Understanding of working mechanism of lithium difluoro(oxalato) borate in Li||NCM85 battery with enhanced cyclic stability
Abstract
Despite the significant advances achieved in recent years, the development of efficient electrolyte additives to mitigate the performance degradation during long-term cycling of high-energy density lithium||nickel-rich (Li||Ni-rich) batteries remains a significant challenge. To achieve a rational design of electrolytes and avoid unnecessary waste of resources due to trial and error, it is crucial to have a comprehensive understanding of the underlying mechanism of key electrolyte components, including salts, solvents, and additives. Herein, we present the utilization of lithium difluoro(oxalate) borate (B) (LiDFOB), a B-containing lithium salt, as a functional additive for Li||LiNi0.85Co0.1Mn0.05O2 (NCM85) batteries, and comprehensively investigate its mechanism of action towards enhancing the stability of both anode and cathode interfaces. The preferential reduction and oxidation decomposition of DFOB- leads to the formation of a robust and highly electronically insulating boron-rich interfacial film on the surface of both the Li anode and NCM85 cathode. This film effectively suppresses the consumption of active lithium and the severe decomposition of the electrolyte. Furthermore, the presence of B elements in the cathode-electrolyte interfacial film, such as BF3, BF2OH, and BF2OBF2 compounds, can coordinate with the lattice oxygen of the cathode, forming strong coordination bonds. This can significantly alleviate lattice oxygen loss and mitigate detrimental structural degradation of the Ni-rich cathode. Consequently, the Li||NCM85 battery cycled in LiDFOB-containing electrolyte displays superior capacity retention of 74% after 300 cycles, even at a high charge cut-off voltage of 4.6 V. The comprehensive analysis of the working mechanisms of LiDFOB offers valuable insights for the rational design of electrolytes featuring multifunctional lithium salts or additives for high energy density lithium metal batteries.
Keywords
INTRODUCTION
Lithium-ion batteries (LIBs) are widely used in various electronic equipment as energy storage devices, while the rapid development of electric vehicles (EVs) has put forward higher requirements for LIBs in terms of energy/power density and cyclic stability[1-6]. To this end, the development of high-specific energy LIBs with lithium metal as the anode (currently the largest energy density) and a suitable cathode is the key to improving the overall energy density of LIBs[7-10]. Among all the identified cathode materials, nickel (Ni)-rich-layered oxide Li[NixCoyMn1-x-y]O2 (x ≥ 0.8) with suitable Ni, Co, and Mn atomic ratios is considered one of the most promising cathode materials due to their high specific capacity of more than 200 mAh g-1 and low cost[11,12]. However, the practical application of the Ni-rich cathode material is limited by severe capacity decay and thermal instability[13]. Several possible reasons have been proposed to explain the performance degradation of Ni-rich materials, mainly including irreversible structural changes and interfacial degradation[14]. On the one hand, at a highly delithiated state, the irreversible phase transition from the second hexagonal phase (H2) to the third hexagonal phase (H3), accompanied by abrupt lattice contraction and rise of internal stress, will result in partial structure collapse at the near-surface area of crystal and serious grain intergranular or intragranular cracking of particles[15,16]. Meanwhile, the structure collapse accompanied by oxygen evolution and transition metal dissolution will cause the spread of this collapse and cracks into the particle core, which leads to fast capacity loss. Additionally, the electrolyte can penetrate into these cracks, which can be catalyzed by highly active Ni4+ and superoxide ions (O-) to form an undesired phase transition layer and excessive deposition of electrolyte decomposition products. This results in a substantial reduction in the kinetics of Li-ion (Li+) migration and electron transfer of the electrode[17-19]. In addition, Ni4+ ions are easily reduced into stable Ni2+ ions in the presence of O-, resulting in capacity loss and O2 release[20]. Meanwhile, due to the similar size of Ni2+ and Li+, it will cause more serious Li+/Ni2+ mixing, which further reduces the stability of the structure[21,22]. Therefore, achieving a stable Ni-rich cathode necessitates the high stability of surficial/interfacial structure, especially at a higher voltage.
To solve these problems, several strategies have been explored in recent studies, such as coherent surface coating[20,23-30] and near-surface element doping or substitution[31-37], to better stabilize the near-surface structure of the material (especially the stability of surface lattice oxygen) and effectively suppress electrolyte decomposition. However, these approaches usually involve complicated synthetic processes, and the introduction of inert compositions reduces the specific capacity of cathodes. More importantly, these methods can only target the cathode alone but cannot simultaneously protect the anode, especially the hyperactive lithium metal anode. Detrimental and persistent interfacial side reactions occur between lithium metal and the electrolyte, which induces the generation of lithium dendrites and inactive lithium, resulting in short cycle life and causing safety issues[38-42].
In this regard, optimizing electrolytes by using interfacial film-formation functional additives to simultaneously regulate Ni-rich cathode and Li anode interfacial properties is a promising strategy[43,44]. Among the diverse electrolyte additives that have been reported so far, borate (B)-containing additives, especially B-containing lithium salts, have been employed as effective salts or additives for high-voltage cathodes or alkali metal anodes[45-60]. For example, Zhang et al. introduced trace (~2%) lithium bis(oxalato)borate (LiBOB) as an electrolyte additive to improve the cyclic stability of Li-rich layered oxide (LRLO) cathode by optimizing the cathode interfacial structure and eliminating the detrimental nucleophilic superoxide attack under the synergy effect of LiPF6[61]. Chen et al. demonstrated lithium difluorobis(oxalato)phosphate (LiDFBOP) as the multifunctional additive can build a stable and robust organic/inorganic hybrid interphase on Na anode and Na3V2(PO4)2F3 cathode surface by the preferential reduction and oxidation of DFBOP-, realizing excellent cyclic stability for high-voltage Na||Na3V2(PO4)2F3 system[62]. Mao et al. configured 1 M lithium difluoro(oxalate) borate (LiDFOB)-based electrolyte, where the LiDFOB can not only in-situ construct a hierarchical and robust cathode/electrolyte interface (CEI) film on the LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode surface but also induce the uniform plating of Li, resulting in greatly improved cyclic stability of Li||NCM811 batteries[63]. While B-containing lithium salts are widely utilized in batteries, a systematic investigation of the differences in the interfacial films formed from their decomposition on the anodes and cathodes is rarely reported. It is crucial to have a comprehensive understanding of the working mechanism of key components in the electrolyte for the rational design of electrolytes to improve the performance and stability of the batteries.
Herein, LiDFOB, as a B-containing lithium salt type additive incorporating the advantages of both lithium tetrafluoroborate (LiBF4) and LiBOB, was selected for high-energy-density Li||LiNi0.85Co0.1Mn0.05O2 (NCM85) battery. Theoretical calculations and experimental studies systematically demonstrated that DFOB- is preferentially reduced on the Li anode surface to form a highly electrochemically stable, electronically insulating, and ion-conductive solid electrolyte interface (SEI) film under the synergistic effect of ethylene carbonate (EC), which effectively promotes the homogenous plating of Li and suppresses the continuous decomposition of the electrolyte and the formation of inactive lithium. On the NCM85 cathode surface, the DFOB- is preferentially oxidized and spontaneously captures the harmful hydrofluoric acid (HF) and O- to form an antioxidant and electronically insulating CEI film, which can effectively suppress the oxidative decomposition of the electrolyte and the erosion of HF to electrodes. Meanwhile, the B element in CEI film will form a strong bond with the lattice O of the cathode surface, thereby stabilizing the phase structure of NCM85 and inhibiting the subsequent irreversible structural transition and O2 release. Consequently, the cyclic stability and rate capability of Li||NCM85 batteries are significantly improved, even at a high charge cut-off voltage of 4.6 V. The comprehensive examination of LiDFOB in this work offers valuable insights and helpful guidance for the design of B-contenting electrolyte additives or lithium salts for high-energy-density lithium metal batteries.
EXPERIMENTAL
Preparation of electrolytes and electrodes
The electrolytes and electrodes were both prepared in the Mbraun glove box (Ar atmosphere) with moisture and oxygen levels less than 0.1 ppm. 1 M LiPF6 in EC and ethyl methyl carbonate (EMC)
Electrochemical measurement
The fabricated cells were charged and discharged after standing for 8 h at room temperature, measured by the LAND system (CT2001A, Wuhan, China). For regular cycles, each Li||NCM85 battery was charged/discharged galvanostatically at 0.3 C (1 C = 200 mAh g-1) for two cycles before testing, then set the charge-discharge rate at 1C to analyze cyclability, voltage ranging from 3.0 V to 4.3 V/4.6 V. After two formation cycles at 0.2 C, the rate test was carried out at 1 C, 3 C, 5 C, 7 C, 10 C, and 0.2 C again for five cycles, respectively. A high current charge and discharge test was carried out to further assess the cyclic stability of the batteries at the charge/discharge rate of 2 C/5 C. In the galvanostatic intermittent titration test (GITT), the parameter pulse current was 1 C, the pulse charge/discharge time was 90 s, and the resting time was 2 h. The Li+ diffusion coefficient is calculated by Equation (1)[64]:
where DLi+ is the chemical diffusion coefficient, S is the surface area of the electrode (1.13097 cm2 in our case), τ is pulse duration, ΔEs is the steady-state voltage change, ΔEt is the transient voltage change, and m, M, and Vm is the mess, molecular weight (97.28 g mol-1), and molar volume (19.3491 cm3 mol-1) of the electrode, respectively. For Li||Li symmetric cells and Li||Cu cells, Li foils with a diameter of 10 mm and
where Qs is the final stripping capacity, Qp is the initial plating capacity, Qc is the constant plating/stripping capacity for each cycle, and n is the cycle number[66]. Li||Li symmetric cells were cycled at a current density of 0.5 mA cm-2 and a capacity of 1.0 or 3.0 mAh cm-2. Linear sweep voltammetry (LSV) and electrochemical impedance spectrum (EIS) analysis were both performed on an electrochemical workstation (Autolab, PGSTAT-302N, Metrohm, Netherlands), with the sweep scan rate at 1 mV s-1 for LSV and a 5 mV amplitude and a frequency between 105 Hz and 10 mHz for EIS.
Material characteristic
The batteries were dissembled after cycling, and the electrodes were rinsed and soaked with dimethyl carbonate (DMC) to remove the residual electrolyte and dried naturally in the glove box. Scanning electron microscopy (SEM, Zeiss GeminiSEM 500, Germany) was used to analyze the morphology of Li anodes and NCM85 cathodes. The transmission electron microscope (TEM, JEM-2100HR, Japan) was carried out to analyze the evolution of NCM85 surface microstructure before and after cycling. The ion beam polished with Cross Section Polisher (Leica, EM TIC 3X, Germany) was used to prepare the cross-section samples. The crystal structure and phase transformation of NCM85 were detected by ex-situ X-ray diffraction (XRD, Rigaku Ultima IV diffractometer, Japan) and in-situ XRD (Bruker D8 discover diffractometer, German) at a scan rate of 5o/min with Cu Kα radiation over 2θ range of 20o to 60o. As for the components on the surface of the Li anode and NCM85 cathode, X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific, UK) was employed with the binding energy in the measured spectrum calibrated based on C1s at 284.8 eV. The fitting results of XPS peaks were obtained using XPSPEAK Version 4.0. In-situ differential electrochemical mass spectrometry (DEMS) experiments were carried out to test the CO2 emission during charging using a commercial quadrupole mass spectrometer (Hiden Analytical) equipped with a digital mass flow meter (Bronkhorst). The homemade Swagelok-type cell was assembled in the glove box and then charged to 4.6 V at 0.2 C after resting for 3 h. Time of flight secondary ion mass spectrometry (TOF-SIMS, IONTOF, German) was employed to investigate the surface structure evolution of both NCM85 cathode and Li anode. All detected secondary ions of interest in the TOF-SIMS analysis had a mass resolution of over 17,000. The samples were prepared in a glove box and transferred to the instruments using an air-tight holder during TOF-SIMS characterizations.
Calculation methods
The first-principles calculations were implemented using Vienna Ab-initio Simulation Package (VASP) version 5.4.1[67] and Gaussian 09 package[68], with strongly constrained and appropriately normed (SCAN) and Perdew-Burke-Ernzerhof (PBE) functional, respectively. The (104) and (100) surfaces of the layered LiNiO2 and Cubic lithium metal crystal were simulated by four-layer slab Li32Ni32O64 and Li100, respectively. The plane-wave cut-off energy was set to be 520 eV, and the convergence criteria were 10-4 eV and
where EX-slab, Ex, and Eslab are the total energy of the interfacial supercell, the adsorbed redox products, and the bottom layer (LiNiO2 (104) or Li (100)), respectively. The calculated oxidation potential (Eox) and reduction potential (Ere) were converted from the absolute oxidation/reduction potential of the species (vs. Li/Li+), according to Equation (4):
where G(M) and G(M+) are the free energies of the species M and its oxidized/reduced form M+ at 298.15 K, respectively, and F is the Faraday constant.
RESULTS AND DISCUSSION
Redox activity of LiDFOB
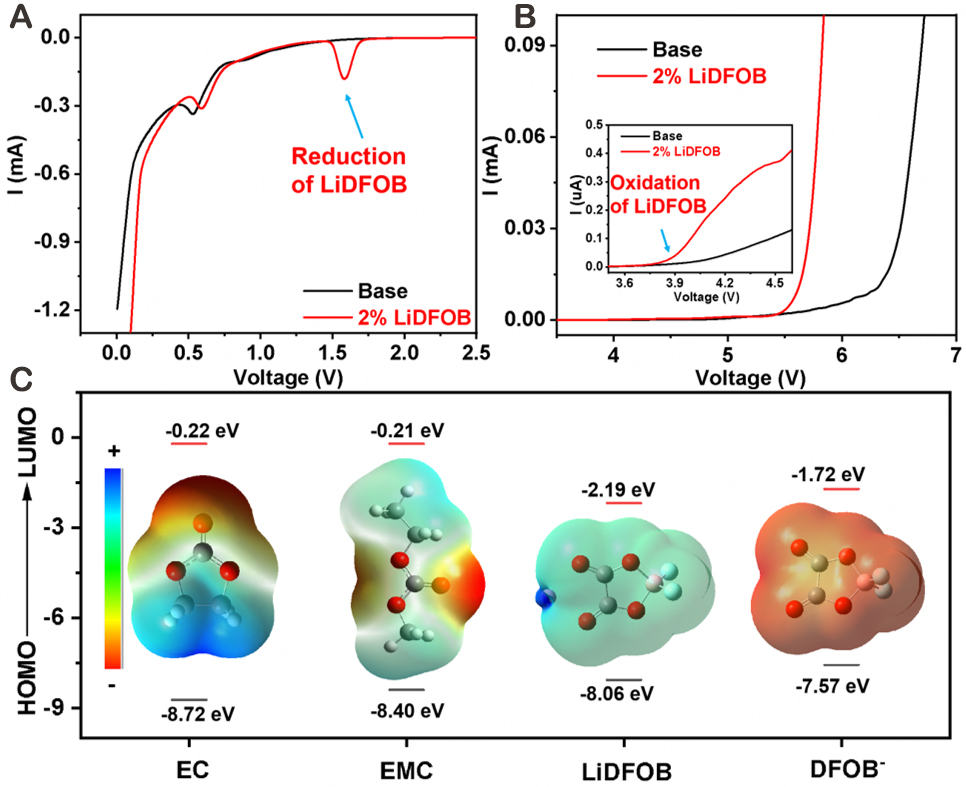
Li/graphite [Figure 1A] and Li/Pt [Figure 1B] cells were assembled to analyze the electrochemical stability of the electrolytes with and without LiDFOB additive, respectively. As shown in Figure 1A, an additional reduction peak at ~1.6 V can be observed before lithiation of graphite in the 2% LiDFOB-containing electrolyte, corresponding to the preferential reduction of LiDFOB compared to the solvent molecules. Besides, a significant negative shift in the onset decomposition potential observed in the LSV curves
Figure 1. LSV curves of (A) Li/graphite and (B) Li/Pt cells cycled in the electrolytes with & without LiDFOB additive. The inset is the enlarged view within the range of 3.5-4.6 V of (B); (C) Molecular orbital models and calculated HOMO/LUMO energy levels of EC, EMC, Li+-DFOB-, and DFOB-, respectively.
Effects of LiDFOB on Li anode
The effect of the LiDFOB additive on the reversibility of Li plating/stripping was first evaluated using Li||Cu and Li||Li cells. It can be noted from Figure 2A that the CE (evaluated by Aurbach’s method[65]) of the Li||Cu cells increases significantly from 53.95% to 83.82% with the addition of LiDFOB and is accompanied by a decreased nucleation overpotential at a current density of 0.5 mA cm-2, indicating that LiDFOB-derived SEI film can effectively regulate Li plating/stripping with the lower barrier and enhance the reversibility of active lithium. Besides, the cells with the prelithiated Cu foil deposited with 4.0 mAh cm-2 Li can stably cycle for more than 60 h in the LiDFOB-containing electrolyte, compared to the sudden voltage fluctuations after cycling for 15 h in the base electrolyte [Supplementary Figure 1]. The voltage fluctuations are related to interfacial instability during the Li platting/stripping process, which results in irreversible loss of active Li in the base electrolyte[65]. In comparison, the SEI film derived by the addition of LiDFOB is robust to guarantee a stable interface for the homogenous plating/stripping of Li. As a result, the Li||Li symmetric cells with the LiDFOB-containing electrolyte exhibited superior cyclic stability at either 1.0 mAh cm-2 or the higher platting/stripping capacity of 3.0 mAh cm-2 [Figure 2B and C]. Then, the morphology of the lithium metal after cycling was studied using SEM. As shown in Figure 2D, a sparse and needle-like morphology on the surface of the cycled Li anode in the base electrolyte is observed. This porous structure with a high surface area intensifies the reaction with electrolytes, thus resulting in continuous consumption of electrolytes and active lithium. Differently, a compact and planar structure is presented with the addition of LiDFOB [Figure 2E], further implying that adding LiDFOB can induce the homogeneous plating/stripping of the Li and significantly inhibit the growth of lithium dendrites.
Figure 2. (A) CE in Li||Cu cells by Aurbach’s test method with Li stripping/plating at 0.5 mA cm-2 and 1 mAh cm-2. The lithium plating/stripping behaviors of Li||Li symmetric cells at (B) 0.5 mA cm-2 and 1 mAh cm-2 and (C) 1 mA cm-2 and 3 mAh cm-2. SEM image of the Li anode after Li plating/stripping at 1 mA cm-2 and 3 mAh cm-2 in (D) the base electrolyte and (E) LiDFOB-contenting electrolytes. (F) Nyquist plots of Li||Li cells after cycling for 400 h at 1 mA cm-2 and 3 mAh cm-2 in the electrolyte with and without LiDFOB additive. (G) XPS spectra of O 1s, C 1s, F 1s, P 2p, and P 2s/B1s for the cycled Li anode in different electrolytes.
The interfacial impedance of the Li anode after cycling for ~400 h at 1 mA cm-2 with an areal capacity of
Protection of microstructure for Ni-rich cathode
To evaluate the compatibility of LiDFOB with Ni-rich cathodes, various concentrations of the LiDFOB additive were added to the base electrolyte and evaluated in Li||NCM85 batteries with the cut-off voltages of 4.3 V. As shown in Supplementary Figure 4, Li||NCM85 batteries show improved capacity retention with the addition of LiDFOB additive, from 85.5% (utilizing the base electrolyte) to 91.9 % (with 1 wt% LiDFOB-added electrolyte), 100% (with 2 wt% LiDFOB-added electrolyte), and 88.4% (with 3 wt% LiDFOB-added electrolyte). These results suggest that 2 wt% is the optimized content of LiDFOB for Li||NCM85 batteries. It can be noted from Figure 3A that the Li||NCM85 battery cycled in the 2 wt% LiDFOB-containing electrolyte demonstrates a notable initial CE of 85.7% and an average CE of 99.9% throughout the 6th cycle to the 200th cycle, surpassing those observed in the base electrolyte. These findings suggest that the CEI film derived from LiDFOB effectively mitigates interfacial parasitic reactions. Consequently, the capacity retention of the battery after 250 cycles is substantially higher at 90.9% compared to the base electrolyte at 64.6% [Figure 3B, vs. the discharge capacity at the 3rd cycle]. Accordingly, by comparing the GITT curves of the battery after the first and 150 cycles, the corresponding overpotentials, and Li+ diffusion coefficients (DLi+), the battery cycled in the base electrolyte exhibits a significantly increased overpotential, accompanied by a rapidly reduced DLi+ [Figure 3C and Supplementary Figure 5]. This may be related to the increasing electrode polarization due to the continuous accumulation of electrolyte parastic reaction products and the structural degradation of the NCM85 cathode[64]. In contrast, the NCM85 electrodes maintained a lower overpotential and a higher DLi+ during cycling with the addition of LiDFOB, which is attributed to the stable NCM85 interfacial structure in the presence of the LiDFOB-derived CEI film
Figure 3. Electrochemical performance of the Li||NCM85 batteries with the base electrolyte and LiDFOB-containing electrolyte. (A) Typical voltage profiles of Li||NCM85 batteries with different electrolytes. (B) Cyclic stability of batteries at 1 C in the voltage range of 3.0-4.3 V at selected electrolytes. GITT curves and corresponding overpotential of (C) charging process and (D) discharging process after 150 cycles. (E) Cyclic stability of batteries at 1 C with a cut-off voltage of 4.6 V at selected electrolytes. The dQ/dV curves of Li||NCM85 batteries (F) without and (G) with 2 wt% LiDFOB additive. Nyquist plots for batteries (H) after the first cycle and (I) after 300 cycles, respectively. The inset is the enlarged view of the high-frequency area in the red dotted box of (I). (J) Rate capability of Li||NCM85 batteries in the voltage range of 3.0-4.6 V. (K) Cyclic stability and coulombic efficiency of Li||NCM85 batteries at the charge/discharge rate of 2 C/5 C in the voltage range of 3.0-4.6 V.
Interfacial impedance was analyzed by EIS measurements to explore the interfacial evolution of the NCM85 electrode. The Nyquist plots of the battery mainly consist of two semicircles at high-to-medium frequency, corresponding to interfacial resistance (Rf) and charge transfer resistance (Rct), while the slope of the line at low frequency provides the Warburg impedance (Wo) associated with the diffusion of Li+[74,75]. The fitting results listed in Supplementary Table 3 were obtained via the equivalent circuit model (inset in Figure 3H). It is worth noting that both Rf and Rct values obtained from the battery cycled with the base electrolyte exhibit a rapid increase, which can be attributed to the overgrowth of the interfacial film with high resistance that is formed due to electrolyte decomposition and the exacerbated structural degradation at the near-surface of the NCM85 crystal [Figure 3H]. In contrast, the NCM85 cathode cycled in the LiDFOB-containing electrolyte exhibits a less resistant interface and faster Li+ transport kinetics with the slow growth of Rf and Rct [Figure 3I], indicating that the CEI film derived by LiDFOB can effectively mitigate the excessive decomposition of electrolyte and structural degradation at the near-surface of NCM85 crystal. The fast Li+ transport kinetics facilitates the rate performance of the battery. As shown in Figure 3J and K, the discharge capacity of the battery at 10 C increases significantly from 137 mAh g-1 to 176 mAh g-1 with the addition of LiDFOB. Besides, the battery cycled in the LiDFOB-containing electrolyte exhibits a high capacity retention of 51.6% after 1,000 cycles at the charge/discharge rate of 2 C/5 C, compared with capacity retention of only 6% after 1,000 cycles in the base electrolyte with a more rapid capacity decay during the first 300 cycles.
More details about the evolution of structure were investigated by XRD (in/ex-situ XRD). The evolution of the (003) peak in in-situ XRD is related to the variation of the unit cell along the c-axis direction[21,76]. It can be noted from the results of in-situ XRD [Figure 4A and B] that the interlayer spacing along the c-axis direction expands as Li+ is continuously extracted (with charging voltage ≤ 4.16 V) and responds by the shift of the (003) peak to a lower angle. When the charging potential is higher than 4.16 V, the sharp shift of the (003) peak to a high angle indicates a rapid contraction of the layer spacing along the c-axis, corresponding to the structural transformation from the H2 phase to the H3 phase, which leads to an increase in internal stress and structural degradation[77]. For the battery cycled in the LiDFOB-containing electrolyte, the evolution angle of the (003) peak during the transition from the H2 phase to the H3 phase is observed to be lower than that in the base electrolyte [Figure 4A and B], especially when the current rate increases from the initial 0.1 C to 0.2 C in the second cycle (1.0774° vs. 1.1820°). This result indicates that the LiDFOB-derived CEI film is robust enough to mitigate the irreversible structural degradation of the cathode, which could be beneficial for improving the cycling stability of the battery. Similar conclusions can be drawn by testing the structural evolution before and after long cycles by ex-situ XRD [Supplementary Figure 7]. The variation range of the (003) peak in the base electrolyte (0.35°) after 300 cycles is larger than that in LiDFOB-containing electrolyte (0.28°), suggesting the superior structural reversibility of the NCM85 cathode cycled in the LiDFOB-containing electrolyte.
Figure 4. In-situ XRD patterns during the first two cycles of Li||NCM85 batteries (A) without and (B) with LiDFOB. DEMS test of Li||NCM85 batteries charging to 4.6 V in (C) the base electrolyte and (D) 2 wt% LiDFOB-containing electrolyte.
The phase transition process at high voltage is often accompanied by the oxidation of lattice oxygen to form O-[78]. The high catalytic activity of O- and the released oxygen will exacerbate the electrolyte decomposition, accompanied by the generation of CO2[79]. Therefore, the CO2 content obtained by in-situ DEMS is used to monitor the gas evolution at the electrodes during cycling. It can be observed from Figure 4C that the battery cycled in the base electrolyte displays a sharply rising CO2 signal when the charging cut-off voltage is higher than 4.5 V, suggesting the intensification of the lattice oxygen loss and electrolyte decomposition. In comparison, the CO2 evolution in the LiDFOB-containing electrolyte is significantly suppressed, indicating that the introduction of LiDFOB can effectively stabilize lattice oxygen and suppress interfacial side reactions [Figure 4D].
The morphologies and interfacial components of the cycled NCM85 electrode in the base and LiDFOB-containing electrolytes were analyzed by the SEM (top-view and cross-section), TEM, and XPS, as shown in Figure 5. Compared with the fresh NCM85 electrode with an intact structure and clean and smooth surface [Figure 5A-C], severe interior cracks with partial crystal fragments can be observed on the particles after 200 cycles in the base electrolyte, and the electrode surface is also covered with an amorphous and inhomogeneous interfacial film with a thickness of about 30-60 nm [Figure 5D-F], which mainly results from the structural disruption of the NCM85 crystal and the deposition of electrolyte decomposition products. In contrast, the morphology of the NCM85 cathode cycled in LiDFOB-containing electrolyte is well maintained, and only a few cracks are discernible [Figure 5G and H]. Moreover, a relatively thin and uniform CEI film derived from the LiDFOB additive was observed to cover the surface of the NCM85, with a thickness of about 10 nm [Figure 5I], which can effectively protect the NCM85 electrode from electrolyte erosion and particle cracking. In C 1s spectra of the XPS spectra [Figure 5J], the peaks located at 290 eV, 288.5 eV, 286.5 eV, and 284.7 eV are assigned to the Li2CO3, C=O, C-O, and C-C species, respectively, which are mainly formed by the decomposition of organic solvent molecules (EC/EMC) in electrolyte[80-82]. The deposits of C-O and C=O can also be observed at 533.4 eV and 531.8 eV in the O 1s spectra. The peaks located at 685 eV and 686.8 eV in F 1s spectra are related to LiF and LixPOyFz/LixPFy, arising from the decomposition of lithium salt (LiPF6) in the electrolyte[71,83]. The intensity of these peaks, represented by the peak area [Supplementary Figure 8], is significantly lower in the LiDFOB-containing electrolyte compared to the base electrolyte, providing evidence for the effective suppression of severe electrolyte decomposition by the application of LiDFOB additive. In addition, additional B-F (684.5 eV in F 1s spectra and 192.7 eV in B 1s spectra) and B-O peaks (190.5 eV in B 1s spectra)[52,73] can only be observed in the LiDFOB-containing electrolyte, indicating the participation of LiDFOB in the construction of the protective CEI film. Furthermore, a peak signal was also detected in B 1s spectra on the NCM85 cathode surface cycled in the base electrolyte [Figure 5J], similar to B 1s spectra observed on the Li anode surface [Figure 2G]. However, SEM and energy dispersive spectrometer (EDS)-mapping results [Supplementary Figure 9] clearly indicate a significantly higher amount of P element on the NCM85 cathode surface cycled in the base electrolyte, while the presence of B element is negligible. Therefore, the observed B 1s signal primarily arises from the overlap with the P 2s spectra peak positions. The robust signal intensity observed at the M-O (530.3 eV) peak in the LiDFOB-containing electrolyte, relative to the base electrolyte, provides evidence for the formation of a thinner and protective CEI film on the NCM85 electrode. This observation is consistent with the findings from TEM. In fact, the formation of a thinner CEI film has some advantages as it enables faster Li+ transport in CEI film and reduced interfacial resistances compared to the progressively thickening deposits resulting from the decomposition of the base electrolyte. This conclusion is also supported by the results that the battery cycled in the LiDFOB-containing electrolyte exhibits significant improvement in rate performance than in the base electrolyte [Figure 3J and K].
Figure 5. (A, D, G) Top-view, (B, E, H) cross-section SEM and (C, F, I) TEM images of (A-C) fresh and (D-I) cycled NCM85 electrodes after 200 cycles in the base and LiDFOB-containing electrolytes. (J) XPS spectra of C1s, O1s, F1s, and P 2s/B1s for the cycled NCM85 electrodes in the base and LiDFOB-containing electrolytes.
Mechanism of the LiDFOB on SEI/CEI film formation
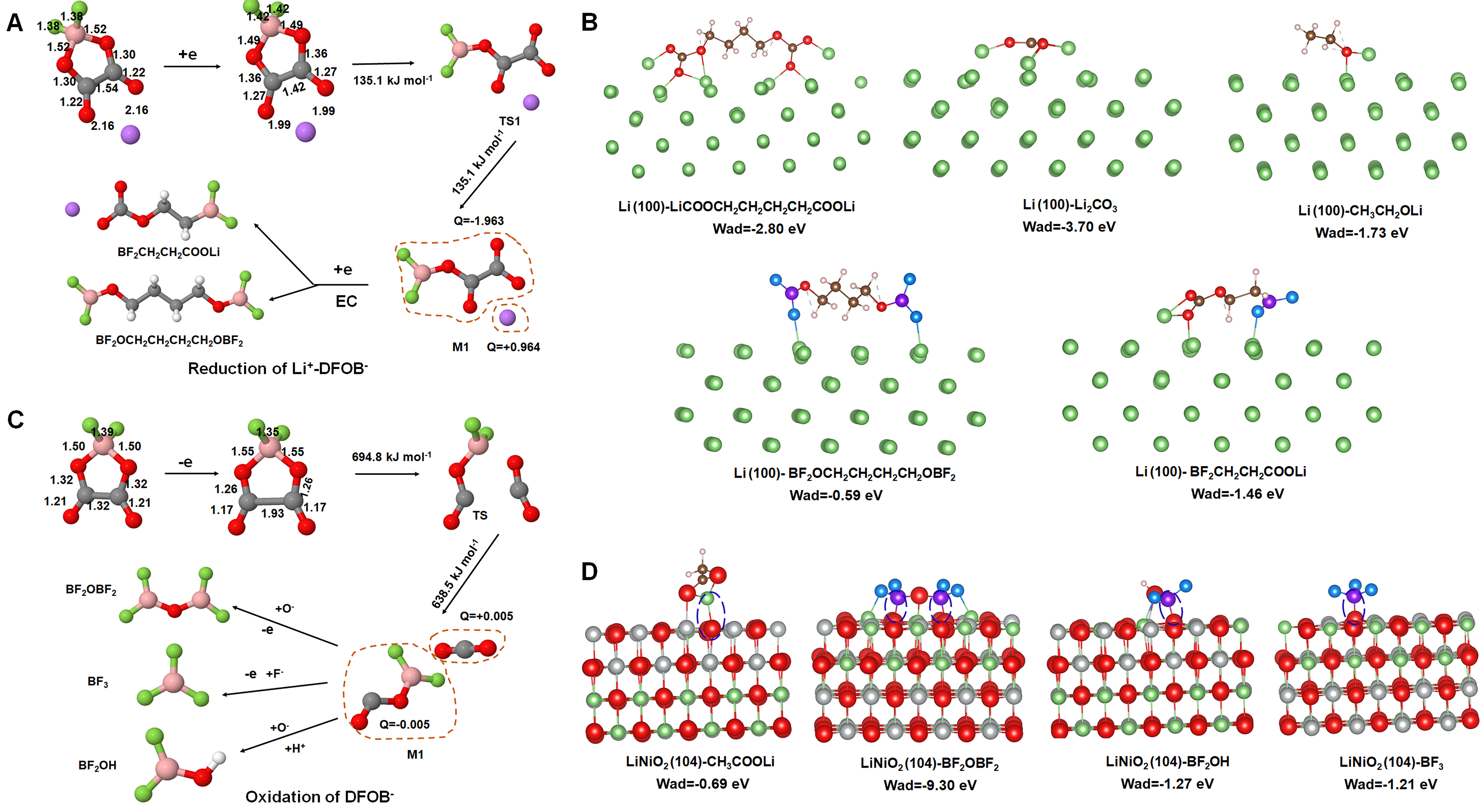
The possible redox decomposition pathways and the corresponding decomposition products of solvent (EC) and additive molecule (DFOB-) were investigated by density functional theory (DFT) calculation. It shows that the preferential reduction of DFOB- triggers the decomposition of EC on the anode side [Figure 6A]. Under the synergistic effect of the two, BF2CH2CH2COOLi and BF2OCH2CH2CH2CH2OBF2 polymers are formed, which are the main components of the SEI film, as confirmed by the B-O-C and B-F peaks detected in XPS [Figure 2G]. The reduction potentials of these polymers are comparable to those of the Li2CO3, LiOOC(CH2)4COOLi, and CH3CH2OLi products generated by the reduction decomposition of EC in the base electrolyte [Supplementary Figures 10A and 11], all of which exhibit good electrochemical stability on Li anode. While the higher LUMO-HOMO energy gaps (6.55 eV and 8.73 eV,
Figure 6. The formation mechanism of SEI and CEI films in LiDFOB-containing electrolyte by DFT calculation. (A) Reduction pathways of EC and Li+-DFOB- and corresponding decomposition products on the anode side. (B) The adsorption energy of decomposition products on Li (100) surface. (C) Oxidation pathways of EC and DFOB- and corresponding decomposition products on the cathode side, searched by DFT. (D) The adsorption energy of decomposition products on the LiNiO2 (104) surface.
To further identify the composition and depth distribution of the SEI and CEI films, TOF-SIMS tests were performed for the Li anode [Figure 7A and B] and NCM85 cathodes [Figure 7C and D] after 20 cycles in the base and LiDFOB-containing electrolytes, respectively. The fragments of CH2-, LiO2-, and LiCO3- are mainly derived from the redox decomposition products of carbonate-based solvents in the electrolytes, such as lithium alkyl esters and Li2CO3. The OH- and LiF2- and NiF2- fragments are by-products of the decomposition of the electrolyte or the corrosion of the electrodes by HF, respectively. It can be noted from the depth profiles [Figure 7A-D] that the intensity of these species on both electrodes cycled in the base electrolyte is higher than that in the LiDFOB-containing electrolyte, indicating that the introduction of LiDFOB can effectively suppress the decomposition of electrolytes and the corrosion of HF to both electrodes. In addition, compared to the electrodes cycled in the base electrolyte, the electrodes cycled in LiDFOB-containing electrolyte show significantly enhanced BF2O- fragment signals, suggesting that a stable and BF2-rich interfacial film (SEI/CEI film) is constructed with the participation of LiDFOB on both the Li anode and NCM85 cathode surface, which is consistent with the theoretical calculation and XPS results.
CONCLUSIONS
In this study, the combination of computational and experimental results provides a comprehensive understanding of the working mechanisms of LiDFOB on suppressing the decomposition of electrolyte and stabilizing electrode structure in high voltage Li||NCM85 batteries. Based on the experimental results, it is evident that LiDFOB exhibits preferential reduction and oxidation decomposition on the surfaces of both Li anode and NCM85 cathode, leading to the formation of B-O-C, B-F bonds-containing SEI film and B-O,
DECLARATIONS
Acknowledgments
The authors thank Ningbo Ronbay Technology Co., Ltd. for kindly supplying the cathode material.
Authors’ contributions
Manuscript wrting: Yang X, Huang Y
Manuscript revision: Gong Z, Yang Y
All authors have given approval to the final version of the manuscript.
Availability of data and materials
The data supporting our findings can be found in the Supplementary Materials.
Financial support and sponsorship
This work was financially supported by the National Key R&D Program of China (Grant No. 2021YFB2401800) and the National Natural Science Foundation of China (Grant Nos. 21935009, 22261160570, 22032004, and 22279108).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2023.
Supplementary Materials
REFERENCES
2. Blomgren GE. The development and future of lithium ion batteries. J Electrochem Soc 2017;164:A5019.
3. Kim T, Song W, Son D, Ono LK, Qi Y. Lithium-ion batteries: outlook on present, future, and hybridized technologies. J Mater Chem A 2019;7:2942-64.
5. Scrosati B, Hassoun J, Sun Y. Lithium-ion batteries. A look into the future. Energy Environ Sci 2011;4:3287-95.
7. Choi JU, Voronina N, Sun Y, Myung S. Recent progress and perspective of advanced high-energy Co-less Ni-rich cathodes for Li-ion Batteries: yesterday, today, and tomorrow. Adv Energy Mater 2020;10:2002027.
8. Jiang F, Yang S, Liu H, et al. Mechanism understanding for stripping electrochemistry of Li metal anode. SusMat 2021;1:506-36.
9. Shi P, Zhang X, Shen X, Zhang R, Liu H, Zhang Q. A review of composite lithium metal anode for practical applications. Adv Mater Technol 2020;5:1900806.
10. Liu H, Sun X, Cheng X, et al. Working principles of lithium metal anode in pouch cells. Adv Energy Mater 2022;12:2202518.
11. Yin S, Deng W, Chen J, et al. Fundamental and solutions of microcrack in Ni-rich layered oxide cathode materials of lithium-ion batteries. Nano Energy 2021;83:105854.
12. Yan P, Zheng J, Liu J, et al. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat Energy 2018;3:600-5.
13. Gao Y, Wang X, Geng J, Liang F, Chen M, Zou Z. Research progress on the failure mechanisms and modifications of Ni-rich ternary layered oxide cathode materials for lithium-ion batteries. J Electron Mater 2023;52:72-95.
14. de Biasi L, Schwarz B, Brezesinski T, Hartmann P, Janek J, Ehrenberg H. Chemical, structural, and electronic aspects of formation and degradation behavior on different length scales of Ni-rich NCM and Li-rich HE-NCM cathode materials in Li-ion Batteries. Adv Mater 2019;31:e1900985.
15. Geldasa FT, Kebede MA, Shura MW, Hone FG. Identifying surface degradation, mechanical failure, and thermal instability phenomena of high energy density Ni-rich NCM cathode materials for lithium-ion batteries: a review. RSC Adv 2022;12:5891-909.
16. Li S, Yao Z, Zheng J, et al. Direct observation of defect-aided structural evolution in a nickel-rich layered cathode. Angew Chem Int Ed 2020;59:22092-9.
17. Sun J, Cao X, Zhou H. Advanced single-crystal layered Ni-rich cathode materials for next-generation high-energy-density and long-life Li-ion batteries. Phys Rev Mater 2022;6:070201.
18. Wang X, Ruan X, Du CF, Yu H. Developments in surface/interface engineering of Ni-rich layered cathode materials. Chem Rec 2022;22:e202200119.
19. Niu C, Liu D, Lochala JA, et al. Balancing interfacial reactions to achieve long cycle life in high-energy lithium metal batteries. Nat Energy 2021;6:723-32.
20. Yoon M, Dong Y, Hwang J, et al. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries. Nat Energy 2021;6:362-71.
21. Ryu H, Park K, Yoon CS, Sun Y. Capacity fading of Ni-rich Li[NixCoyMn1-x-y]O2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: bulk or surface degradation? Chem Mater 2018;30:1155-63.
22. Zhang C, Wan J, Li Y, et al. Restraining the polarization increase of Ni-rich and low-Co cathodes upon cycling by Al-doping. J Mater Chem A 2020;8:6893-901.
23. Zhu Z, Yu D, Shi Z, et al. Gradient-morph LiCoO2 single crystals with stabilized energy density above 3400 Wh L−1. Energy Environ Sci 2020;13:1865-78.
24. Wang Y, Zhang Q, Xue Z, et al. An in situ formed surface coating layer enabling LiCoO2 with stable 4.6 V high-voltage cycle performances. Adv Energy Mater 2020;10:2001413.
25. Wang X, Wu Q, Li S, et al. Lithium-aluminum-phosphate coating enables stable 4.6 V cycling performance of LiCoO2 at room temperature and beyond. Energy Stor Mater 2021;37:67-76.
26. Yang X, Wang C, Yan P, et al. Pushing lithium cobalt oxides to 4.7 V by lattice-matched interfacial engineering. Energy Stor Mater 2022;12:2200197.
27. Wang L, Liu T, Wu T, Lu J. Strain-retardant coherent perovskite phase stabilized Ni-rich cathode. Nature 2022;611:61-7.
28. Li J, Zhou Z, Luo Z, et al. Microcrack generation and modification of Ni-rich cathodes for Li-ion batteries: a review. Sustain Mater Technol 2021;29:e00305.
29. Liang L, Zhang W, Zhao F, et al. Surface/interface structure degradation of Ni-Rich layered oxide cathodes toward lithium-ion batteries: fundamental mechanisms and remedying strategies. Adv Mater Interfaces 2020;7:1901749.
30. Zhang C, Jiang W, He W, Wei W. Heteroepitaxial interface of layered cathode materials for lithium ion batteries. Energy Stor Mater 2021;37:161-89.
31. Zhang J, Li Q, Ouyang C, et al. Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V. Nat Energy 2019;4:594-603.
32. Zhu Z, Wang H, Li Y, et al. A surface Se-substituted LiCo[O2-δSeδ] cathode with ultrastable high-voltage cycling in pouch full-cells. Adv Mater 2020;32:e2005182.
33. Wang L, Ma J, Wang C, et al. A novel bifunctional self-stabilized strategy enabling 4.6 V LiCoO2 with excellent long-term cyclability and high-rate capability. Adv Sci 2019;6:1900355.
34. Jiang M, Danilov DL, Eichel R, Notten PHL. A review of degradation mechanisms and recent achievements for Ni-rich cathode-based Li-ion batteries. Adv Energy Mater 2021;11:2103005.
35. Liu J, Wang J, Ni Y, Zhang K, Cheng F, Chen J. Recent breakthroughs and perspectives of high-energy layered oxide cathode materials for lithium ion batteries. Mater Today 2021;43:132-65.
36. Ni L, Zhang S, Di A, et al. Challenges and strategies towards single-crystalline Ni-rich layered cathodes. Adv Energy Mater 2022;12:2201510.
37. Wang X, Ding Y, Deng Y, Chen Z. Ni-rich/Co-poor layered cathode for automotive Li-ion batteries: promises and challenges. Adv Energy Mater 2020;10:1903864.
38. Chen H, Pei A, Wan J, et al. Tortuosity effects in lithium-metal host anodes. Joule 2020;4:938-52.
39. Liu W, Lin D, Pei A, Cui Y. Stabilizing lithium metal anodes by uniform Li-ion flux distribution in nanochannel confinement. J Am Chem Soc 2016;138:15443-50.
40. Jie Y, Ren X, Cao R, Cai W, Jiao S. Advanced liquid electrolytes for rechargeable Li metal batteries. Adv Funct Mater 2020;30:1910777.
42. Zhang JG, Xu W, Xiao J, Cao X, Liu J. Lithium metal anodes with nonaqueous electrolytes. Chem Rev 2020;120:13312-48.
43. Yang X, Xu N, Liu G, et al. Modification and regulation of electrode/electrolyte interface for high specific energy and long life lithium ion batteries. Chin Sci Bull 2021;66:1170-86.
44. Zhao W, Ji Y, Zhang Z, et al. Recent advances in the research of functional electrolyte additives for lithium-ion batteries. Curr Opin Electrochem 2017;6:84-91.
45. Li L, Fu L, Li M, et al. B-doped and La4NiLiO8-coated Ni-rich cathode with enhanced structural and interfacial stability for lithium-ion batteries. J Energy Chem 2022;71:588-94.
46. Birrozzi A, Laszczynski N, Hekmatfar M, von Zamory J, Giffin GA, Passerini S. Beneficial effect of propane sultone and tris(trimethylsilyl) borate as electrolyte additives on the cycling stability of the lithium rich nickel manganese cobalt (NMC) oxide. J Power Sources 2016;325:525-33.
47. Cha J, Han J, Hwang J, Cho J, Choi N. Mechanisms for electrochemical performance enhancement by the salt-type electrolyte additive, lithium difluoro(oxalato)borate, in high-voltage lithium-ion batteries. J Power Sources 2017;357:97-106.
48. Cheng F, Zhang X, Wei P, et al. Tailoring electrolyte enables high-voltage Ni-rich NCM cathode against aggressive cathode chemistries for Li-ion batteries. Sci Bull 2022;67:2225-34.
49. Cui Y, Liang X, Chai J, et al. High performance solid polymer electrolytes for rechargeable batteries: a self-catalyzed strategy toward facile synthesis. Adv Sci 2017;4:1700174.
50. Ehteshami N, Ibing L, Stolz L, Winter M, Paillard E. Ethylene carbonate-free electrolytes for Li-ion battery: study of the solid electrolyte interphases formed on graphite anodes. J Power Sources 2020;451:227804.
51. Han J, Lee JB, Cha A, et al. Unsymmetrical fluorinated malonatoborate as an amphoteric additive for high-energy-density lithium-ion batteries. Energy Environ Sci 2018;11:1552-62.
52. Hu M, Wei J, Xing L, Zhou Z. Effect of lithium difluoro(oxalate)borate (LiDFOB) additive on the performance of high-voltage lithium-ion batteries. J Appl Electrochem 2012;42:291-6.
53. Jurng S, Brown ZL, Kim J, Lucht BL. Effect of electrolyte on the nanostructure of the solid electrolyte interphase (SEI) and performance of lithium metal anodes. Energy Environ Sci 2018;11:2600-8.
54. Liu S, Zhang Q, Wang X, Xu M, Li W, Lucht BL. LiFSI and LiDFBOP dual-salt electrolyte reinforces the solid electrolyte interphase on a lithium metal anode. ACS Appl Mater Interfaces 2020;12:33719-28.
55. Xia L, Lee S, Jiang Y, Xia Y, Chen GZ, Liu Z. Fluorinated electrolytes for Li-ion batteries: the lithium difluoro(oxalato)borate additive for stabilizing the solid electrolyte interphase. ACS Omega 2017;2:8741-50.
56. Ji Y, Li S, Zhong G, et al. Synergistic effects of suberonitrile-LiBOB binary additives on the electrochemical performance of high-voltage LiCoO2 electrodes. J Electrochem Soc 2015;162:A7015.
57. Sun Y, Tao M, Zou Y, et al. 2,2,5,5-tetramethyl-2,5-disila-1-oxacyclopentane as a bifunctional electrolyte additive for Ni-rich
58. Zhang X, Liu G, Zhou K, et al. Enhancing cycle life of nickel-rich LiNi0.9Co0.05Mn0.05O2 via a highly fluorinated electrolyte additive-pentafluoropyridine. Energy Mater 2022;1:100005.
59. Zhao W, Zheng G, Lin M, et al. Toward a stable solid-electrolyte-interfaces on nickel-rich cathodes: LiPO2F2 salt-type additive and its working mechanism for LiNi0.5Mn0.25Co0.25O2 cathodes. J Power Sources 2018;380:149-57.
60. Zhao W, Zheng B, Liu H, et al. Toward a durable solid electrolyte film on the electrodes for Li-ion batteries with high performance. Nano Energy 2019;63:103815.
61. Zhang B, Wang L, Wang X, et al. Sustained releasing superoxo scavenger for tailoring the electrode-electrolyte interface on Li-rich cathode. Energy Stor Mater 2022;53:492-504.
62. Chen J, Peng Y, Yin Y, et al. High energy density Na-metal batteries enabled by a tailored carbonate-based electrolyte. Energy Environ Sci 2022;15:3360-8.
63. Mao M, Huang B, Li Q, Wang C, He Y, Kang F. In-situ construction of hierarchical cathode electrolyte interphase for high performance LiNi0.8Co0.1Mn0.1O2/Li metal battery. Nano Energy 2020;78:105282.
64. Wang C, Li X, Zhao Y, et al. Manipulating interfacial nanostructure to achieve high-performance all-solid-state lithium-ion batteries. Small Methods 2019;3:1900261.
65. Zhou P, Xia Y, Hou WH, et al. Rationally designed fluorinated amide additive enables the stable operation of lithium metal batteries by regulating the interfacial chemistry. Nano Lett 2022;22:5936-43.
66. Adams BD, Zheng J, Ren X, Xu W, Zhang J. Accurate determination of coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv Energy Mater 2018;8:1702097.
67. Hafner J. Ab-initio simulations of materials using VASP: density-functional theory and beyond. J Comput Chem 2008;29:2044-78.
68. Wu B, Liu Q, Mu D, et al. Suppression of lithium dendrite growth by introducing a low reduction potential complex cation in the electrolyte. RSC Adv 2016;6:51738-46.
69. Chen J, Xing L, Yang X, Liu X, Li T, Li W. Outstanding electrochemical performance of high-voltage LiNi1/3Co1/3Mn1/3O2 cathode achieved by application of LiPO2F2 electrolyte additive. Electrochim Acta 2018;290:568-76.
70. Cao Z, Zheng X, Qu Q, Huang Y, Zheng H. Electrolyte design enabling a high-safety and high-performance Si anode with a tailored electrode-electrolyte interphase. Adv Mater 2021;33:e2103178.
71. Li Q, Wang Y, Wang X, et al. Investigations on the fundamental process of cathode electrolyte interphase formation and evolution of high-voltage cathodes. ACS Appl Mater Interfaces 2020;12:2319-26.
72. Fan X, Ji X, Han F, et al. Fluorinated solid electrolyte interphase enables highly reversible solid-state Li metal battery. Sci Adv 2018;4:eaau9245.
73. Parimalam BS, Lucht BL. Reduction reactions of electrolyte salts for lithium ion batteries: LiPF6, LiBF4, LiDFOB, LiBOB, and LiTFSI. J Electrochem Soc 2018;165:A251.
74. Yang X, Lin M, Zheng G, et al. Enabling stable high-voltage LiCoO2 operation by using synergetic interfacial modification strategy. Adv Funct Mater 2020;30:2004664.
75. Yang X, Tang S, Zheng C, et al. From contaminated to highly lithiated interfaces: a versatile modification strategy for garnet solid electrolytes. Adv Funct Mater 2023;33:2209120.
76. Han Y, Jung SH, Kwak H, et al. Single- or poly-crystalline Ni-Rich layered cathode, sulfide or halide solid electrolyte: which will be the winners for all-solid-state batteries? Adv Energy Mater 2021;11:2100126.
77. Ryu H, Namkoong B, Kim J, Belharouak I, Yoon CS, Sun Y. Capacity fading mechanisms in Ni-Rich single-crystal NCM cathodes. ACS Energy Lett 2021;6:2726-34.
78. Yan P, Zheng J, Tang ZK, et al. Injection of oxygen vacancies in the bulk lattice of layered cathodes. Nat Nanotechnol 2019;14:602-8.
79. Zheng W, Shi C, Dai P, et al. A functional electrolyte additive enabling robust interphases in high-voltage Li‖LiNi0.8Co0.1Mn0.1O2 batteries at elevated temperatures. J Mater Chem A 2022;10:21912-22.
80. Ma Y, Chen K, Ma J, et al. A biomass based free radical scavenger binder endowing a compatible cathode interface for 5 V lithium-ion batteries. Energy Environ Sci 2019;12:273-80.
81. Dupin J, Gonbeau D, Vinatier P, Levasseur A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys Chem Chem Phys 2000;2:1319-24.
82. Pereira-nabais C, Światowska J, Chagnes A, et al. Interphase chemistry of Si electrodes used as anodes in Li-ion batteries. Appl Surf Sci 2013;266:5-16.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Yang X, Huang Y, Li J, Huang W, Yang W, Wu C, Tang S, Ren F, Gong Z, Zhou N, Yang Y. Understanding of working mechanism of lithium difluoro(oxalato) borate in Li||NCM85 battery with enhanced cyclic stability. Energy Mater 2023;3:300029. http://dx.doi.org/10.20517/energymater.2023.10
AMA Style
Yang X, Huang Y, Li J, Huang W, Yang W, Wu C, Tang S, Ren F, Gong Z, Zhou N, Yang Y. Understanding of working mechanism of lithium difluoro(oxalato) borate in Li||NCM85 battery with enhanced cyclic stability. Energy Materials. 2023; 3(4): 300029. http://dx.doi.org/10.20517/energymater.2023.10
Chicago/Turabian Style
Yang, Xuerui, Yaxin Huang, Jianhui Li, Weilin Huang, Wen Yang, Changquan Wu, Shijun Tang, Fucheng Ren, Zhengliang Gong, Naigen Zhou, Yong Yang. 2023. "Understanding of working mechanism of lithium difluoro(oxalato) borate in Li||NCM85 battery with enhanced cyclic stability" Energy Materials. 3, no.4: 300029. http://dx.doi.org/10.20517/energymater.2023.10
ACS Style
Yang, X.; Huang Y.; Li J.; Huang W.; Yang W.; Wu C.; Tang S.; Ren F.; Gong Z.; Zhou N.; Yang Y. Understanding of working mechanism of lithium difluoro(oxalato) borate in Li||NCM85 battery with enhanced cyclic stability. Energy Mater. 2023, 3, 300029. http://dx.doi.org/10.20517/energymater.2023.10
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 34 clicks
Cite This Article 34 clicks


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.