capacitance of 121 F g-1 for CsPbBr3 microrods on a nickel foam substrate in aqueous KOH electrolytes[19]. However, Ni metal, which is oxidised to NiOOH, is known to have low potential (0.4 V vs. saturated calomel reference electrode) for water splitting in the potential range where the supercapacitor operates[20,21]. It is also known metal halide perovskites are extremely sensitive to their chemical environment, including the polarity of the solvent and the supporting ions in electrolyte[9]. A polar solvent can decompose the perovskite compound, while small cations such as K+ can enter the crystal structure of the perovskite[22]. Therefore, to obtain meaningful and accurate results, it is critical to select an electrolyte that does not cause decomposition of the perovskite as well as a stable substrate that does not exhibit electrochemical reactions within the operational voltage window of the supercapacitors. The limited studies mentioned above all have certain drawbacks, such as low specific capacitance, poor cycle stability, and absence of mechanism explanation. Our material shows an outstanding performance among the other reported perovskite electrode materials
In this work, we investigated the intrinsic electrochemical properties of CsPbBr3 perovskite by using an electrode of pure CsPbBr3 nanocrystals coated on a FTO glass substrate without any additives in an organic electrolyte solution composed of tetrabutylammonium hexafluorophosphate (TBAPF6) in dichloromethane (DCM). We found that this perovskite material showed outstanding stability in this electrolyte and demonstrated the characteristic behaviour of an electrical double-layer capacitor with a nearly ideal rectangular shape in the cyclic voltammetry (CV) plots and a triangle shape in galvanic charge-discharge plots (GCD). Using optimal concentration of TBAPF6 in the electrolyte, the potential window increased from 0.8 V to 1 V, and the specific capacitance of the CsPbBr3 electrode reached 528 mF g-1 (264 mF cm-2 based on the area of active material) at a current density of 100 mA g-1. A symmetrical supercapacitor using the CsPbBr3 perovskite exhibited remarkable capacitance retention of 90% after 10,000 charge and discharge cycles. An energy density and power density of 33.3 mWh kg-1 and 25.0 mW kg-1, respectively, were obtained at a current density of 100 mA g-1. The device is more suitable for electrical components that need small current (µA or mA) and long cycle life, such as signal lights, sensors, watches, signal receivers, controllers, and current buffers[23-25].
EXPERIMENTAL
Synthesis of the CsPbBr3 perovskite
CsPbBr3 nanocrystals were synthesized by a hot injection method, as reported by us previously[26-28]. Briefly, a precursor solution composed of 203 mg caesium carbonate (Cs2CO3), 10 mL 1-octadecene (ODE) (95%, Sigma-Aldrich), and 1.5 mL oleic acid (OA) (90%, Sigma-Aldrich) was heated in a round bottom flask under vacuum at 120 °C for 1 h. The temperature was then increased to 160 °C and the solution was heated for 20 min in a nitrogen atmosphere until the solid was completely dissolved to form a Cs-oleate solution that is kept at 120 °C for further use. 207 mg lead bromide (PbBr2), 15 mL ODE, 1.5 mL oleic acid, and
Characterization of CsPbBr3
The morphology and structure of the as-synthesized CsPbBr3 nanocrystal powder was measured with scanning electron microscopy (SEM, JEOL 7001F) and transmission electron microscopy (TEM, JEOL 2100). The composition of the perovskite was measured by energy dispersive X-Ray spectroscopy (EDS). The voltage used in the SEM and EDS measurements were 5 kV and 15 kV, respectively, to avoid damaging the perovskite material. The optical properties of the CsPbBr3 nanocrystals were measured by a UV-VIS spectrophotometer (CARY 60 Romulus) and photoluminescence (PL) spectrophotometer (Cary Eclipse Argus) by dispersion of the as-synthesized material in hexane. X-ray diffraction (XRD, CuKα) patterns and X-ray photoelectron spectroscopy (XPS) measurements of thin films of CsPbBr3 nanocrystals. The films were made by spin coating a solution containing 0.58 mg of the as-synthesised CsPbBr3 in 10 mL hexane on the surface of a FTO substrate. Inductively coupled plasma mass spectrometry (ICP-MS) was used to quantitatively analyze the composition of the electrolyte solution after the electrochemical cycling test, and the standard deviation and residual standard deviation of the results are shown in Supplementary Table 2.
Preparation of a CsPbBr3 electrode and a symmetric supercapacitor
A CsPbBr3 electrode was made by spin coating the dispersion of CsPbBr3 nanocrystals in hexane (5.8 mg CsPbBr3 nanocrystals in 10 mL hexane) on a cleaned FTO substrate. The FTO glass substrate was washed with Decon 90 solvent under sonication for 20 min, followed by a mixed solvent of acetone, ethanol, and isopropanol at a ratio of 1:1:1 under sonication for another 20 min. After this, the FTO substrate was treated under UV light in an ozone atmosphere for 30 min to remove organic residuals and increase the surface wettability. The process for spin coating was performed at 1,000 rpm s-1 for 10 s with an acceleration rate of 100 rpm s-1 with a spin coater (CY-SPC8-HAR, Zhengzhou CY Scientific instrument Co., Ltd). After this, the spinning speed was reduced at a rate of 100 rpm s-1 for 10 s. The substrate with the sample was dried in a vacuum oven for 12 h. This process was repeated several times until a uniform film with a thickness of around 700 nm was obtained. The excess CsPbBr3 was wiped off with a cotton swab moistened with DMF, leaving the film with a size of 1.0 × 2.0 cm2 [Supplementary Figure 1]. The mass loading (around 1 mg) of the activated material was determined by weighing the difference between the unmodified FTO and the FTO with the CsPbBr3 film. A symmetric CsPbBr3 supercapacitor was assembled by immersing the two CsPbBr3 electrodes with similar mass loading of activated materials in an electrolyte solution composed of TBAPF6 at different concentrations in DCM. In comparison, we also tested the stability of the perovskite electrode in a 1 M KOH aqueous solution.
Electrochemical characterization
The electrochemical properties of the CsPbBr3 perovskite material were measured using a three-electrode configuration composed of a CsPbBr3-based working electrode, a saturated calomel reference electrode, and a platinum plate counter electrode. The electrolyte solution was composed of TBAPF6 in DCM solvent with different concentrations. The Cyclic voltammetry (CV) at different scan rates (2 mV s-1, 5 mV s-1, 10 mV s-1, 20 mV s-1, and 50 mV s-1) in a potential window of 0.0 to 1.0 V, galvanostatic charge-discharge (GCD) at different current densities (40-100 mA g-1) and impedance spectrum of the perovskite electrode were recorded with an electrochemical workstation (BioLogic) in air. The electrochemical impedance spectroscopy (EIS) measurements were performed over a frequency range from 0.1 Hz to 10 kHz. The EIS data were fitted by an equivalent circuit via Zview software.
The specific mass capacitance of the electrode was calculated according to the GCD plots specifically by the equation of
RESULTS AND DISCUSSION
Effect of electrolyte solution on the stability of CsPbBr3 perovskite
KOH aqueous solution is one of the most used electrolytes in supercapacitors. However, an aqueous electrolyte is not suitable for metal halide perovskite electrode materials because exposure to water can cause material degradation and ultimately destroy the CsPbBr3 structure[29]. To confirm this, we investigated the stability of the CsPbBr3 electrode in a 1 M KOH aqueous solution. We found that the yellow color of the perovskite film quickly disappears after contact with the 1 M KOH electrolyte within 3 s
In order to address the moisture instability of CsPbBr3, we used a non-polar organic solution composed of TBAPF6 in low polarity DCM for the electrochemical characterization of CsPbBr3[9]. The large size of both the cation and anion in TBAPF6 benefits the structural stability of CsPbBr3 in solution as they are too bulky to intercalate into the CsPbBr3 crystal structure[9]. In addition, the much lower polarity of DCM (polarity index = 3.1) compared to water (polarity index = 10.2) is expected to hinder the decomposition of the perovskite material. Indeed, we found that the CsPbBr3 film was almost completely intact after 2,000 cycles of electrochemical charge/discharge based on the fluorescence image of the film under a UV light exposure [Supplementary Figure 2D]. These results gave us confidence that TBAPF6 in DCM is a compatible electrolyte for studying the electrochemical properties of CsPbBr3. This is the first time the electrolyte was used in a supercapacitor rather than in other applications.
Electrochemical performance characterization
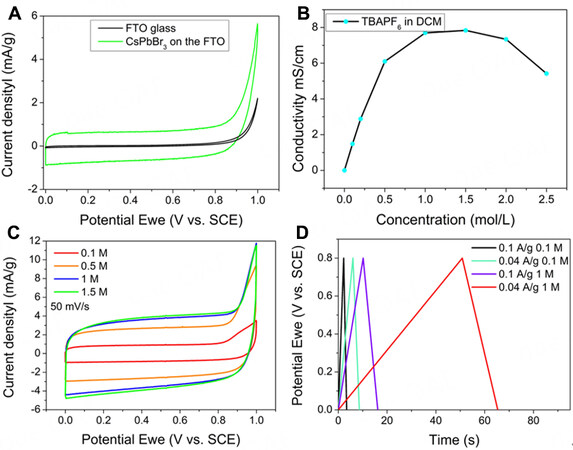
The CV behaviour of a CsPbBr3 electrode in a three-electrode configuration in an electrolyte with different concentrations of TBAPF6 in DCM is shown in Figure 1. Figure 1A compares the CV curves of a bare FTO electrode and CsPbBr3 coated FTO. The bare FTO electrode showed negligible capacitance and can be regarded as only a current collector. In contrast, a nearly rectangular-shaped CV curve was obtained with the CsPbBr3 electrode over a wide potential window of 0-0.8 V [vs. standard calomel electrode (SCE)] [Figure 1A]. The complete absence of any redox peaks from a faradaic process indicates that the perovskite material operates through an electric double-layer energy storage mechanism that relies on electrolyte ion adsorption/desorption on the electrode surface [Supplementary Figure 3][19,31]. We further investigated the ionic conductivity of the electrolyte as a function of concentration of TBAPF6 in DCM. The results show that the highest conductivity was obtained with 1.5 M TBAPF6 [Figure 1B]. However, such a high concentration of TBAPF6 can result in precipitation of TBAPF6 on the surface of the electrode due to oversaturation of the salt in DCM. The CV curves of the CsPbBr3 electrode in a three-electrode system with different concentrations of TBAPF6 are shown in Figure 1C. The capacitance of the CsPbBr3 electrode increased significantly when the TBAPF6 concentration increased from 0.1 M to 1 M. Beyond this, the capacitance of the CsPbBr3 electrode does not change. The GCD curves [Figure 1D] also confirm the superior properties of the 1 M electrolyte compared to 0.1 M TBAPF6. The 1 M electrolyte exhibits a much longer discharge time at both high (100 mA g-1) and low (40 mA g-1) current densities, which implies a higher specific capacitance. Therefore, 1 M TBAPF6 electrolyte was used in the following experiments because of its high conductivity and lack of precipitation from solution.
Figure 1. (A) Comparison of CV curves of CsPbBr3/FTO electrode and bare FTO in 0.1 M TBAPF6 at a scan rate of 50 mV s-1.
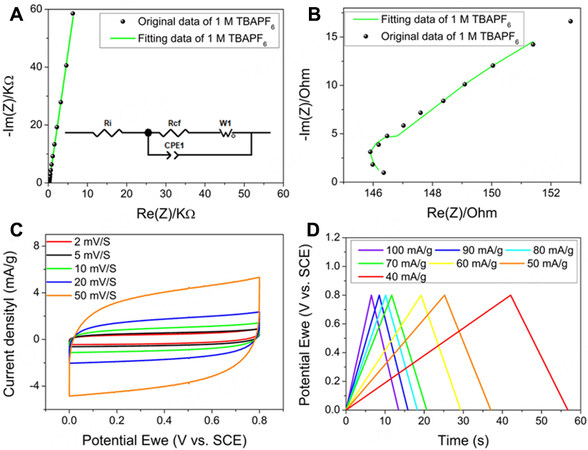
Nyquist plots of the CsPbBr3 electrode in a three-electrode setup employing 1 M TBAPF6 electrolyte over a frequency range from 0.1 Hz to 100 KHz are shown in Figure 2A and B. A nearly vertical line with a slope of 84° indicates that the CsPbBr3 nanocrystals possess an electric double-layer capacitor (EDLC) capacitive behaviour, which is determined by the diffusion of electrolyte ions between the electrode surface and the electrolyte [Figure 2A][32,33]. Fitting the high-frequency region of the Nyquist plot by using the equivalent circuit shown in the inset of Figure 2A clearly shows the resistance from each part of the system [Figure 2B]. The 1 M TBAPF6 electrolyte had a charge transfer resistance (Rct) of 0.5 Ω and an internal resistance (Ri) of 146 Ω. The Warburg impendence is 165 Ω and the CPE (constant phase element) is
Figure 2. Nyquist plot of the CsPbBr3 electrode in 1 M TBAPF6 electrolyte in (A) Low and (B) high-frequency region. The insert is the enlarged curve at the high-frequency region and the equivalent electric circuit. (C) the CV curves of CsPbBr3 electrode in 1 M TBAPF6 at different scan rates. (D) the charge and discharge curves of CsPbBr3 electrode in 1 M TBAPF6 at different current densities. All the tests were performed in a three-electrode system in air.
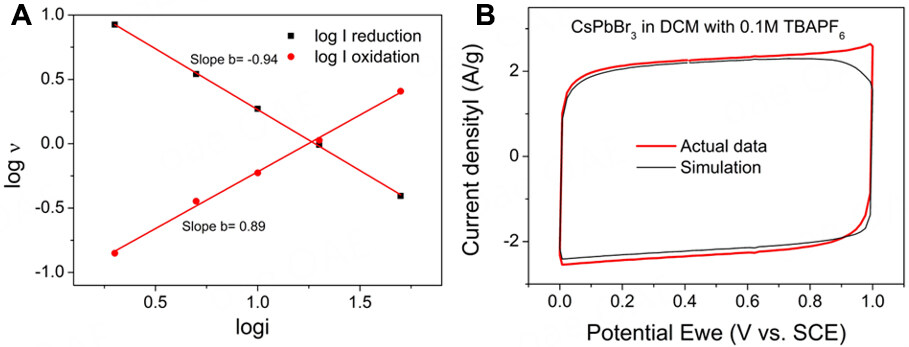
To further confirm the energy storage mechanism of the perovskite electrode, a relationship based on a power law between the current (i) and the scan rate (ν) was investigated according to
Figure 3. (A) Demonstration of the Trasatti method. (B) the simulation calculation of CV curve CsPbBr3 electrode in three-electrode system in 0.1 M TBAPF6 at a scan rate of 50 mV s-1 (Dunn method).
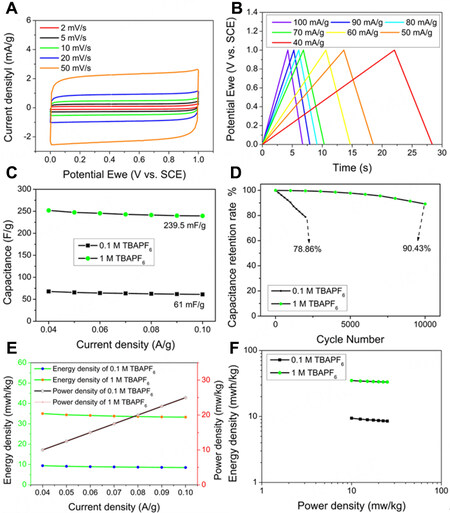
Symmetrical supercapacitors (SCs) composed of two identical CsPbBr3 electrodes with similar active material loading were fabricated to assess the energy and power density of the device as well as cycling stability. To demonstrate the importance of the TBAPF6 concentration on the device performance, we compared supercapacitors using 0.1 M and 1 M TBAPF6 in DCM electrolytes. The SC using 0.1 M TBAPF6 is named SS-0.1, while the one using 1 M TBAPF6 in DCM is named SS-1, respectively. The CV curves of SS-1 [Figure 4A] at different scan rates over a potential window of 0-1.0 V are even closer to a rectangle than the CV curves in a three-electrode system, further confirming the EDLC behaviour of the CsPbBr3 nanocrystals[10,13]. The shape of the CV is well maintained at a high scan rate of 50 mV s-1, indicating good rate performance. Figure 4B shows the charge and discharge curves of the SS-1 at different current densities over a current density range from 40 to 100 mA g-1. It was found that the shape of the curves remained triangular with no noticeable voltage drop upon an increase in the current density. The calculated specific capacitances of SS-1 and SS-0.1 are 119.8 mF g-1 (60.88 mF cm-2) and 30.5 mF g-1 (17.75 mF cm-2), respectively, at a current density of 100 mA g-1. Figure 4C compares the specific capacitances of SS-0.1 and SS-1 at different current densities, where it is worth noting that the specific capacitance of SS-1 is nearly
Figure 4. (A) The CV curves of SS-1 under different scan rates. (B) the charge and discharge curves SS-1 at different current densities. (C) the specific capacitance of SS-0.1 and SS-1 at different current densities. (D) cycling stability of SS-0.1 and SS-1 after 2,000 and 10,000 cycles at a current density of 100 mA g-1. (E) energy density and power density of SS-0.1 and SS-1 at different current densities. (F) relationship between energy density and power density of SS-0.1 and SS-1 (Ragone plot).
Figure 4D shows the cycle stability of SS-0.1 and SS-1. It was found that the specific capacitance of SS-0.1 dropped to 78.86% of its initial value after 2,000 cycles at a current density of 100 mA g-1 [Figure 4D]. In contrast, SS-1 showed superior cycle stability with negligible change in the specific capacitance in the first 2,000 cycles. Even after 10,000 cycles, a retention rate of 90.43% was obtained. The energy density and power density of SS-0.1 and SS-1 are shown in Figure 4E, where SS-0.1 and SS-1 show the same power density which increases from 10 mW kg-1 to 25 mW kg-1 upon an increase in the current density. On the other hand, the energy density of SS-1 is four times higher than that of SS-0.1 at the same applied current density. The small decreases in energy density from 9.4 mWh kg-1 to 8.5 mWh kg-1 for SS-0.1 and from
Understanding the cycle stability of the CsPbBr3 electrode
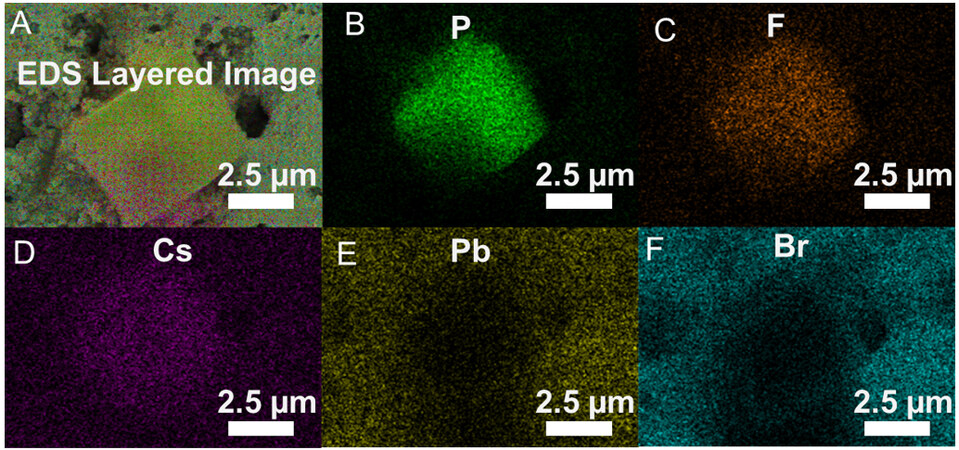
It is clear that the performance of SS-1 is significantly better than SS-0.1, in particular, the cycle stability. Therefore, further research was undertaken to investigate the underlying reasons. After the electrochemical cycling test, characterization of the electrode material in SS-0.1 by EDS shows the formation of larger crystals that are different from the pristine CsPbBr3 crystals on the surface of the CsPbBr3 electrode [Figure 5]. EDS mapping [Figure 5A-F] shows that F and P elements are concentrated on the large crystals while Br and Pb elements are distributed around the large crystals while Cs exists across the whole area. Therefore, this infers that the large crystals are mainly composed of three elements, Cs, P, and F. The EDS line scan also confirms these findings [Supplementary Figure 6A and B]. Supplementary Figure 6B shows the position of the EDS line scan on the large crystal. The concentration of F and P is higher in the region of the large crystals than in their surroundings, while the opposite trend is found for Br and Pb elements. The distribution of Cs is largely uniform. The atomic ratio of P and F is very close to 1:6, proving the existence of the PF6- functional group [Supplementary Figure 6C]. Therefore, we suspect that the large crystal is probably a secondary phase, such as CsPF6 formed on the electrode during the electrochemical charge-discharge process. The XRD pattern in Figure 6A confirms that, indeed, besides CsPbBr3, CsPF6 also exists in the electrode. This is consistent with previous reports where CsPF6 was formed in CsPbBr3 in the presence of a TBAPF6 electrolyte[9]. On the other hand, Samu et al. postulated that the phase transformation of CsPbBr3 is triggered by the reduction of Pb2+ at -1.4 V vs. Ag/AgCl reference electrode[9]. However, the reduction potential of Pb2+ is -0.13 V vs. standard hydrogen electrode, and -0.37 V vs. saturated calomel electrode)[39-41], respectively. In addition, our results show that the transformation from CsPbBr3 to CsPF6 can occur even at a low voltage of 0.4 V rather than -1.4 V[6]. Supplementary Figure 7A and B show the SEM of the CsPbBr3 electrode in SS-0.1 after 40 CV cycles at a scan rate of 5 mV s-1 over a potential window of
Figure 5. EDS mapping of CsPbBr3 electrodes in SS-0.1 after the electrochemical test. (A) the layered image of mapping. Mapping result of different elements (B) F; (C) P; (D) Cs; (E) Br; (F) Pb.
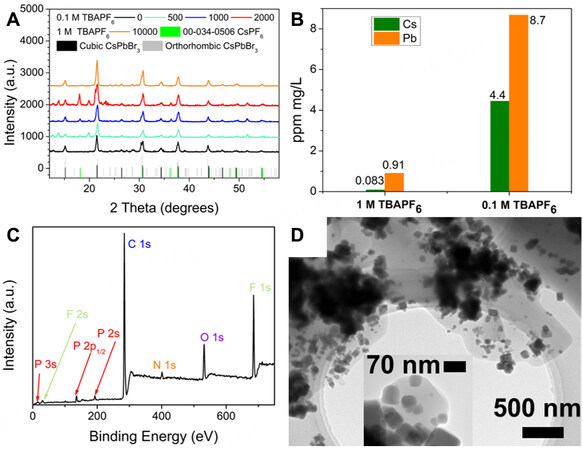
Figure 6. (A) The XRD scan of SS-0.1 and SS-1 after the cycles of charge/discharge. (B) the ICP-MS results of SS-0.1 and SS-1 after 2,000 cycles of charge/discharge. (C) XPS of CsPbBr3 electrode in SS-1 after 2,000 cycles of charge and discharge without etching process. (D) TEM of TBAPF6 covered CsPbBr3 crystal obtained from CsPbBr3 electrode after 2,000 cycles of charge and discharge.
The content of each crystal phase in the electrode at different cycling stages was then analyzed quantitatively based on XRD measurements [Figure 6A] using TOPAS software, as shown in Supplementary Table 3. Before the test, CsPbBr3 electrodes contain 89% cubic CsPbBr3 and 11% orthorhombic CsPbBr3. Upon electrochemical cycling, the amount of CsPF6 in the perovskite electrode of SS-0.1 increased with the increase of cycle numbers and reached 24.48% after 2,000 cycles of charge/discharge. In contrast, the contents of CsPF6 in the CsPbBr3 electrode of SS-1 is only 4.01% even after 10,000 cycles, which is much lower than the amount of CsPF6 in SS-0.1. Therefore, a high concentration of TBAPF6 can effectively hinder the dissolution of the perovskite in the electrolyte, preventing the formation of CsPF6. To confirm this hypothesis, we measured the content of Cs and Pb in the electrolyte of the SS-0.1 and SS-1 devices after 2,000 cycles by ICP-MS. The results [Figure 6B] show that the concentrations of Cs and Pb ions in the electrolyte of SS-0.1 are 4.4 ppm (mg L-1) and 8.7 ppm (mg L-1), respectively, while their concentrations in the electrolyte of SS-1 are significantly lower with values of 0.083 and 0.91 ppm (mg L-1), respectively. This confirms the importance of having a high concentration of TBAPF6 in the electrolyte to stabilize the perovskite electrode materials. This could be related to suppressed decomposition or reorganization of CsPbBr3 surrounded by a high concentration of large electrolyte ions, because of the formation of the nano-scaled film on the surface of the CsPbBr3 electrode, thereby increasing the overall cycling stability of the supercapacitor. The XPS wide scan without etching for the CsPbBr3 electrode in SS-1 after 2,000 cycles of charge and discharge in Figure 6C indicates that the elements present in the film on the CsPbBr3 electrode surface only include C, N, O, P, and F rather than Cs, Pb, and Br. For further investigation, the components on the electrode in SS-1 were redispersed in hexane for TEM measurements. TEM images in Figure 6D visually show that the CsPbBr3 nanocrystals still keep a cubic shape and were wrapped in an amorphous substance, which could be TBAPF6. Further investigations carried out by FTIR [Supplementary Figure 8] proved the existence of the PF6- functional group on the electrode. The absence of TBAPF6’s peaks in the XRD pattern [Figure 6A] also indicates that the TBAPF6 is amorphous, which is consistent with the TEM results. Therefore, the dramatically increased stability resulted from the coverage of amorphous TBAPF6 on the CsPbBr3 electrode, which only happens at high concentrations. Additionally, although the TBAPF6 layer (solid electrolyte interphase) covered on the surface of the electrode prevents the perovskite from decomposing, it also reduces the coulombic efficiency because it hinders the ion transfer from electrolyte to the surface of CsPbBr3 for adsorption. Moreover, side reactions such as the formation of CsPF6 could reduce the coulombic efficiency to some extent, especially at low current densities. Supplementary Figure 9 shows the cycle stability of SS-1 at a current density of 40 mA/g and Cs ions concentration in the electrolyte after 2,000 cycles. The retention rate of SS-1 at a current density of 40 mA/g after 2,000 cycles is 89.77%
Specifically, it is known that the chemical bonding strength between the Cs+cation in the perovskite crystal structure with the [PbBrx]- the framework is weak, and the energy barrier for migration of Cs+ in the perovskite crystal structure is 0.3-0.7 eV[42-44]. Experimental evidence has shown that Cs+ can drift away from the perovskite crystal structure under certain electrical fields which are high enough to overcome the energy barrier for Cs+ migration. Under low current density, it takes a longer time for the electrode to reach the maximum voltage compared to the situation under higher current density. Specifically, according to the GCD plots, it takes 45 s for the charging plot to reach the maximum at 40 mA/g, whereas the charging time is much shorter (55 s) under 100 mA/g. The much higher content of Cs+ ions in the electrolyte at 40 mA/g than that at 100 mA/g indicates that Cs+ ions have sufficient time to drift out of the perovskite crystal structure when the current density is low (40 mA/g), which eventually leads to the collapse of the perovskite structure because Cs+ plays a role in stabilizing the crystalline lattice in perovskite[45-47]. The high concentration of Cs+ will also improve the formation of CsPF6 (side reaction). In comparison, the faster charging/discharge at the larger current density means Cs+ ions might not have sufficient time to leave the perovskite.
CONCLUSIONS
In conclusion, we synthesized CsPbBr3 nanocrystals by a hot injection method and investigated the electrochemical properties of the materials in a three-electrode system and symmetrical supercapacitor. Our research has shown that 1 M TBAPF6 in DCM electrolyte is the most suitable electrolyte for ensuring the stability of CsPbBr3. CV, GCD, and XPS experiments demonstrated the electric double-layer energy storage mechanism of CsPbBr3. The specific capacitance of CsPbBr3 can reach as high as 528 mF g-1, while the specific capacitance of the symmetrical supercapacitor is 119.8 mF g-1 at a current density of 100 mA g-1 with a potential window of 0-1.0 V. A high retention rate of 90.43% was achieved after 10,000 cycles of charging and discharging at a current density of 100 mA g-1. The power density and energy density of the CsPbBr3 symmetric supercapacitor are 25 mWh kg-1 and 33.3 mW kg-1, respectively, at a current density of
DECLARATIONS
AcknowledgmentsWe thank Dr. Osama Yousef Ali Ghidan (Sam) for the ICPMS measurement.
Authors’ contributionsConception and the design of the study: Wang H, Pang L
Methodology, experiment, data acquisition, data analysis, data interpretation and writing: Pang L
Technical and material support: Hoang MT
Chemicals and instrument support: O’Mullane AP
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was supported by the Australian Research Council (ARC) Discovery Project (DP210102580, DP190102252), ARC Linkage Project (LP210100217) Strategic Research.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
1. Kumar R, Bag M. Hybrid halide perovskite-based electrochemical supercapacitors: recent progress and perspective. Energy Technol 2022;10:2100889.
2. Narayanan S, Parikh N, Tavakoli MM, et al. Metal halide perovskites for energy storage applications. Eur J Inorg Chem 2021;2021:1201-12.
3. Wang R, Huang T, Xue J, Tong J, Zhu K, Yang Y. Prospects for metal halide perovskite-based tandem solar cells. Nat Photon 2021;15:411-25.
4. Kostopoulou A, Kymakis E, Stratakis E. Perovskite nanostructures for photovoltaic and energy storage devices. J Mater Chem A 2018;6:9765-98.
5. Xia HR, Sun WT, Peng LM. Hydrothermal synthesis of organometal halide perovskites for Li-ion batteries. Chem Commun 2015;51:13787-90.
6. Kostopoulou A, Vernardou D, Makri D, Brintakis K, Savva K, Stratakis E. Highly stable metal halide perovskite microcube anodes for lithium-air batteries. J Power Sources 2020;3:100015.
7. Paul T, Maiti S, Chatterjee BK, et al. Electrochemical performance of 3D network CsPbBr3 perovskite anodes for Li-ion batteries: experimental venture with theoretical expedition. J Phys Chem C 2021;125:16892-902.
8. Liu S, Zhang K, Tan L, Qi S, Liu G, Chen J, et al. All-inorganic halide perovskite CsPbBr3@CNTs composite enabling superior lithium storage performance with pseudocapacitive contribution. Electrochim Acta 2021;367:137352.
9. Samu GF, Scheidt RA, Kamat PV, Janáky C. Electrochemistry and spectroelectrochemistry of lead halide perovskite films: materials science aspects and boundary conditions. Chem Mater 2018;30:561-9.
10. Zhou S, Li L, Yu H, Chen J, Wong C, Zhao N. Thin film electrochemical capacitors based on organolead triiodide perovskite. Adv Electron Mater 2016;2:1600114.
11. Wang T, Lei J, Wang Y, et al. Approaches to enhancing electrical conductivity of pristine metal-organic frameworks for supercapacitor applications. Small 2022;18:2203307.
12. Zhu Y, Murali S, Stoller MD, et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011;332:1537-41.
13. Wang T, Chen HC, Yu F, Zhao XS, Wang H. Boosting the cycling stability of transition metal compounds-based supercapacitors. Energy Stor Mater 2019;16:545-73.
14. Choudhary N, Li C, Moore J, et al. Supercapacitors: asymmetric supercapacitor electrodes and devices. Adv Mater 2017;29:1605336.
15. Popoola I, Gondal M, Oloore L, Popoola A, AlGhamdi J. Fabrication of organometallic halide perovskite electrochemical supercapacitors utilizing quasi-solid-state electrolytes for energy storage devices. Electrochim Acta 2020;332:135536.
16. Pious JK, Katre A, Muthu C, Chakraborty S, Krishna S, Vijayakumar C. Zero-dimensional lead-free hybrid perovskite-like material with a quantum-well structure. Chem Mater 2019;31:1941-5.
17. Li T, Mallows J, Adams K, Nichol GS, Thijssen JH, Robertson N. Thiourea bismuth iodide: crystal structure, characterization and high performance as an electrode material for supercapacitors. Batteries Supercaps 2019;2:568-75.
18. Maji P, Ray A, Sadhukhan P, Roy A, Das S. Fabrication of symmetric supercapacitor using cesium lead iodide (CsPbI3) microwire. Mater Lett 2018;227:268-71.
19. Thakur S, Paul T, Maiti S, Chattopadhyay KK. All-inorganic CsPbBr3 perovskite as potential electrode material for symmetric supercapacitor. Solid State Sci 2021;122:106769.
20. Chen L, Dong X, Wang Y, Xia Y. Separating hydrogen and oxygen evolution in alkaline water electrolysis using nickel hydroxide. Nat Commun 2016;7:11741.
21. Li J, Wang S, Chang J, Feng L. A review of Ni based powder catalyst for urea oxidation in assisting water splitting reaction. Adv Powder Technol 2022;1:100030.
22. Yao D, Zhang C, Pham ND, et al. Hindered formation of photoinactive δ-FAPbI3 phase and hysteresis-free mixed-cation planar heterojunction perovskite solar cells with enhanced efficiency via potassium incorporation. J Phys Chem Lett 2018;9:2113-20.
23. Wang Y, Zhou W, Kang Q, et al. Patterning islandlike MnO2 arrays by breath-figure templates for flexible transparent supercapacitors. ACS Appl Mater Interfaces 2018;10:27001-8.
24. Chu D, Li F, Song X, et al. A novel dual-tasking hollow cube NiFe2O4-NiCo-LDH@rGO hierarchical material for high preformance supercapacitor and glucose sensor. J Colloid Interface Sci 2020;568:130-8.
25. Zhang L, Hu X, Wang Z, Sun F, Dorrell DG. A review of supercapacitor modeling, estimation, and applications: a control/management perspective. Renew Sustain Energy Rev 2018;81:1868-78.
26. Hoang MT, Pannu AS, Tang C, et al. Potassium doping to enhance green photoemission of light-emitting diodes based on CsPbBr3 perovskite nanocrystals. Adv Opt Mater 2020;8:2000742.
27. Hoang MT, Pannu AS, Yang Y, et al. Surface treatment of inorganic CsPbI3 nanocrystals with guanidinium iodide for efficient perovskite light-emitting diodes with high brightness. Nano-Micro Lett 2022;14:69.
28. Kostopoulou A, Brintakis K, Nasikas NK, Stratakis E. Perovskite nanocrystals for energy conversion and storage. Nanophotonics 2019;8:1607-40.
29. Hsieh YT, Lin YF, Liu WR. Enhancing the water resistance and stability of CsPbBr3 perovskite quantum dots for light-emitting-diode applications through encapsulation in waterproof polymethylsilsesquioxane aerogels. ACS Appl Mater Interfaces 2020;12:58049-59.
30. Zhu Y, Cao C, Tao S, Chu W, Wu Z, Li Y. Ultrathin nickel hydroxide and oxide nanosheets: synthesis, characterizations and excellent supercapacitor performances. Sci Rep 2014;4:5787.
31. Barbieri O, Hahn M, Herzog A, Kötz R. Capacitance limits of high surface area activated carbons for double layer capacitors. Carbon 2005;43:1303-10.
32. Mohanadas D, Mohd Abdah MAA, Azman NHN, Ravoof TBSA, Sulaiman Y. Facile synthesis of PEDOT-rGO/HKUST-1 for high performance symmetrical supercapacitor device. Sci Rep 2021;11:11747.
33. Lin T, Chen IW, Liu F, et al. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015;350:1508-13.
34. Yen MC, Lee CJ, Liu KH, et al. All-inorganic perovskite quantum dot light-emitting memories. Nat Commun 2021;12:4460.
35. Gomez CM, Pan S, Braga HM, et al. Possible charge-transfer-induced conductivity enhancement in TiO2 microtubes decorated with perovskite CsPbBr3 nanocrystals. Langmuir 2020;36:5408-16.
36. Chen C, Fu Q, Guo P, et al. Ionic transport characteristics of large-size CsPbBr3 single crystals. Mater Res Express 2019;6:115808.
37. Pu X, Zhao D, Fu C, et al. Understanding and calibration of charge storage mechanism in cyclic voltammetry curves. Angew Chem Int Ed 2021;60:21310-8.
38. Jiang Q, Chen M, Li J, et al. Electrochemical doping of halide perovskites with ion intercalation. ACS Nano 2017;11:1073-9.
39. Hwang JH, Islam MA, Choi H, et al. Improving electrochemical Pb2+ detection using a vertically aligned 2D MoS2 nanofilm. Anal Chem 2019;91:11770-7.
40. Zhang X, Lin S, Chen Z, Megharaj M, Naidu R. Kaolinite-supported nanoscale zero-valent iron for removal of Pb2+ from aqueous solution: reactivity, characterization and mechanism. Water Res 2011;45:3481-8.
41. Pala IR, Brock SL. ZnS nanoparticle gels for remediation of Pb2+ and Hg2+ polluted water. ACS Appl Mater Interfaces 2012;4:2160-7.
42. Woo YW, Jung Y, Kim GY, Kim S, Walsh A. Factors influencing halide vacancy transport in perovskite solar cells. Discov Mater 2022;2:8.
43. Nur’aini A, Lee S, Oh I. Ion migration in metal halide perovskites. J Electrochem Sci Technol 2022;13:71-7.
44. Li N, Jia Y, Guo Y, Zhao N. Ion migration in perovskite light-emitting diodes: mechanism, characterizations, and material and device engineering. Adv Mater 2022;34:e2108102.
45. Cai J, Zhao T, Chen M, et al. Ion migration in the all-inorganic perovskite CsPbBr3 and its impacts on photodetection. J Phys Chem C 2022;126:10007-13.
46. Hussain T, Fatima K, Anjum A, et al. Experimental evidence of ion migration in aged inorganic perovskite solar cells using non-destructive RBS depth profiling. Mater Adv 2022;3:7846-53.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Pang L, Hoang MT, O’Mullane AP, Wang H. Revealing energy storage mechanism of CsPbBr3 perovskite for ultra-stable symmetric supercapacitors. Energy Mater 2023;3:300012. http://dx.doi.org/10.20517/energymater.2022.81
AMA Style
Pang L, Hoang MT, O’Mullane AP, Wang H. Revealing energy storage mechanism of CsPbBr3 perovskite for ultra-stable symmetric supercapacitors. Energy Materials. 2023; 3(2): 300012. http://dx.doi.org/10.20517/energymater.2022.81
Chicago/Turabian Style
Pang, Le, Minh Tam Hoang, Anthony P. O’Mullane, Hongxia Wang. 2023. "Revealing energy storage mechanism of CsPbBr3 perovskite for ultra-stable symmetric supercapacitors" Energy Materials. 3, no.2: 300012. http://dx.doi.org/10.20517/energymater.2022.81
ACS Style
Pang, L.; Hoang MT.; O’Mullane AP.; Wang H. Revealing energy storage mechanism of CsPbBr3 perovskite for ultra-stable symmetric supercapacitors. Energy Mater. 2023, 3, 300012. http://dx.doi.org/10.20517/energymater.2022.81
About This Article
Copyright
Data & Comments
Data
 Cite This Article 19 clicks
Cite This Article 19 clicks













Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.