Ultra-long Zn3V2O7(OH)2·2H2O nanowires grown on carbon cloth as cathode material for aqueous zinc-ion batteries
Abstract
Enhancing the performance of the cathode materials is one of the key issues for aqueous zinc-ion batteries (AZIBs). Layered vanadium-based compounds are considered to be a candidate cathode material for AZIBs owing to their advantages of variable crystal structures and high-theoretical capacity. Nevertheless, the inherent low conductivity of V-based compounds leads to their sluggish kinetics and serious capacity degradation of AZIBs. Here, we proposed a strategy that combined morphology regulation with self-supporting electrodes to build an efficient electron/ion transport network and prepared Zn3(OH)2V2O7·2H2O (ZVO) nanowires (ZVNW) on carbon cloth (CC) by a hydrothermal method. As expected, the ZVNW-CC electrode showed excellent electrochemical performances of a high specific capacity of 361.8 mAh g-1 (50 mA g-1), high-rate capability (145.9 mAh g-1 discharge capacity at 1,000 mA g-1), and long cycling life (96.7% capacity retention after 1,010 cycles at 1,000 mA g-1). The Zn2+/H2O co-intercalation mechanism for ZVNW-CC electrodes was demonstrated by ex-situ XPS and ex-situ TGA.
Keywords
INTRODUCTION
Nowadays, there are two determining factors in the development of lithium-ion batteries (LIBs): safety and cost price. The rechargeable aqueous Zn-ion batteries (AZIBs) are considered as a preferred solution due to their high level of safety, low cost, and environmental friendliness[1-4]. However, the wide application of AZIBs has been seriously hampered by the lack of high-performance cathode materials. Layered vanadium-based compounds with an open framework structure can facilitate the fast Zn-ion intercalation/de-intercalation and enhance the specific capacities. Besides, the multiple oxidation states of vanadium can further contribute to increasing the specific capacity[5-9].
Zn3(OH)2V2O7·2H2O (ZVO) materials became a powerful competitor among all vanadium-based compounds. This was because the structural water located between the V-O layers, which was attached by hydrogen bonds to hydroxyl groups, enlarged the interlayer spacing, leading to high capacity and high-rate capability[10,11]. While they were commonly used as cathode electrodes in LIBs, only a few studies reported their usage in AZIBs. For example, Li et al. developed hexagonal ZVO nanoplates by a hydrothermal route and applied them as a cathode material for AZIBs[12]. The ZVO electrode displayed a discharge capacity of 117.69 mAh g-1 at a current density of 50 mA g-1. Xia et al. reported ultra-long ZVO nanowires (ZVNW) by a microwave method[13]. The ZVNW were utilized as a cathode for AZIBs and showed a high capacity of
To address these issues, the carbon cloth (CC) with high conductivity, light weight, and excellent structural stability has been used as the growth template to ensure the orientation of active materials and as collectors[15-18] to fasten the electron transport due to the strong attachment between the active materials and CC. In this study, we first prepared the ultra-long ZVO nanowires on CC (ZVNW-CC) through a hydrothermal route. ZVNW-CC was directly utilized as a cathode for AZIBs and exhibited a high specific capacity of 361.8 mAh g-1 (at 50 A g-1). ZVNW-CC electrodes displayed higher rate performance and more favorable cycle retention than pristine ZVNW, indicating that the construction of an efficient electron/ion transport path of ZVNW-CC cathode for AZIBs was beneficial to the improvement of electrochemical performance.
EXPERIMENT
Preparation of Zn3(OH)2V2O7·2H2O nanowires grown on carbon cloth
All the chemicals used in this study were analytical grade without any further purification. They were purchased from Sinopharm Group Chemical Reagent Co. CC (3 × 3 cm) was pretreated by immersing it in a mixture solution of 20% nitric acid and sulfuric acid (v% 1:1) for 12 h. Then NH4VO3 (2 mmol) and
Sample characterization
For crystallographic analysis, the as-obtained samples were characterized by the powder X-ray diffraction (XRD, Cu Kα radiation, λ = 1.5418 Å). Surface elemental oxidation states were identified by X-ray photoelectron spectroscopy (XPS, Thermo Scientific Escalab 250Xi) using Al Kα as the X-ray source. The spectrometer was calibrated by the C 1s peak with a binding energy of 284.6 eV. The surface structure was obtained using field-emission scanning electron microscopy (FE-SEM, S-4700 Hitachi). The lattice fringes were analyzed using field-emission transmission electron microscopy (FE-TEM, Philips Tecnai F20 at
Electrode preparation and electrochemical performance
A ZVNW-CC electrode disk (diameter: 12 mm) was used directly as a cathode, zinc foil (diameter: 16 mm) as an anode, and a Whatman CF/F separator was placed between the electrodes. The electrodes were assembled using a 2016-type coin cell and tested in 3 M Zn(CF3SO3)2 solution. The active material loading was approximately 3 mg cm-2. For comparison, a ZVNW cathode was fabricated by casting a slurry mixture of 70 wt% active material, 20 wt% acetylene black, and 10 wt% polyvinylidene fluoride (PVDF) binder in N-methylpyrrolidone (NMP) on Ti foil. The mixture was then dried at 60 °C for 12 h under vacuum. Cyclic voltammetry (CV) was performed over a potential range of 0.2 to 1.6 V (vs. Zn2+/Zn) at 0.1 mV s-1. Galvanostatic charge/discharge (GCD) cycling was conducted at various current rates. CV tests were performed on a CHI 600E electrochemical station. All the tests were performed at room temperature.
RESULTS AND DISCUSSION
Structural and phase analysis
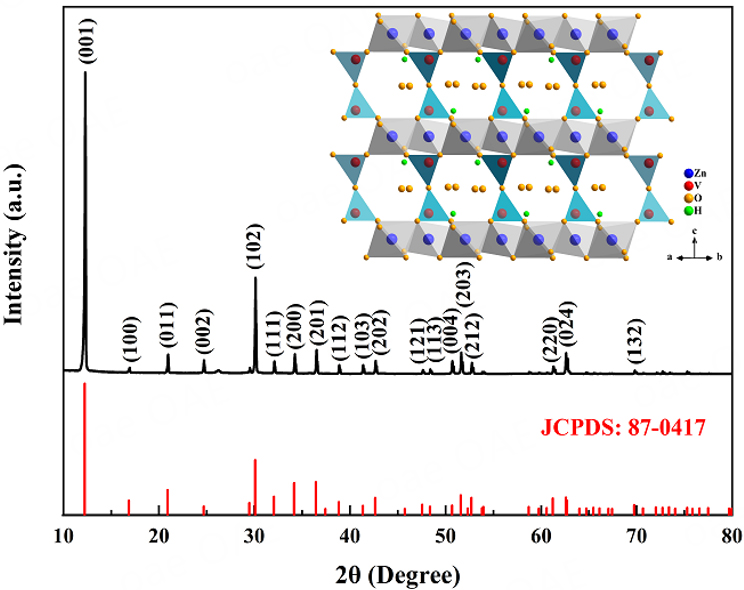
The as-obtained sample was characterized by XRD. Figure 1 shows that the diffraction peaks at 12.3°, 16.9°, 20.9°, 24.7°, 30.1°, 32.1°, 34.2°, 36.5°, 38.9°, 42.7°, 51.6°, and 62.6°corresponded to (001), (100), (011), (002), (102), (111), (200), (201), (112), (202), (203), and (204) planes of hexagonal Zn3(OH)2V2O7·2H2O (JCPDS NO. 87-0417), respectively. In addition, the diffraction peak intensity of (001) is much higher than that of (102), suggesting the [001] direction (c-axis) crystallographic preferred orientations of ZVNW. The inset image in Figure 1 shows the crystal structure of Zn3V2O7(OH)2·2H2O, which consists of a Zn-O layer formed by edge-sharing [ZnO6] octahedra and a V-O layer. The Zn-O layers were separated by V-O-V pillars formed by corner-sharing [VO4] tetrahedra along the c-axis. Water molecules were randomly filled in the large cavities[19]. Supplementary Figure 2 indicates the XRD pattern of ZVNW-CC. The carbon peaks at 26° were clearly observed because the content of ZVNW was lower than that of CC.
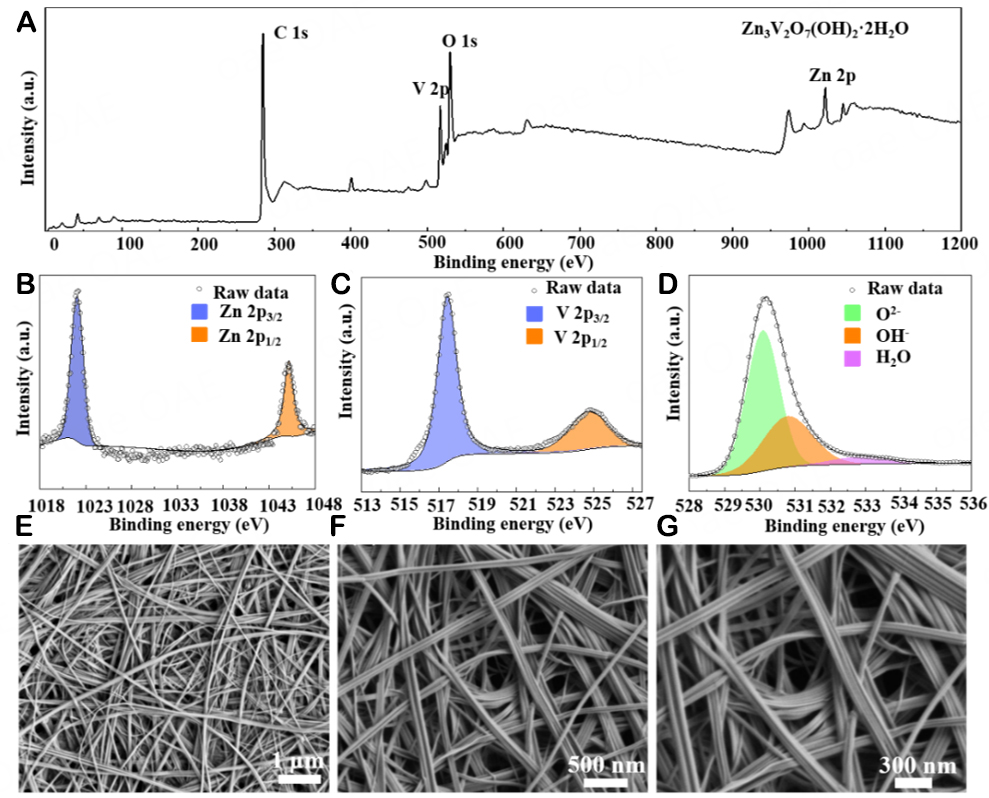
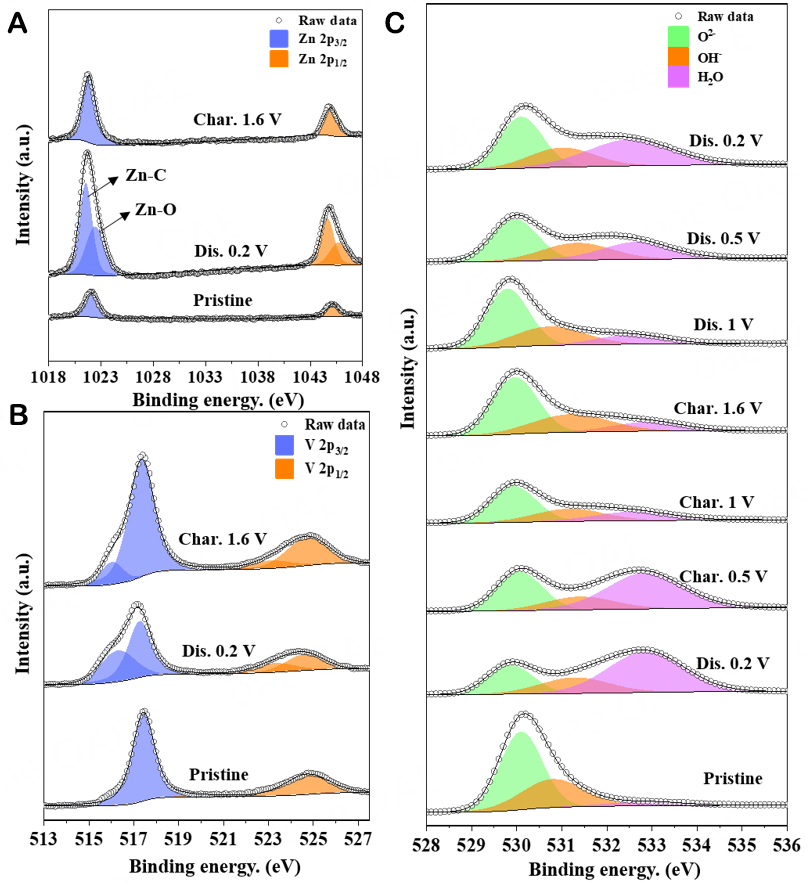
To further characterize the valence state and composition of ZVNW-CC, the XPS techniques were performed. Figure 2A shows the overall XPS spectrum of ZVNW-CC, illustrating that it is composed of Zn, V, O, and C elements. Figure 2B shows the high-resolution XPS spectrum of Zn 2p. The peaks centered at binding energies of 1045.0 and 1021.9 eV were attributed to Zn 2p1/2 and Zn 2p3/2, respectively. And their binding energy difference was about 23.1 eV, proving the existence of Zn2+[13,20]. Figure 2C is a high-resolution V 2p XPS spectrum of ZVNW-CC. The binding energies of the fitted peaks corresponding to
Morphology and microstructure analysis
The morphologies of ZVNW-CC and ZVNW were characterized by SEM in Figure 2E-G and supplementary Figure 3. The ZVNW with tens of microns grown on CC in parallel can be clearly observed in Figure 2E. The length of each nanowire can reach the micron level. This structure was beneficial for increasing the contact area between ZVNW and CC, which enhanced the structural stability of the ZVNW-CC electrode. And 1D nanostructure can shorten the diffusion pathway of Zn2+ during the charging and discharging process, promoting the electrochemical performance of the ZVNW-CC electrode. SEM images in Figure 2F and G show a higher-resolution view of the sample. ZVNW, ranging in size from 20-200 nm, was stacked and interlaced grown on CC, forming a multi-void network structure.
The microstructure of ZVNW, peeled from CC through prolonged sonication, was further characterized by TEM. Figure 3A shows a TEM image of a single ZVO nanowire. The surface of the ZVNW was rough, which increased the contact area between the electrode and electrolyte. Figure 3B is its HRTEM image. The inter-planar distances of 2.01 and 2.31 Å were attributed to (210) and (112) planes of ZVO, respectively. Figure 3C shows the fast Fourier transform electron diffraction (FFT-ED) pattern of the HRTEM image, which suggests the single-crystal feature. Figure 3D shows the EDS analysis of a single ZVO nanowire. It was further confirmed that the ZVNW were composed of Zn, V, and O elements, which were uniformly distributed in the ZVNW.
Electrochemical performances
The electrochemical properties of ZVNW and ZVNW-CC electrodes were evaluated by CV and GCD. Figure 4A shows the CV curves of these two electrodes at 0.1 mV s-1. The surrounding CV curve area of the ZVNW-CC electrode was much larger than that of the ZVNW electrode, indicating that the ZVNW-CC electrode exhibited higher specific capacity. Figure 4B presents the first three cycles of the CV plot for the ZVNW-CC electrode at a scan rate of 0.1 mV s-1 in the voltage range of 0.2-1.6 V (vs. Zn/Zn2+). Three pairs of redox peaks were observed at 0.56/0.76, 0.85/1.07, and 1.35/1.46 V, which were attributed to the three-step reaction of Zn2+ insertion and extraction into the ZVO lattice structure[21]. Among the reduction reactions at 0.56, 0.85, and 1.35 V showed that the vanadium element in ZVNW-CC was gradually reduced from +5 valence to +α (α < 5), and the oxidation reactions at 0.76, 1.07, and 1.46 V corresponded to the gradual oxidation of Vα+ to V5+. The cyclic scanning process of the first three loops almost overlapped, indicating that the storage process of Zn2+ was highly reversible. GCD tests were conducted between 0.2 and 1.6 V (vs. Zn/Zn2+). Figure 4C and D indicates the GCD profiles of ZVNW-CC and ZVNW electrodes for the first five and 50th cycles at 50 mA g-1. In the discharge curve, there were two distinctive potential plateaus within the range of 0.69-1.00 and 0.45-0.69 V, which corresponded to the two main oxidation peaks of CV. Similarly, another two potential plateaus in the charge curve within 0.88-1.15 and 0.6-0.88 V corresponded to the reduction peaks. The related charge-discharge curve of ZVNW-CC electrodes at
Figure 4. (A) CV curves of ZVNW and ZVNW-CC electrodes. (B) CV curves of ZVNW-CC electrode in the first three cycles at
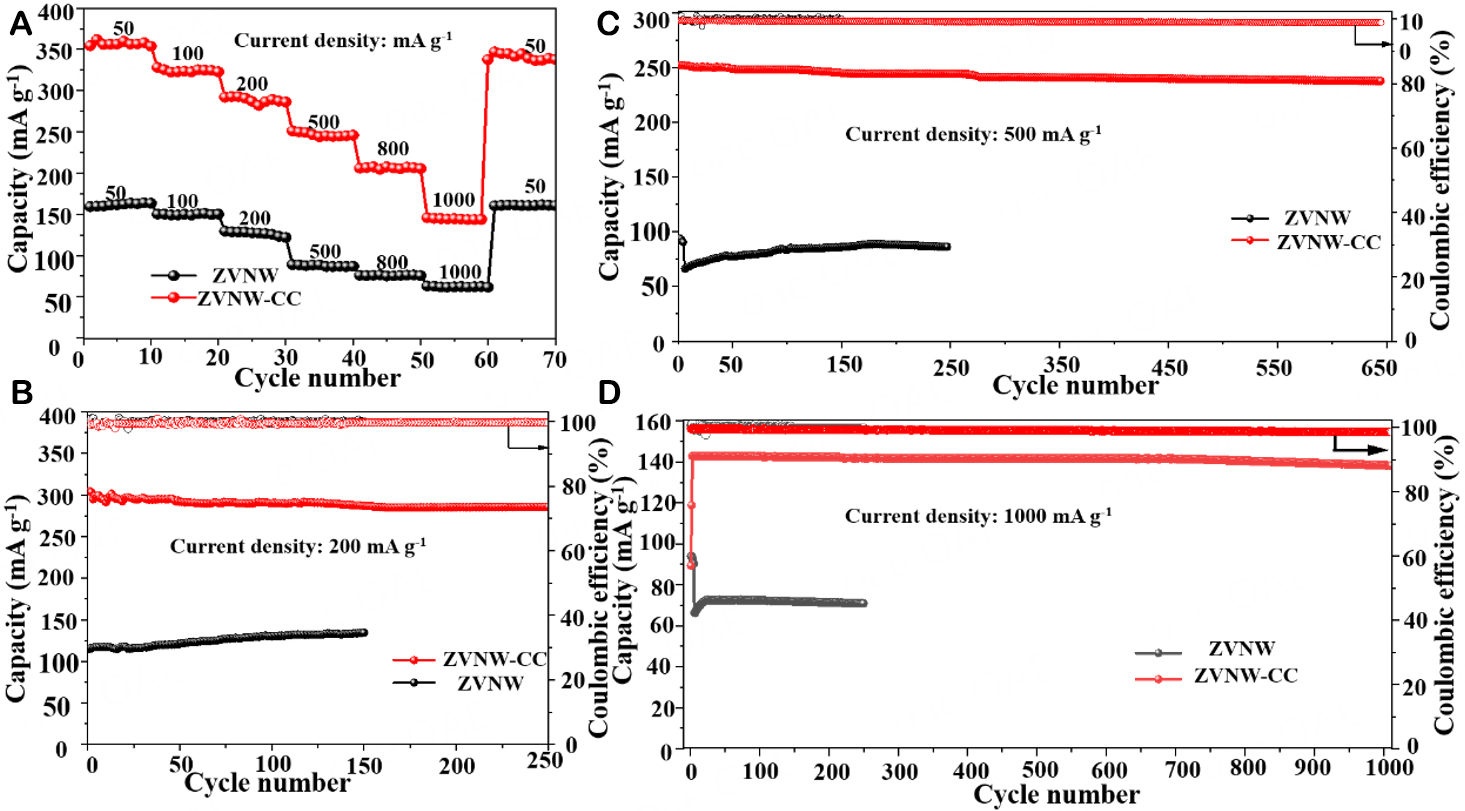
The rate performances are presented in Figure 5A, where the current density increases from 50 to
Figure 5. (A) The rate capability of ZVNW-CC and ZVNW electrodes. The cycling stability of ZVNW-CC and ZVNW electrodes at (B) 200, (C) 500, and (D) 1,000 mA g-1.
Comparison of the specific capacity and cycling performance of ZVNW-CC electrode with other reported cathodes in AZIBs
| Samples | Specific capacity (mAh g-1) | Capacity retention | Refs |
| ZVO-CC | 361.8 (0. 05 A g-1) | 80% (650 cycles) | This work |
| Zn3V2O7(OH)2·2H2O | 213 (0.05 A g-1) | 68% (300 cycles) | [13] |
| V2O5 | 319 (0.02 A g-1) | 81% (500 cycles) | [22] |
| LiV3O8 | 256 (0.016 A g-1) | 75% (65 cycles) | [23] |
| KV3O8 | 249 (0.025 A g-1) | 82.8% (500 cycles) | [24] |
| Zn2V2O7 | 203 (0.3 A g-1) | 85% (1,000 cycles) | [25] |
| K2V6O16·2.7H2O | 329.6 (0.2 A g-1) | 82% (500 cycles) | [26] |
| VS2 | 190.3 (0.05 A g-1) | 98% (200 cycles) | [27] |
| Na3V2(PO4)3 | 97 (0.05 A g-1) | 74% (100 cycles) | [28] |
| VS4@rGO | 180 (1 A g-1) | 93.3% (165 cycles) | [29] |
Electrochemical kinetic properties and zinc storage mechanism
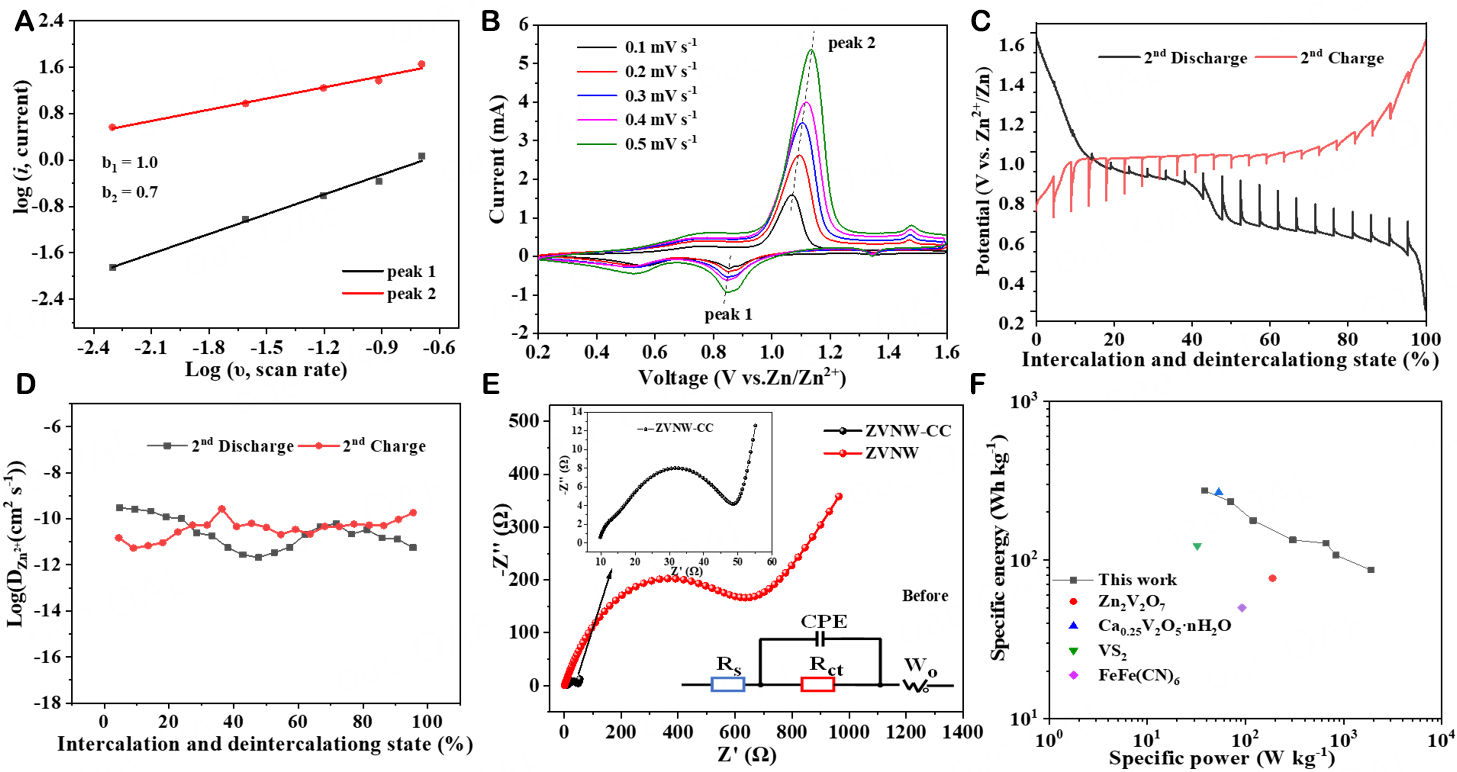
The Zn-ion storage behavior of the ZVNW-CC electrode was further studied by CV. Figure 6A presents the CV curves of the ZVNW-CC cathode measured at different scan rates. The CV curves retained the same shape with the increase of scan rates from 0.1 to 0.5 mV s-1, indicating good reversibility of the ZVNW-CC cathode. A power law relationship with the sweep rate: i = avb, or log i = b log v + log a (peak current: i, scan speed: v, and adjustable parameters: a and b), was used to calculate the contributions of capacitive and diffusion-controlled processes in AZIBs. Among them, if the value of b was close to 1.0, then the electrochemical process was controlled by the capacitive effect; when b = 0.5, the process was controlled by ion diffusion; if 0.5 < b < 1.0, both ion diffusion and capacitance effects were involved. Figure 6B shows the plot of log (i) versus log (v) for calculation of the b value. The b values of peak (1) and peak (2) in Figure 6A were 0.7 and 1.0, respectively, indicating a contribution process dominated by capacity control. To further clarify the details of electrochemical behavior, GITT measurement was implemented to study the Zn2+ diffusion coefficients (DZn2+) of the ZVNW-CC electrode. DZn2+ can be estimated according to Supplementary Figure 4 and Eq. 1 in the supporting information. Each cycle consisted of charging to 1.6 V at a rate of 50 mA g-1 for 20 min, relaxing for 2 h, and discharging to 0.2 V at the same rate. Figure 6C shows the GITT data at the second cycle taken from the charge-discharge curve. DZn2+ was calculated between 10-9 and 10-11 cm2 s-1 (as shown in Figure 6D), which is superior to those of some reported V-based cathode materials in AZIBs[30-33].
Figure 6. (A) CV curves of the ZVNW-CC electrode at different scan rates. (B) b value of the redox peak in the CV curve. (C) GITT curves during discharge and charge. (D) Corresponding Zn2+ diffusion coefficient during the 2nd cycle of charge-discharge. (E) Nyquist plots of ZVNW-CC and ZVNW electrodes before cycling in the frequency range of 0.01 Hz to 100 kHz. (F) The Ragone plot comparison of Zn/ZVNW-CC battery with other cathode materials for AZIBs.
The electrochemical impedance spectroscopy measurement was performed to evaluate the charge transfer resistance of the ZVNW-CC and ZVNW electrodes before and after cycling (as shown in Figure 6E and Supplementary Figure 5). Figure 6E displays the Nyquist plots of these two cathodes before cycling and their equivalent circuit models. The diameter of the semi-circle in the Nyquist plots of ZVNW-CC, associated with the contact resistance and charge transfer processes (Rct), was far less than that of the ZVNW electrode. It meant that the Rct value of the ZVNW-CC electrode was significantly decreased without any additives[34,35]. Rct values of ZVNW-CC and ZVNW electrodes before (after) cycling were 41.4 (34.3) and 603.5 (109.1) Ω, respectively, indicating the improved conductivity of the self-supporting electrode. The above results further proved that both the 1D ZVNW-CC cathode and self-supporting electrode together fastened the Zn2+ immigration, resulting in high capacity and high cycle stability. Moreover, the energy density and power density of the ZVNW-CC electrode were calculated to be 273.5 Wh kg-1 and
Ex situ XPS analyses were used to analyze the valence-state change of the ZVNW-CC electrode and further revealed the possible Zn2+ storage mechanism during the charge and discharge processes. Figure 7A shows the Zn 2p spectra of the fully charged and fully discharged states of the ZVNW-CC electrode. In the fully discharged state, Zn 2p3/2 peaks at 1,022.48 and 1,021.58 eV or Zn 2p1/2 peaks at 1,045.76 and 1,044.67 eV were due to the formation of Zn-O and Zn-C bonds[37]. However, in the fully charged state, only the XPS peak of the Zn-O bond remained and the Zn-C peak disappeared, which suggested that Zn2+ of Zn-C was more easily de-intercalated and led to an increase in capacitance. Moreover, with the interaction of Zn2+, the V5+ of V-O-V pillars underwent a reduction reaction to Vα+ (α < 5), as shown in Figure 7B. In addition, the high-resolution XPS spectra of O 1s (as shown in Figure 7C) revealed its evolution at the pristine, fully discharged/charged states, respectively. Notably, the relative intensity of the crystal H2O peak in the fully discharged ZVNW-CC decreased significantly during charging, further confirming the co-intercalation mechanism of Zn2+ and H2O. Subsequently, thermogravimetric tests (nitrogen atmosphere) were also performed on ZVNW-CC electrodes under different charge and discharge conditions, as shown in Supplementary Figure 6. It was consistent with the XPS results. Based on the above analysis and discussion, the electrochemical reactions that occurred inside the Zn/ZVNW-CC batteries can be described by the following equations:
Zn2+ + 2e- ↔ Zn (1)
Zn3(OH)2V2O7·2H2O + xZn2+ + yH2O + 2xe- ↔ Zn3+x(OH)2V2O7·(2+y) H2O (2)
CONCLUSIONS
We have designed the “self-supporting” electrode by 1D ZVNW grown on CC as the cathode material in AZIBs. Integrating the excellent electronic conductivity of CC, 1D ZVO nanowire with layered framework structure in ZVNW-CC can facilitate Zn2+ diffusion and fasten the electron transport, improving the electrochemical reaction kinetics of the electrode. Consequently, the ZVNW-CC electrode showed excellent electrochemical performances of a specific capacity of 361.8 mAh g-1 (50 mA g-1), a high-rate capability (145.9 mAh g-1 discharge capacity at 1,000 mA g-1), and long cycling life (96.7% capacity retention after 1,010 cycles at 1,000 mA g-1). The Zn2+/H2O co-intercalation mechanism in ZVNW-CC electrodes was demonstrated by ex-situ XPS and ex-situ TGA. The novel strategy proposed in this study may provide guidance for exploiting high-performance energy storage systems.
DECLARATIONS
Authors’ contributionPreparing the manuscript draft: Cui Y
Carried out the synthesis and characterization, performed the electrochemical measurements and characterizations: Cui Y, Ding Y, Guo L
Conceived the concept and directed the research, Writing-review & editing: Guo C, Liu Y
Provided the research advice: Liu Y, Bai Y
Supervision, Resources: Li G, Wang K
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis study was supported by the National Nature Science Foundation of China (Grant nos. U1910210, U1810204, 22278291, and 22105142) and Research Foundation for the Returned Overseas in Shanxi Provence (2020-048).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
1. Xu C, Li B, Du H, Kang F. Energetic zinc ion chemistry: the rechargeable zinc ion battery. Angew Chem Int Ed 2012;51:933-5.
2. Hwang B, Cheong JY, Matteini P, Yun TG. Highly efficient phthalocyanine based aqueous Zn-ion flexible-batteries. Mater Lett 2022;306:130954.
3. Yang W, Yang Y, Yang H, Zhou H. Regulating water activity for rechargeable zinc-ion batteries: progress and perspective. ACS Energy Lett 2022;7:2515-30.
4. Zhang Y, Huang R, Wang X, et al. Facile large-scale preparation of vanadium pentoxide-polypyrrole composite for aqueous zinc-ion batteries. J Alloys Compd 2022;907:164434.
5. Chen R, Luo R, Huang Y, Wu F, Li L. Advanced high energy density secondary batteries with multi-electron reaction materials. Adv Sci 2016;3:1600051.
6. Tang H, Peng Z, Wu L, et al. Vanadium-based cathode materials for rechargeable multivalent batteries: challenges and opportunities. Electrochem Energy Rev 2018;1:169-99.
7. Wan F, Niu Z. Design strategies for vanadium-based aqueous zinc-ion batteries. Angew Chem Int Ed 2019;58:16358-67.
8. Liu N, Li B, He Z, Dai L, Wang H, Wang L. Recent advances and perspectives on vanadium- and manganese-based cathode materials for aqueous zinc ion batteries. J Energy Chem 2021;59:134-59.
9. Yuan Z, Chen X, Liu X, Feng C. Synthesis of Zn3V2O7(OH)2·2H2O microspheres as novel anode material for lithium-ion battery application. Ionics 2020;26:1703-10.
10. Bulbul B, Beyaz S, Akyol M, Ekicibil A. Simple manufacturing and metal type-dependent properties of M3(OH)2V2O7·nH2O (M; Co, Ni, Cu, Zn) nanostructures. Nanochem Res 2022:2;154-67.
11. Du Y, Liu X, Wang X. et al. Freestanding strontium vanadate/carbon nanotube films for long-life aqueous zinc-ion batteries. Rare Metals 2021;41:415-24.
12. Li L, Jia T, Pei X, et al. A study on the properties of hexagonal Zn3(OH)2V2O7·2H2O as cathode material for zinc-ion battery. Ionics 2022;28:283-93.
13. Xia C, Guo J, Lei Y, Liang H, Zhao C, Alshareef HN. Rechargeable aqueous zinc-ion battery based on porous framework zinc pyrovanadate intercalation cathode. Adv Mater 2018;30:1705580.
14. Wang F, Wang Q, Dong S, Wang S. An aqueous zinc pyrovanadate nanowire cathode doped by nitrogen-doped carbon from PANI calcination for capacity and stability enhancement. Ionics 2022;28:295-305.
15. Zhao S, Guo J, Jiang F, Su Q, Du G. Porous CoFe2O4 nanowire arrays on carbon cloth as binder-free anodes for flexible lithium-ion batteries. Mater Res Bull 2016;79:22-8.
16. Storan D, Ahad SA, Forde R, et al. Silicon nanowire growth on carbon cloth for flexible Li-ion battery anodes. Mater Today Energy 2022;27:101030.
17. Corpuz RD, Juan-Corpuz LM, Nguyen MT, et al. Binder-free α-MnO2 nanowires on carbon cloth as cathode material for zinc-ion batteries. Int J Mol Sci 2020;21:3113.
18. De Juan-corpuz LM, Corpuz RD, Somwangthanaroj A, et al. Binder-free centimeter-long V2O5 nanofibers on carbon cloth as cathode material for zinc-ion batteries. Energies 2020;13:31.
19. Jia X, Liu C, Neale ZG, Yang J, Cao G. Active materials for aqueous zinc ion batteries: synthesis, crystal structure, morphology, and electrochemistry. Chem Rev 2020;120:7795-866.
20. Biesinger MC, Lau LW, Gerson AR, Smart RS. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl Surf Sci 2010;257:887-98.
21. Xia C, Guo J, Li P, Zhang X, Alshareef HN. Highly stable aqueous zinc-ion storage using a layered calcium vanadium oxide bronze cathode. Angew Chem Int Ed 2018;57:3943-8.
22. Chen X, Wang L, Li H, Cheng F, Chen J. Porous V2O5 nanofibers as cathode materials for rechargeable aqueous zinc-ion batteries. J Energy Chem 2019;38:20-5.
23. Alfaruqi MH, Mathew V, Song J, et al. Electrochemical zinc intercalation in lithium vanadium oxide: a high-capacity zinc-ion battery cathode. Chem Mater 2017;29:1684-94.
24. Kim HJ, Jo JH, Choi JU, Voronina N, Myung S. KV3O8 with a large interlayer as a viable cathode material for zinc-ion batteries. J Power Sources 2020;478:229072.
25. Sambandam B, Soundharrajan V, Kim S, et al. Aqueous rechargeable Zn-ion batteries: an imperishable and high-energy Zn2V2O7 nanowire cathode through intercalation regulation. J Mater Chem A 2018;6:3850-6.
26. Sambandam B, Soundharrajan V, Kim S, et al. K2V6O16·2.7H2O nanorod cathode: an advanced intercalation system for high energy aqueous rechargeable Zn-ion batteries. J Mater Chem A 2018;6:15530-9.
27. He P, Yan M, Zhang G, et al. Layered VS2 nanosheet-based aqueous Zn ion battery cathode. Adv Energy Mater 2017;7:1601920.
28. Li G, Yang Z, Jiang Y, et al. Towards polyvalent ion batteries: a zinc-ion battery based on NASICON structured Na3V2(PO4)3. Nano Energy 2016;25:211-7.
29. Qin H, Yang Z, Chen L, et al. A high-rate aqueous rechargeable zinc ion battery based on the VS4@rGO nanocomposite. J Mater Chem A 2018:6;23757-65.
30. Tang B, Fang G, Zhou J, et al. Potassium vanadates with stable structure and fast ion diffusion channel as cathode for rechargeable aqueous zinc-ion batteries. Nano Energy 2018;51:579-87.
31. Guo X, Fang G, Zhang W, et al. Mechanistic insights of Zn2+ storage in sodium vanadates. Adv Energy Mater 2018;8:1801819.
32. He P, Quan Y, Xu X, et al. High-performance aqueous zinc-ion battery based on layered H2V3O8 nanowire cathode. Small 2017;13:1702551.
33. Zhang N, Jia M, Dong Y, et al. Hydrated layered vanadium oxide as a highly reversible cathode for rechargeable aqueous zinc batteries. Adv Funct Mater 2019;29:1807331.
34. Tatara R, Karayaylali P, Yu Y, et al. The effect of electrode-electrolyte interface on the electrochemical impedance spectra for positive electrode in Li-ion battery. J Electrochem Soc 2019;166:A5090-8.
35. Dong L, Yang W, Yang W, et al. High-power and ultralong-life aqueous zinc-ion hybrid capacitors based on pseudocapacitive charge storage. Nanomicro Lett 2019;11:94.
36. Liu Z, Bertram P, Endres F. Bio-degradable zinc-ion battery based on a prussian blue analogue cathode and a bio-ionic liquid-based electrolyte. J Solid State Electrochem 2017;21:2021-7.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Cui Y, Ding Y, Guo L, Guo C, Liu Y, Bai Y, Li G, Wang K. Ultra-long Zn3V2O7(OH)2·2H2O nanowires grown on carbon cloth as cathode material for aqueous
AMA Style
Cui Y, Ding Y, Guo L, Guo C, Liu Y, Bai Y, Li G, Wang K. Ultra-long Zn3V2O7(OH)2·2H2O nanowires grown on carbon cloth as cathode material for aqueous
Chicago/Turabian Style
Cui, Yu, Yi Ding, Lingfan Guo, Chunli Guo, Yanzhen Liu, Yulin Bai, Gang Li, Kaiying Wang. 2023. "Ultra-long Zn3V2O7(OH)2·2H2O nanowires grown on carbon cloth as cathode material for aqueous
ACS Style
Cui, Y.; Ding Y.; Guo L.; Guo C.; Liu Y.; Bai Y.; Li G.; Wang K. Ultra-long Zn3V2O7(OH)2·2H2O nanowires grown on carbon cloth as cathode material for aqueous
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 9 clicks
Cite This Article 9 clicks















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.