Research on carbon-based and metal-based negative electrode materials via DFT calculation for high potassium storage performance: a review
Abstract
The key R&D concern in the domain of new energy in recent years has been the large-scale development of electrochemical energy storage. However, the steep increase in pricing has constrained the further expansion of lithium-ion batteries, primarily due to the ongoing depletion of their scarce lithium supplies. A potential candidate material at the moment is the potassium-ion battery (KIB), which has an anode made of carbon and/or an alloy and rich reserves, offering an excellent theoretical capacity and ideal working voltage. More significant advancements are still required to achieve long life and high energy density, despite the fact that some significant breakthroughs have been reported. The most recent findings from research on carbon-based [graphite, hard carbon (HC), and nanoporous carbon] and alloy-based (mainly including Sb, Sn, P, and its compounds) anodes for KIBs are compiled in this document. Numerous simulations at the atomic level based on particular chemical interactions, phase transitions, ion/electron transport dynamics, and conduction band spin utilizing density functional theory (DFT) calculations have been conducted to thoroughly investigate the storage mechanism of K+ on various electrode materials. Moreover, this paper examined contemporary structural modification techniques used in carbon- and alloy-based anode electrode materials and applied DFT calculations to confirm the advancement of its thorough tests. To promote the manufacturing of rechargeable KIBs, the challenges and potential of KIBs were also explored in future research.
Keywords
INTRODUCTION
The massive consumption of fossil fuels and the growing environmental problems have prompted the rapid growth of the demand for clean energy in human society[1,2]. Therefore, it is imperative to develop secondary batteries to promote the progress of large-scale energy storage technology and realize the efficient use of energy[3]. Lithium-ion batteries (LIBs), as secondary batteries, have been commercialized in electric vehicles, energy storage, and other fields due to their high reversible specific capacity, cycle stability, and other advantages[4]. However, lithium resources in the earth are low in reserves and expensive, which is not suitable for large-scale energy storage devices. In various alternative battery systems, the abundant reserves of sodium (Na) and potassium (K) have become research hotspots due to their chemical properties and working principles similar to LIBs. However, as an alkali metal, K-ion batteries (KIBs) have faced more competition than LIBs and sodium-ion batteries (NIBs) due to the low-cost and suitable standard potential of K/K+, which has been extensively investigated and considered as the promising energy storage technology.
For KIBs, the most outstanding benefit is the low redox potential of K/K+, which gives rise to a high energy density and makes it possible to design high output voltage[5]. In addition, due to a much larger ionic radius of K+ (0.138 nm) compared to Li+ (0.076 nm) and Na+ (0.102 nm), KIBs exhibit low charge density and Lewis acidity, resulting in weak interactions and low desolvent activation energy. But in addition to allowing for high ionic transport, weak interactions between K+ ions and solvent molecules with smaller Stokes radii can also result in fast rates due to lower desolvent activation energies. Thanks to the aforementioned advantages, anode materials for KIBs, such as carbon-based anodes, alloys, oxides, sulfides, selenides, and more, exhibit excellent potassium storage capacity and cycling stability.
In the past few decades, systematic research on KIBs has become the main focus of researchers both domestically and internationally. As the next alkali metal after sodium, potassium has a lower oxidation-reduction potential. Since KIBs work at a higher potential, it is conducive to improving energy density. In addition, there is a weak interaction between K+ and solvent molecules with a smaller Stokes radius, and a lower desolvent activation energy can improve the ion transport rate. On the other hand, the intercalation/detachment mechanism of K+ with graphite is believed to be similar to lithium, mainly through the formation of graphite intercalation compounds in stages. With the increase of K concentration, KC8 is ultimately formed[6]. Among them, anode materials, as an important component of KIBs, including carbon materials [graphene, carbon microspheres, carbon nanofibers (CNFs)], metal alloys, oxides, sulfides, and other materials[7], have been proven to contribute to the development of high-performance KIBs [Figure 1A]. In terms of cost [Figure 1B], carbon-based materials stand out among a group of candidate materials due to their advantage of very low cost, becoming one of the preferred anode materials for commercial KIBs. Unfortunately, the larger ionic radius makes it more difficult for K+ to be embedded in the electroactive material, resulting in a slow kinetic behavior of the K+ reaction in the solid electrode. Controlling the size of the material and preparing nanostructured materials can effectively improve the K+ reaction kinetics and further enhance the rate capability[8]. On the other hand, the insertion/removal of large-sized K+ can easily cause significant volume expansion of the electrode material during the potassium process[9]. Thus, researchers continuously attempt to design and optimize electrode materials, electrolytes, and electrode components and structures in a reasonable manner[10,11].
Figure 1. (A) Overview of the proposed architectures for serving as the anodes of PIBs. (B) Price comparison of various metals for potential applications in PIBs. Copyright 2021, Nano Research[7].
Moreover, the comprehension and identification of potassium storage materials can be assisted by simulations[12,13]. In this review, the following quantities of interest can be calculated via systematic density functional theory (DFT) computations[14,15]: the structure-property correlation, interfacial stability, phase transition, K+ adsorption and diffusion mechanisms of electrode materials, along with spin correlation effects triggered by some metal atom doping[16,17]. Since the creation of KIBs, there has been a lot of emphasis on the development of ultra-high-performance anodes, such as intercalation type carbonaceous-based anodes manufactured of graphite, HC, and soft carbon, as well as alloy-based materials made of transition metal nitrides, oxides, and sulfides. From this perspective, we examine the modeling literature for two significant groups of potassium storage materials, carbon-based systems and alloy-based systems. Finally, we present a future outlook in anode material development for KIBs.
CARBON-BASED ANODES FOR KIBS
Anode materials, as critical components of KIBs, directly affect the battery performance, including operating potential and cyclability. Owing to a significantly higher ionic radius of K+, seeking appropriate potential electrode materials has become crucial for the development of KIBs. Among them, carbon-based materials have been successfully used as anode materials in KIBs due to their plentiful sources, environmentally friendly nature, low cost, and good chemical inertia, which is due to the similar physical and chemical properties of lithium and potassium. In this section, we divided anode materials into three categories according to the storage mechanisms of K+, which consist of graphite-based anodes, HC-based anodes, and nanoporous carbon composite anodes.Graphite-based anode for KIBs.
Nowadays, graphite has been the dominant supplier for LIB anodes attributed to its low cost, low
At the same time, plenty of literature indicated that graphite can intercalate potassium, which prompted more studies into the creation of KIBs. Furthermore, Komaba et al. have demonstrated the production of KC8 GICs in stage I based on the potassium reaction of graphite in carbonate-based electrolytes, which can provide a high specific capacity of 244 mAh/g[27]. In 2015, Jian et al. conducted the first research focusing on the electrolytic K+ insertion behavior in graphite for non-aqueous electrolytes[9]. The (de-)potassiation mechanism in graphite was revealed and shown in Figure 2A, and the quasi-equilibrium potentials of
Figure 2. (A) Galvanostatic intermittent titration technique (GITT) curves of graphite at C/10. (B) Galvanostatic potassiation/depotassiation (GPD) profiles of graphite for the initial two cycles at C/40. (C) Ex-situ XRD patterns of (de-)potassiation of graphite corresponding to the voltage points in (D). (D) The first charge-discharge curve of graphite at C/10. (E) Schematic diagram of different K-GICs. Copyright 2015, American Chemical Society[9].
Ex situ X-ray diffraction (XRD) has been employed to support the potassium storage mechanism in graphite, which contains the formation of several stages of K-GICs, including stage-3 KC36, stage-2 KC24, and stage-1 KC8 [Figure 2C]. The number of graphene layers that separate the neighboring layer of the
Through DFT calculations, a related investigation on K+ insertion into graphite reveals a distinct manner for potassium insertion. Despite the fact that intercalation happens within three phases, Luo et al. posits that the development of stage-III (KC24) accompanied by stage-II (KC16) and stage-I (KC8) is firmer than that of stage-III (KC36) to stage-II (KC24) and stage-I (KC8), which is similar to lithium [Figure 3A][29]. It is vital to recognize that this is the first time to produce KC8 at room temperature utilizing an electrochemical approach. Figure 3B shows a rather smooth potential slope ranging from 0.35 V to 0.18 V, corresponding to a capacity of only 93 mA h/g, which is connected to the stage-III (KC24). A closer look reveals that the conversion of stage-II (KC16) occurs at another shift around 0.14 V. Roughly 270 mA h/g is the whole first intercalation capacity, which points to the synthesis of stage-I (KC8). These results seem to declare that the stage of K intercalation into graphite behaves similarly to that of Li intercalation. According to DFT analysis, the most stable stoichiometry is KC8. As shown in Figure 3C, the potential must be negative to produce potassium metal; hence KC6 cannot form in the potential window (2-0.01 V vs. K+/K). When examining the staging process Stage-II (KC24), Stage-I (KC8) with results to DFT calculation (green line), we find that not just Stage-II (KC24) (theory b) about 0.1 eV less solid than Stage-III (KC24) (theory a), but the potential profile also exhibits a higher voltage from Stage-II (KC24) to Stage-I KC8, which is not observed in the experiment. Conversely, the energy grading mechanism from stage-III (KC24) to stage-II (KC16) and then to stage-I (KC8) can maximize the separation of coulomb repulsion between ions (blue line). This fits the prospective profile we discovered through our experiments extremely well (black solid line and averaged red dotted line). Nevertheless, plenty of efforts have been devoted to solving challenges for KIBs, such as serious volume expansion and high irreversible capacity in the initial cycles due to large ionic radius. In 2022,
Figure 3. (A) Diagram illustrating the several stages of K-intercalated graphite, with K represented in blue and C shown in yellow. (B) The charge-discharge curves of K+ and Li+ embedded in graphite at ambient temperature and pressure, respectively. (C) Potential distribution of K+ embedded in graphite at different stages based on DFT calculation. The interlayer stage is represented by the blue line [Theory (a)]: Stage-III (KC24) → Stage-II (KC16) → Stage-I (KC8). The dotted green line [Theory (b)] represents the computed value of the phase that was previously reported: Stage-II (KC24) → Stage- I (KC8). The red dotted line is used to correct the capacitance contribution formed by SEI. Copyright 2015, Nano Letters[29]. (D) Schematic diagram of the potassium/dipotassium process in the GDY layer. Copyright 2022, ACS Nano[30]. (E) Schematic illustration of the preparation process for MG. Copyright 2021, Carbon[31].
Graphite has been successfully discovered and marketed as an anode for LIBs. However, the insertion and removal of K+ during the electrochemical process limit the practical application of KIBs to poor cycling stability and severe volume expansion. Therefore, it is urgent to develop new modification strategies to improve the stability of graphite anodes. Hence, Capone et al. reported red phosphorus (RP)-graphite composite manufactured by a two-step ball-milling process in order to regulate particle size and maximize the carbon coating[32]. Raman measurements suggest that the volume expansion of RP particles causes the carbon covering to reversibly expand and shrink. Then, Ma et al. used triphenylphosphine and graphite oxide to prepare active phosphorus (P) and O double-doped graphene (PODG) for KIB anodes by a thermal annealing process[33]. Due to larger interlayer spacing, which accelerates the insertion and extraction of K+, PODG exhibits high specific capacity and ultra-long cycling stability. In addition, the electrochemical performance of KIB graphite carbon anodes regulated by defect engineering has been further confirmed by DFT calculations. The preferred technique is nitrogen (N) doping since cutting-edge N dopants may efficiently increase the adsorption energy towards K+ ions. A number of modification strategies for N-doped carbon-based materials have been applied over the last five years to create KIBs with superior K+ storage performance. In order to establish a special symbiosis between great reversible K ion storage capacity and excellent recycling capacity, Lee et al. discovered activated crushed stone graphene (A-CG) with morphological and functional flaws through the aerosol spraying process and continuous reduction and activation technology[34]. The unique defect engineering enables A-CG materials to exhibit a high reversible capacity of 340 mAh/g. The greater K+ adsorption energy at the defect site is one of the primary factors contributing to the improved performance of electrode materials, according to DFT simulation data. Single vacancy (SV), double vacancy (DV), and Stone-Wales (SW) are typical flaws[35]. Furthermore, Zeng et al. synthesized flexible N and O dual-doped carbon-coated graphene foam films (denoted as NOC@GF) that achieve exceptional electrochemical performance for KIBs, delivering outstanding rate capability and reversible capacity[11]. Additionally, DFT analysis demonstrates that N-O dual-doping offers additional active sites, which is significant in enhancing the K+ migration rate. As shown in Figure 4A, various values of the corresponding adsorption energies (Eads) show that N, O co-doped structures are substantially more suitable for adsorbing K atoms than graphene alone. Aside from that, the differential charge density map reveals the charge transfer between K atoms and adjacent carbon atoms, while the accumulation of more charges near the N and O doping sites in the NOC layer provides a theoretical basis for improving the potassium storage performance of the electrode material [Figure 4B]. Figure 4C is the diffusion barrier study of K ions on graphene and doped graphene systems. These findings indicate that the lower Ed value on Graphene makes the electrolyte ions have a faster migration rate, and the excellent electrochemical performance of NOC@GF materials positions them as one of the candidate materials for KIBs.
Figure 4. Theoretical simulations of K atom adsorption and diffusion in different structures. (A) Top view of K+ adsorbed in graphene, along with N, O doping; N doping and O doping structures, and the related adsorption energies. (B) The corresponding side view of the electron density changes of the K atom adsorbed on these structures, and yellow and blue spots represent raised and reduced electron densities, respectively. (C) Top view of the structures with K atom diffusion and associated diffusion energies. The atoms C, N, O, and K are represented by the brown, silver, red, and purple balls, respectively. Copyright 2020, Nano Energy. Theoretical simulations of K adsorption in different structures[11].
To further interpret the relationship between electrochemical properties and the K+ storage mechanism,
Hard carbon-based anode for KIBs
Given its affordable price, eco-friendliness, and high cycle stability, non-graphite carbon, which includes HC, soft carbon, and amorphous carbon, has become one of the potential candidate anodes in KIBs. Even if it is carbonized at an annealing temperature higher than 3,000 °C, HC is difficult to be graphitized and is typically produced from a precursor with a strong cross-linked structure. The distinctive “pseudo graphite”[36] HC structure featured by disordered nanostructure with turbostratic nanodomains increases the structural stability by adding extra rigidity and buffer room to prevent volume expansion. The larger (002) peaks, increased interlayer spacing, and smaller grains that are provided by this disordered structure in the c-direction assist in preserving the structural integrity of materials[37]. Additionally, HC has a high standard potential of K+/K, which helps to prevent the growth of potassium dendrites and enhance safety.
There is no denying that SIBs are developing quickly, and several HC materials have been presented with distinctive structures and outstanding performance. In 2016, Jian et al. successfully added HC microspheres (HCS) to KIBs for the first time by pyrolyzing the hydrothermally treated sucrose[38]. With remarkable long-term stability, the typical HCS voltage curves showed a sloping form in the high potential area and a quasi-plateau region in the low potential area. In 2018, He et al. created highly disordered HC from degreased cotton, which displayed high initial coulomb efficiency (73%)[39]. This explains the resultant distinctive porous structure, huge specific surface area, and extremely high electrochemical performance of the disordered HC[40].
Furthermore, the electrochemical energy storage mechanism of HC as KIB anode material has been widely discussed. The unique structure of HC at the molecular level provides many active sites for the storage of Na+/K+, which makes its real capacity limit as an electrode material immeasurable. Based on this, different storage mechanisms have emerged. In 2000, the “insertion absorption” concept, additionally referred to as the “house of cards model,” was initially reported by Stevens et al. after they researched the storage mechanism of Na+ in nanoporous HC[41]. In this model, Na+ can be inserted into the interlayer of carbon microdomains at high potential and filled with pores at low potential. Since then, a lot of research has been conducted on the Na+ storage mechanism. Kim et al. developed HC with two distinct crystallinities as anode materials for SIBs[42]. Microcrystalline cellulose (MCC) with more micropores formed by removing amorphous regions exhibits higher specific capacity (300 mAh/g) and rate performance. The impact of amorphous pore structure on the reversible storage ability of sodium was then further investigated on the basis of Na MAS NMR analysis, as shown in Figure 5A. The Na ions can be stored more efficiently in the nanopores created by larger and more open pores, which also increases the pace at which ions diffuse. In 2015, Bommier et al. proposed a novel Na+ storage mechanism: the “intercalation filling” mechanism
Figure 5. (A) Schematic diagram of the adsorption and pore filling mechanism of sodium ions in nanopores based on NMR analysis. Copyright 2021, The Journal of Physical Chemistry C[42]. (B) Visualization of the “intercalation pore filling mechanism” of sodium ion storage in HC. Copyright 2015, American Chemical Society[43]. (C) Ion storage mechanisms of Li+ and K+ in LPG materials. Copyright 2019, Electrochimica Acta[45].
Recently, the adsorption mechanism in the high potential zone and the embedding mechanism in the low potential region were the two separate K+ storage mechanisms that Li et al. discovered in porous HC
To further interpret the K-storage mechanism in HC, Yuan et al. has successfully created a number of chemically active HC spheres (AHCS) with adjustable micro/mesoporous structures to investigate the connection between K migration behavior and micro/mesoporous structure[46]. They also revealed that the storage mechanism of K ions in the HC system can be divided into two stages: adsorption and embedding. There is still much to learn about the fundamental K+ storage process in HCs and the internal factors impacting K+ storage behavior, despite the fact that research on HC materials as KIB anodes has advanced greatly over the years.
Due to its inherent characteristics, HC provides good bearing capacity and structural integrity[47]. Among them, Bin et al. prepared functional porous hollow carbon materials using molecular/environmental variable-controlled growth processes [Figure 6A][48]. And their extremely low density and low ICE, which results in a low bulk density, restrict their usefulness. In order to overcome the poor diffusion dynamics in KIBs, Chen et al. developed a graded N-doped HC microsphere (NCS) composite material with high porosity[49]. The unique porous structure provides more buffer space for volume expansion in the electrochemical process [Figure 6B]. The charge distribution was examined, as shown in Figure 6C-E, in order to systematically analyze the impact of N doping on the K+ storage performance in NCS materials. The nearly full transfer of Valence electron density from the carbon atom to K+ demonstrates its ionic characteristics. Among them, the density of states (DOS) diagram in Figure 6F shows that more electrons in the carbon materials doped with graphitic N (GN) and pyridinic N (PN) are conducive to improving the DOS value at the Fermi level (EF), thereby increasing the electronic conductivity. Controlling the microstructure of HC (interlayer, defect, heteroatom doping) is thought to be a successful method for enhancing K+ storage performance. Zhang et al. successfully prepared N-doped CoSe/C composite materials with egg yolk-shell structure, as shown in Figure 7A[50]. CoSe nanoparticles are completely surrounded by a carbon skeleton with high N content and graphitization degree, exhibiting high capacity and excellent rate performance. Lu et al. prepared porous N-doped CNF aerogel from chitin as the anode material of SIBs, which shows a high reversible capacity of 282 mAh/g at 0.1 A/g and excellent cycle stability[51]. DFT calculations were used to investigate the effect of N-doped on the electrochemical performance of SIB anodes. Figure 7B shows different types of N-doped graphene groups and high electron density regions, mainly including defect pyridine-N (N-6), defect N-6, pyrrole-N (N-5), and large defect (pyridine/pyrrole/graphite-N) (N6-N5-NG). Among them, the binding energy of Na+ adsorption on the corresponding structure is -0.89 eV,
Figure 6. (A) Schematic illustration of the different shape evolution paths for hollow structures. Grey, yellow, and blue represent carbon, insoluble component, and dissolvable component, respectively. Copyright 2017, Journal of the American Chemical Society[48]. (B) Schematic illustration for the large-scale fabrication of hierarchically porous nitrogen-doped carbon microspheres. (C-E) The electronic density differences of K+ ion adsorbed on pristine carbon, GN-doped carbon, and PN-doped carbon, respectively. (F) The density of states of corresponding carbon materials. Copyright 2017, Energy Storage Materials[49].
Figure 7. (A) Schematic illustration of the preparation of the nitrogen-doped CoSe/C composites. Copyright 2017, ACS Applied Materials and Interfaces[50]. (B) The DFT optimized structure of N6, N6-N5-1, and N6-N5-NG-1 N-doped graphene and the corresponding electron density. The blue and red regions correspond to the electron-rich and electron-deficient regions, respectively. (C) Optimized top and side views of Na+ adsorption on corresponding structures. Copyright 2019, Applied Surface Science[51]. Top views of the K-absorption models (D) of defect C, P-3, P-2, pyrrolic N, graphite N, and pyridinic N frameworks, the Ead, and the differential charge density distribution maps. (E-G) The TDOS and the PDOS before and after K adsorption. Copyright 2021, Applied Surface Science[52].
Attributed to the introduction of N doping has a favorable impact on the Na ion absorption and diffusion, Gao et al. further produced a novel KBT-7 electrode with exceptional electrochemical performance from kitchen biological waste of 700 °C carbonized waste black tea by layered porous structure and co-doping effect[52]. The impact of defects and N, P co-doping on the kinetics of K adsorption capability was further explained using DFT analysis. The differential charge density distribution and adsorption energies of the systems with and without the carbon defect, the carbon defect, the N-doped NC3 (graphite N), the NC2 (pyrrole N and pyridine N), the P-doped PC3 (P-3) and the P-doped PC2 (P-2), are each shown in Figure 7D. The charge transfer between the K atom and the carbon-based framework is what causes the charge density at C to fall when the K atom is absorbed by the surface, and electrons gather on the carbon atom nearby. Additionally, the variation in charge densities at various sites following K adsorption suggests that the inclusion of N, P doping or defects will dramatically increase the adsorption energy of the structure and intensify the electron transfer event. Figure 7E and F displays the total density of states (TDOS) before and after K adsorption for the systems with defect C, defect C, P-3, P-2, pyrrole N, graphite N, and pyridine N (designated as a-TDOS and b-TDOS, respectively), as well as the corresponding partial density of states (PDOS) of K 4s and K 3p in the K adsorption model. By comparing the TDOS before and after K adsorption, it can be shown that the addition of K+ will trigger the DOS value guide band at the EF to shift in a certain direction, increasing the relative electronic conductivity, which is mostly caused by the contribution of K atomic orbital. The pyridine N model also exhibits the largest TDOS at the EF, which is further subdivided into two peaks, as seen in Figure 7F and G. The findings demonstrate that the sample with a moderate amount of pyridine nitrogen has improved conductivity when compared to other heteroatom doping models. Different N doping strategies can provide different electrochemical properties for HC materials. Thus, Yang et al. created a hollow biomass carbon sphere (NOP-PB), where N/O/P ternary doping increased the interlayer distance on the graphite surface and added more defect sites[4]. The special solid hollow porous structure can buffer the volume expansion of the potassium insertion process, promote the charge transfer of K ions and electrons, and greatly improve the electrochemical performance of the material. The adsorption and diffusion mechanism of K+ in HC and NOP-PB electrodes can be better understood by simulating the effects of N, O, and P doping on K+ adsorption behavior via DFT simulations. N-5 and P/O doping are the most effective at enhancing the electronic conductivity of electrode materials, according to a number of calculations and analyses. As a result, NOP-PB electrodes perform better in terms of potassium storage.
HC exhibits good speed capabilities and excellent structural integrity owing to its loose structure[53]. It should be underlined that HC produced during pyrolysis always performs poorly for K ion storage in carbon-based materials. Their extremely low density and low ICE result in low bulk density, limiting their usefulness. As a result, designing a carbon-based anode with a decent degree of graphitization, a finely tuned pore structure, and a chosen surface function is extremely optimal. Surprisingly, not as many individuals are aware of how the solid electrolyte interface (SEI) film impacts the electrode reactivity and changes during the cycle. The commercialization of HC as a high-performance KIB anode will be greatly influenced by this.
Nanoporous carbon composite anode for KIBs
Compared with traditional carbon materials, nanoscale carbon-based materials with high conductivity and specific surface area, such as carbon nanotubes (CNTs), carbon nanospheres, and CNFs[54], can store more charges at the interface and improve the specific capacity and rate performance of KIBs[55,56]. Due to the restrictive C@C, It has incredible elasticity, which increases its mechanical properties of strength, stiffness, and durability[57]. CNTs become one of the most viable carbon materials among potential KIB anodes because of their inherent mechanical properties and connected conductive network, which makes them become a crucial part in sustaining electrode integrity during the (de-)potassium process for alkali metal ion batteries[58]. The cautious alteration of the shape and size of nanoscale carbon materials is a rational and efficient modification method since nanomaterials with various geometric configurations can enhance the ion storage performance of electrode materials to diverse degrees. Given its established use in LIBs, some typical examples of success may be applied to KIBs. In 2017, freestanding porous CNF paper was used as an anode for KIBs by Zhao et al., which showed excellent reversible capacity (211 mAh/g after 1,200 cycles at 0.2 A/g) and rate performance[59]. As said by Cao et al., a carbon nanocage (CNC) can greatly lessen anisotropy and, as a result, prevent layer slippage[60]. Based on their distinctive cage structure, CNCs have outstanding discharge capacity and capacity retention as an anode material for KIBs.
Numerous modification techniques of carbon fiber materials have been documented in an effort to further enhance the efficacy of K ion storage in CNFs. High-performance KIBs utilized unique anodes made by Wang et al., which consisted of highly active hollow carbon nanosphere composite materials (AHCS) with large interlayer spacing, high specific surface area, and surface oxygen-containing functional groups (OCFGs)[61]. The introduction of OCFGs further improves the pseudo-capacitive behavior and specific capacity. After chemical activation, the interlayer gap (0.408 nm) of AHCS is significantly larger than that of hollow carbon nanospheres (HCSs), which can accommodate more reversible K+ insertion and removal to adapt to larger volume expansion during the (de-)potassium process. Moreover, OCFGs offer additional active sites, which can increase the capacity of reversible K storing. As the anode of the KIB, AHCS exhibits exceptional initial specific capacity, ultra-long cycle life, and extraordinary magnification performance as a result of the synergistic impact of structural properties. The interlayer spacing, surface oxygen functionalization, and layered porosity of carbon-based anodes are highlighted in this investigation as crucial factors in potassium storage. Furthermore, Figure 8A illustrates the porous carbon fibers (PCFs) that Sun et al. created utilizing the electrospinning technique as independent electrode materials for high K ion storage[62]. These PCFs have a high reversible capacity of 256 mAh/g under circumstances of 50 mA/g. Corresponding to the potassium storage process [Figure 8B], larger holes increase the surface area, enabling PCFs to absorb and hold more K+. Therefore, the synergistic effect of K embedding and K clustering is responsible for the extremely reversible potassium storage performance. And Wu et al. further fabricated macroporous honeycomb CNFs (MHCNFs), where poly(tetrafluoroethylene) emulsion (PTFE) is primarily involved in the creation of porous materials[63]. Extremely high potassium storage capacity and competitive rate capacity are displayed by the adhesive-free electrode [Figure 8C]. Additionally, a porous structure can offer enough gaps to offset a significant volume shift while attaining prolonged cycling stability[64,65]. Besides, Wen et al. prepared PNF-Co3O4 as a KIB anode via a solutions-combustion process and proposed a “bubble gum fracture effect” as its main growth mechanism
Figure 8. (A) Schematic illustration of the synthesis process of PCF. (B) schematic of K-ion storage mechanism for CF and PCF. Copyright 2022, Chemical Engineering Journal[62]. (C) Schematic illustration of the synthesis process of MHCNFs. Copyright 2021, ACS Applied Materials & Interfaces[63]. (D) Schematic presentation of proposed growth mechanism of porous nanoflaked Co3O4. Copyright 2020, Journal of Power Sources[66]. (E) The discharge curve (solid line) of the third cycle of HG-CNFs and the theoretical capacity contribution of each K-GIC transition reaction marked by dotted lines. Copyright 2020, Journal of Power Sources[67].
Several modification strategies have been constructed so as to utilize nanocarbon materials as an alternative alkaline metal ion insertion, adsorption, and diffusion electrode material. Doping heteroatoms [N, O, sulfur (S), P, etc.] in nano carbon materials have also been shown to be a successful method to enhance the efficacy of K ion storage for the following reasons: (1) Heteroatom doping technology can accumulate charges by altering the local electronic structure on the surface of carbon lattice, thereby raising the electronic conductivity of carbon nanomaterials[68]; (2) Heteroatom doping can provide a large number of active sites for K+ adsorption[69] and; (3) Due to the large covalent radius of nano carbon materials, heteroatom doping can regulate its interlayer distance[70]. The carbon source generally originates from polyacrylonitrile (PAN) carrying N element, which is the most frequent doped element in CNFs. Thereby N-doped CNFs are often synthesized in situ during carbonization. It has been demonstrated that adding more defects due to N doping increases reactivity and that adding better local electronic configurations due to N doping increases electronic conductivity[71]. In 2019, as separate anodes for high-performance potassium-based dual-ion batteries (KDIBs), Zhang et al. created graded porous N-doped carbon fibers (HPNCF) with distinct hierarchical architectures (micro, medium, large pores, and nanochannels)[72]. The distinctive structure offers the HPNCF not only an excellent ion transport channel and an intrinsic electronic channel but also adequate buffer space to survive the volume shift of the cycle, which favors enhancing electrochemical efficiency. Moreover, calculations based on the DFT theory are commonly used to examine how N-doped porous carbon materials affect the performance of potassium storage. Through a single carbonization of N-rich energetic metal-organic frameworks (EMOFs), Tong et al. created N-doped porous carbon frameworks (NPCF) with a large N content (MET-6)[73]. The greater porosity and specific surface area are responsible for the optimized potassium storage ability (327 mAh/g). To further research the effect of pyrrole and pyridine N-doped porous carbon structures on the K+ adsorption mechanism, the K ion adsorption abilities and the relative adsorption energy (ΔEa) at three different models were constructed and calculated by DFT, including pristine C-doping, graphitic N-doping, pyrrolic N-doping. It is evident that the endothermic reaction occurs in the graphite N and C co-doped system, and the extremely unstable potassium insertion reaction may be a result of the electronic abundance of graphite
Figure 9. (A) Top view of a single K atom absorbed in quaternary-N (N-Q), pyrrolic-N (N-5), and pyridinic-N (N-6). (B) The adsorption energy of different amounts of one N-6, two N-6, and three N-6. (C) The DOS of the defect site without N-doping, one, two, and three N-6 doping sites. (D) The barrier energy of one N-6, two N-6, and three N-6 doping sites. Copyright 2021, Electrochimica Acta[75].
Due to the extensive study that has been done on the diatomic co-doping modification technology in CNFs, it is anticipated that high-performance CNF anodes will be produced thanks to the synergistic interaction of two distinct heteroatoms. In 2019, Ma et al. created sodium polyacrylate as the carbon precursor for graded N, S co-doped porous carbon (NSPC)[68]. The structure of nitro and S co-3D doping makes it easier to inject K ions, increases layer gap, and hence enhances electronic conductivity. NSPC has exceptionally high reversible capacity (289.7 mAh/g after 70 cycles at 50 mA/g) and outstanding cycling performance as the anode material for KIBs. Besides, several studies showed that the introduction of P improved the electrochemical performance of the carbon anode of KIBs. Thus, Gong et al. successfully constructed an independent P/N co-doped porous carbon monolithic (PN-PCM) anode for KIBs using supercritical CO2 foaming technology and a number of pretreatment steps to increase the potassium storage capacity (including amidation, phosphorylation, and heat treatment)[76]. The PN-PCM anode has good reversible specific capacity (396 mAh/g at 0.1 A/g after 300 cycles), initial coulomb efficiency (63.6%), and magnification performance owing to the 3D macroporous open structure and the heterodimer of high P/N ratios. According to theoretical calculations, P-C bonds are more useful for enhancing potassium storage and improving carbon conductivity through adsorption in P/N co-doped carbon, whereas P-O bonds are more beneficial for extending the distance between carbon layers and lowering the ion diffusion barrier. Based on this, further research has been done to determine how P-C and P-O functional groups affect the electrochemical performance of P/N co-doped carbon materials (the most typical N-6 bond is used to represent N doping). The layer spacing of original carbon, N-6 doped carbon, N-6/P-C co-doped carbon, and N-6/P-O co-doped carbon is depicted in Figure 9E. This graph demonstrates how the layer spacing of carbon may be greatly increased by the addition of P, particularly for the P-O form. Additionally, the inclusion of N-6, P-C, and P-O bonds increases the Ead of carbon, as seen by the corresponding adsorption energies (Ead) of each structure following K+ intercalation [Figure 9F]. N-6/P-C co-doped carbon exhibits a lower value than N-6/P-O co-doped carbon (-0.97 eV), indicating that the P-C bond contributes most to the capacitance of P/N co-doped carbon. Then again, Figure 9G displays the DOS of four carbon structures.
Consequently, N doping is the correct strategy since it may efficiently increase the adsorption energy toward K+ ions[77]. To achieve high magnification capacity, the creation of KIBs will also need appropriate nanocarbon materials[78,79]. The design of nanostructures is typically accompanied by an increase in the conductivity of the material, which can significantly shorten the electron transmission path and speed up the diffusion of K+ in the bulk phase[80,81]. One of the most successful methods for enhancing the electrochemical performance of carbon materials is the production of CNTs, carbon nanowires, and other nanomaterials. The high throughput electrochemical performance may also be directly predicted by DFT calculations, accelerating the screening and practical design of KIBs anode materials. At the same time, it can give us fresh perspectives on how various anode materials behave from a molecular to a macro level. Additionally, it offers new insights into how to create high-performance KIBs.
ALLOY-BASED ANODES FOR KIBS
With the creation of polyanion compounds and layered transition metal oxides on the cathode side, significant advancements have been realized[82,83]. Despite having a high reversible specific capacity of
Nowadays, the high theoretical specific capacity, good conductivity, and appropriate working potential of alloy-based anode materials, such as P[92], tin (Sn)[93], antimony (Sb)[5], bismuth (Bi)[94], Si[95], and Ge[96], and their oxides, sulfides, and selenides, and so on, have recently attracted significant attention to ensure the high energy density and safety of secondary batteries[97]. For instance, the theoretical capacities of Si, Ge, Sn, and Sb for LIBs are high, at 4,200 mAh/g, 1,600 mAh/g, 994 mAh/g, and 660 mAh/g, whereas P and Sn can form alloys with sodium, with theoretical capacities of 2,596 mAh/g and 847 mAh/g, respectively[98]. As a result, it is anticipated that the alloy-based anode employed for high-energy-density KIBs would receive careful consideration. Theoretically, alloy-based anodes made of S, Si, P, Ge, Sn, Sb, and Pb can be alloyed with potassium in a variety of ratios, as determined by Kim et al.[99]. Among them, the sulfur electrode has become one of the candidate electrode materials in metal lithium-ion batteries since their affordable and enormous theoretical capacity[100]. However, the difficult electrochemical reactions between cyclo-S8 and K+, such as the reduction from K2S3 to K2S, are extremely challenging, thus excluding it from the field of candidate electrode materials for KIBs. Some studies have shown that reforming the chemical structure of S can effectively improve the electrochemical performance of S and regulate the electrochemical behavior of S[101]. Using this information as a foundation, Tian et al. proposed a S-modified doped biomass bamboo charcoal (S-BC) negative electrode material that shows remarkable reversible specific capacity (339.3 mAh/g at 50 mA/g) as a KIB anode material[102]. The remaining O atoms and doped S of the biomass materials render an abundance of active sites for electrode materials, significantly raising the electrical conductivity. However, during the cycle, due to the multi-electron reaction, the deep potassification of the S-based anode material will produce a large volume expansion, and the active material will fall off, resulting in battery failure[103,104]. In addition, the large volume expansion leads to multiple continuous exposures of specific surface areas, which accelerates the loss of active substances and the consumption of electrolytes[105], reduces the coulomb efficiency and overall energy density of the battery, and makes the development of high-capacity electrode materials more difficult. Thus, Yang et al. prepared multi-shell hollow nanospheres (ASHCs) with a high content (about 32 wt%) of single-atom S doping[106]. Based on the superior reaction kinetics of single-atom S, ASHCs exhibit upgraded reversible capacity and fantastic rate capability as KIB anode materials. Furthermore, the special nanoporous structure of the carbon-based material may efficiently reduce volume expansion during the electrochemical reaction and guarantee the structural integrity of the substance.
Furthermore, a large number of studies have shown that the creation of nanostructures and internal spaces, heteroatom doping, protective coating, introduction of conductive buffer media, and application of appropriate materials can effectively improve the structural stability of materials and promote fast
Sb and its derivative anode for KIBs
With its abundant resources, low price, excellent theoretical capacity (660 mA h/g), and adequate voltage window, Sb has drawn a lot of interest as an alternative anode for Li+ and Na+ storage[13]. Then, Han et al. described the binary phase diagram of the K-Sb system[10]. The phase diagram demonstrates that Sb will go through a series of potassium alloying transformations, including KSb2, KSb, and K5Sb4, before the development of the K3Sb phase. The thermodynamic stability of the K-Sb phase can also be investigated using the binary K-Sb phase diagram. In an attempt to shorten the ion diffusion path, 1D nanowires, nanorods, and nanotubes have all been investigated extensively as part of the nano-engineering profile strategy of alloy-based anode materials. As a KIB anode, Yi et al. created nanoscale Sb particles with high reversible capacity (381 mAh/g at 100 mA/g) and capacity retention rate (210 mAh/g at 500 mA/g after
Figure 10. (A) Schematic diagram of potassium mechanism of Bi/K and Sb/k systems in KIBs. Copyright 2018, The Journal of Physical Chemistry C[110]. (B) The XRD patterns for electrodes at various charge/discharge states. Copyright 2022, Applied Surface Science[111]. (C) Schematic presentation of the formation and (de-)potassiation process of Sb-C-rGO composite. (D) Crystal structure of cubic K3Sb (c-K3Sb) and hexagonal K3Sb (h-K3Sb). (E) Formation energies of KxSb obtained by the ionic substitution method. Phase stability is a relative energy of a given phase compared to all other materials with different compositions. (F) Atomic migration barriers in the cubic A3Sb phase (A = K, Na, and Li). Copyright 2019, ACS Applied Materials & Interfaces[117].
Besides, Shi et al. successfully prepared 3D flowered antimony oxychloride (Sb4O5Cl2) as the anode material of PIBs by a hydrothermal method[111]. As depicted in Figure 10B, the XRD results of Sb4O5Cl2 electrodes show that the typical peak of Sb4O5Cl2 weakens with the formation of KCl, KSb, and K3Sb products and reversibly recovers from 0.01 V to the original strength at 3.0 V during (de-)potassification process. The presence of KCl and K-Sb alloys further confirms the synergistic effect of conversion type and alloying reaction. Therefore, the reversible electrochemical mechanism of storing K+ ions by Sb4O5Cl2 can be established: Sb4O5Cl2 + k+ + e- ⇔ KCl + K3Sb + K2O.
Unfortunately, the unavoidable high volume strain of the cyclic operation causes the rupture of the active material and insufficient electrical contact, which immediately causes a quick attenuation of capacity and prevents the practical use of KIBs[112]. Thus, numerous modification methods have been developed till now with a view of balancing the (de-)alloying process of Sb-based materials, which include nanoparticles[113], the inclusion of carbon components[114], the improvement of adhesives[115], and the creation of open structures[116]. Surprisingly, mixing alloy anodes with carbon materials may dramatically boost their cycling stability. The investigation on the electrochemical behavior of Sb-C composite electrodes as KIB anodes, which deliver 650 mAh/g high reversible capacity and good coulomb efficiency, was initially reported by McCulloch et al.[5]. According to in-situ XRD and electrochemical analysis, the discharge result is the final cubic K3Sb phase. Additionally, SEM inspection at various voltage cut-off points revealed that the Sb-C electrode underwent significant expansion during the potassium alloying event. Therefore, carbonaceous materials are frequently utilized in Sb-based systems as a volume change buffer to conduct ion conduction in the full potassium alloying/dealloying reaction. In the research of Ko et al. the multi-component electrode [Sb-C-rGO (reduced graphene oxide)] with uniform and fine Sb particles was prepared from Sb-based composite materials containing Sb nanoparticles, amorphous carbon, and rGO [Figure 10C][117]. And the side reactions in potassium metal and the electrolyte, not the degradation of Sb nanoparticles, are to blame for the capacity decline in the Sb-C-rGO cell. And three intermediate phases, K0.5Sb, K1.0Sb, and K1.25Sb, which are congruent with the experimental phase diagram of the K-Sb system, exist between the two reaction end members (Sb and K3Sb). Among the stable phases, cubic and hexagonal K3Sb polycrystalline phases are more stable than other crystalline phases. As can be shown in Figure 10D, the two polymorphic forms of K3Sb are structurally identical. The hexagonal system (h-K3Sb) is more stable than the cubic system between the two polymorphs [Figure 10E]. The relative stability of K3Sb polymorphs is the same at a higher theoretical calculation level. This finding demonstrates that the electrochemically produced phase of c-K3Sb is metastable. One intriguing aspect is the ability of the final phase, the c-K3Sb phase, to change into a stable h-K3Sb phase after forming. However, no phase change was noticed while the battery was in use, indicating that dynamics are hampered during the phase transition from c-K3Sb to h-K3Sb. This cubic to hexagonal phase shift may necessitate both the vertical migration of K and the lateral sliding of the K-Sb layer. In actuality, the c-K3Sb before h-K3Sb creation is still present. After several hours of heating at the samples between 160 °C and 170 °C, the resultant c-K3Sb can likewise be changed into h-K3Sb. According to the aforementioned findings, the c-K3Sb to the h-K3Sb phase transition is dynamically non-spontaneous under environmental conditions, and the h-K3Sb phase is more stable. The migration potential barrier of each component atom can be a suitable signal to estimate the phase transition kinetics since the phase transition necessitates the reorganization of atoms. The migration barriers for Li, Na, and K in each cubic A3Sb phase are 0.09 eV, 0.16 eV, and 0.26 eV, respectively, as shown in Figure 10F. The occurrence of the metastable phase in the K-Sb system and its simple nucleation properties are explained by the higher K atom migration barrier. The inclusion of carbon substrates and nanoscale materials still lowers their energy density despite the tremendous progress that has been accomplished thus far; therefore, in-depth modification exploration continues to be the main focus of research. For example, Ge et al. employed Sb@CNFs as the alloy anode to investigate the dynamic volume expansion during the charging and discharging process by in-situ transmission electron microscopy (TEM) in order to explore the structural changes of carbon-coated conversion anode materials[118]. Studies indicate that Sb@CNF compound materials can successfully buffer the volume growth of Sb nanoparticles during the K ion insertion/extraction process, which enhances the electrochemical performance[90]. Owing to the aforementioned study, porous structures may successfully prevent the accumulation of Sb nanoparticles and respond to the significant volume variations of Sb during charging and discharging while preserving structural stability. The K+ diffusion distance can also be minimized via porous structures, which also enable ions to transfer charges quickly along 3D skeletal structures.
Sb also displays considerable volume variations during the alloying/dealloying process, similar to other alloy-based anode materials[119]. Severe battery polarization issues can also be caused by the poor K+ diffusion rate within Sb crystals[120]. To address this concern, Luo et al. created a novel form of N-doped carbon hollow nanotube-wrapped Sb nanorod composite (Sb@N-C) via the nanostructure technique[121]. The hollow structure of 1D conductive carbon covering takes advantage of its advanced structural advantages, including the high conductivity brought on by heteroatom doping, and makes room for the superior ion transfer capacity Sb@N-C, which performs electrochemically quite well. In 2018, An et al. reported a novel 3D nanoporous Sb material (NP-Sb) which is created by vacuum distilling the zinc (Zn) atoms out of a commercial Zn-Sb alloy[116]. It is also discovered that altering the Zn-Sb ratio and distillation temperature can change the shape and porosity of NP-Sb materials. As shown in Figure 11A, the unique nanoporous structure can effectively reduce the volume expansion caused by electrochemical cycling, accelerate the diffusion of K+, and achieve high electrochemical performance. In the same year, by perfecting the crystal structure and ion storage mechanism, Huang et al. reported the heterostructure of In2S3-Sb2S3 formic acid microspheres and enhanced their electrochemical performance[122]. After a series of characterization methods, it is known that when the voltage drops to 0.9 V, the conversion reaction occurs and generates metal Sb, metal In, and sodium sulfide. Strong pseudocapacitive effects can be produced because In2S3-Sb2S3 is highly reversible during the charging process, and the InSb phase retained in the Sb2S3 gap leaves enough room for later conversion and alloy processes. The synergy of In2S3 and Sb2S3 also maintains the primary structure and improves electronic conductivity throughout the cycle, reducing the likelihood of a devastating collapse brought on by the structural expansion zone. A CNT-coated electrode is also advantageous for enhancing cycle and rate performance. Therefore, a hollow material that was created using the self-template procedure Sb2Se3@C microtubules was reported by Yi et al.[123]. The type of alloying in the microtubule is conventional, as confirmed through Sb2Se3@C electrochemical behavior and in-situ Raman spectroscopy. The original electrode exhibits significant Raman signals at 187 cm-1 and 253 cm-1, which are closely related to the production of Sb2Se3. These peaks gradually diminish and eventually vanish with the discharge. The rapidly growing signals of the newly discovered peaks at 112 cm-1, 148 cm-1, and
Figure 11. (A) Schematic of potassiation process for bulk-Sb and NP-Sb anodes. Copyright 2018, ACS Nano[116]. (B) XRD diagram of the first two cycles for BiSb@C composite anode. The corresponding CV curve (10 mA/g) is shown on the right. (C) Schematic diagram of potassium/dipotassium process of BiSb@C composite anode. Copyright 2019, ACS Nano[124]. (D) Schematic illustration of Ti3C2-Sb2S3 during cycling. Copyright 2020, ACS Applied Materials & Interfaces[125]. (E) Schematic illustration of the potassium storage mechanism for the in situ XRD results. Copyright 2020, ACS Nano[94].
What is more, following previous research, double heteroatom doping is a common modification method for Sb-based compounds, which improves their electrical activity. Han et al. employed a method of
Nevertheless, certain theories and experiments have confirmed that stable rare earth-free MnBi materials can generate a ferromagnetic low-temperature phase employing Sb dopant. Thereby, Nguyen et al. adopted DFT theoretical calculations to carry out phonon dispersion and DOS analysis of the doping system in order to further clarify the implications of Sb doping on the structural stability, internal magnetism, and electronic structure of MnBi components[127]. Figure 12A-C displays the phonon dispersion and phonon DOS of MnBi, MnBi0.5Sb0.5, and MnSb along the high symmetry direction. This figure demonstrates the instability of the MnBi material, which is indicated by the abundance of imaginary frequencies. The number of imaginary frequencies in the system diminishes when Sb is introduced. The imaginary frequencies in the MnSb system vanish, and the system energy becomes more stable when Sb totally replaces Bi. By lowering the magnetic moment, it is possible to stabilize MnBi while Bi is exchanged by Sb. The calculation of the DOS reveals that doping causes a decline in the DOS at the EF. And hence, to investigate how Sb substitution doping influences the electronic structure of MnBi, DOS calculations of spin and lower spin states are employed, along with calculations of energy band structure, which is shown in Figure 12D. Due to their shared crystal structure and the isomorphism of Bi and Sb, the energy band structures of MnBi and MnSb and the related DOS-s data exhibit the same trend. Subsequently, the energy band structure of doped materials splits along some k-paths, and the band within the energy range is divided into two separate bands [Figure 12E]. Along Г MK Г k-path, the dispersion curve of the k-path becomes flatter. In contrast, the dispersion curve along the ALHA k-path is split into a unique double dispersion curve, meaning that there is a linkage between the split band and the s orbital, p orbital, and d orbital of Mn and between the split band and the non-split band. Analysis of the DOS concerning MnBi1-xSbx demonstrated that the reduction of DOS through the substitution doping from MnBi to MnSb was another effect on the band structure
Figure 12. (A-C) Phonon dispersion, (D) phonon density of state, (E) up-spin state band structure, (F) density of states of MnBi,
In accordance with the studies mentioned above and accounts, quite a few solutions have been implemented to lower the volume expansion of Sb-based anode materials and strengthen their potential for potassium storage: (1) To minimize volume changes and shorten the ion diffusion distance, alter the morphology and structure at the nanoscale; (2) To stop the accumulation of Sb nanoparticles, contain volume variations throughout the cycle, improve electrical conductivity, and utilize carbon materials for buffer layers or conductive structures; (3) The addition of heteroatom doping increases the number of active sites on the surface of carbon-based materials and promotes electrical activity. As a consequence, choosing the proper electrolyte component in accordance with the unique properties of the materials will also aid in increasing the ability of Sb-based anode materials to store potassium. And the practical research on the future large-scale production of Sb-based anode KIBs is intimately tied to the selection and design of appropriate electrolyte components.
Sn and its derivative anode for KIBs
Alternative metallic elements that can be alloyed with K have also been investigated as potential KIB anode materials, except that metal Sb. They appear to be a potential option to meet the requirements of the upcoming large-scale energy storage devices due to their low cost and high natural abundance. As a desirable anode material for energy storage devices, tin-based materials offer an extremely specific capacity, a substantial relative concentration, and are environmentally friendly as well[100,128]. In addition, the theoretical capacity of Sn for the LIB and NIB anodes can be up to 990 mAh/g and 847 mAh/g, respectively[129,130]. It is thought that this phenomenon is caused by the contribution of high-density property to the volume energy density of the battery. Sn is, therefore, essential to the investigation of metal-based anode materials for KIBs. Pure Sn also experiences enormous volume changes throughout battery cycling, which may result in electrode material separation and poor battery capacity[131]. Basically, metallic Sn reacts with K through alloying reaction (Sn + K+ + e− ↔ KSn) with a theoretical specific capacity of 225 mAh/g. The first study on the electrochemical behavior of a Sn-based anode in a potassium battery was given by
Ramireddy et al. conducted a thorough investigation of the K+ alloying reaction mechanism of Sn thin film electrodes following a range of in-situ characterization techniques[137]. After an electrode was fully potassiated, the KSn alloy phase was discovered. Till the initial cycle of potassization/depotassization, a reversible capacity of up to 245 mAh/g was noted. It is interesting that just the second potassium reaction stage revealed the creation of SEI membranes. Besides that, in-situ studies of stress variations in Sn film electrodes during potassium/potassium removal cycles prove that mechanical instability happens both during surface reactions and the phase shift caused by potassium alloying and dealloying. Also, research on the cyclic stability of electrode materials at various operating voltage ranges has found that the ideal value is between 0.01 V and 1.2 V. At the same time, Wang et al. reported a study of the K ions storage mechanism of Sn nanoparticles in KIBs using in situ TEM and XRD testing[93]. Sn anodes have a 197 mAh/g capacity; however, they are accompanied by serious capacity decay. The results from TEM and XRD analysis reveal that the final alloy in the production of potassium is KSn, which was in accordance with the work of Ramireddy et al.[137]. According to in-situ TEM, a two-step potassium mechanism with volume changes of 101% and 180% occurred when K4Sn9 and KSn alloys were formed, and the development of reversible nanopores during the cycle of Sn nanoparticles precluded the complete recovery of Sn particles. The intense crushing of Sn particles is responsible for the capacity decline that occurs quickly after several cycles. As an example, the metals Sn and K can be alloyed at room temperature to produce a variety of intermetallic compounds, including K2Sn, KSn, K2Sn3, K2Sn5, KSn2, and K4Sn23[138]. Other charge storage mechanisms have also been proposed in recent years. Despite the fact that these studies highlight some benefits, the Sn element is prone to large volume expansion during the potassium process, which will inevitably result in material crushing and stripping and lower cycling performance and make it more challenging for KIBs to achieve high energy density. Consequently, the Sn-based material modification technique for LIBs and SIBs can also be employed for the K ion system, even though research into Sn-based materials for KIBs has only started. Other approaches to optimizing the capacity of alloy anodes for maintaining potassium, including carbon-based nano-engineering, heteroatom doping, profitable adhesives, and electrolytes, have also made considerable strides.
To further safeguard the active components, graphene can be distributed in CNFs and used as a soft buffer substrate. Additionally, it can improve the conductivity and magnification of materials. Therefore, one of the methods to address this issue is to limit Sn-based alloys to a carbon matrix and introduce the method of dealloying to create porous metal particles by alloy corrosion[112,139]. Ge et al. expressed Sn nanoparticles in the non-graphitized carbon in the Sn-C composite made by mechanical ball milling for the first time, and they have a capacity of 150 mA h/g as the anode of NIBs[140]. The KIB anode is, therefore, predicted to have a reversible capacity of between 150 mA h/g and 190 mA h/g when the carbon content of an electrode is taken into account. Qin et al. developed a 3D porous graphene network using 3D self-assembly as a template, encasing Sn nanoparticles inside a graphene shell (Sn@G-PGNWs), as shown in Figure 13A[141]. In addition to successfully preventing Sn from coming into direct contact with the electrolyte, the highly elastic graphene shell created by Chemical Vapor Deposition (CVD) also helps to preserve the structure and interface stability of Sn nanoparticles. Sn@G is a tightly fixed 3D porous graphene network with great mechanical flexibility, huge surface area, and good conductivity. It is possible to considerably increase the conductivity and structural integrity of the entire electrode. As such, porous carbon materials that serve as the buffer layer may assist in maintaining the integral design of composite electrodes. Huang et al. built a 3D carbon/Sn porous polymer (3D-HPCS) anode utilizing a NaCl template[142]. The performance of potassium storage is improved by the enormous surface area of Sn nanoparticles and composites uniformly placed in 3D porous carbon. This can somewhat reduce the volume expansion during the (de-)potassium process. The initial Coulombic efficiency (ICE) exceeding 96% and the reversible capacity of up to 276.4 mAh/g imply that non-uniform structures are also vital for the efficacy of potassium storage. Via the sol-gel technique, Yang et al. created N-doped porous carbon materials (Sn/NPC)[143]. Given their distinctive shape, strong conductivity, and substantial surface area, Sn/NPC electrodes have an amazing ability to store potassium. As illustrated in Figure 13B, Li et al. described the hierarchically porous carbon-supported Sn4P3@C composite as an anode material for KIBs first[144]. According to a number of characterization techniques and electrochemical performance research, Sn4P3@C undergoes a three-phase transformation into Sn, KSn, and K4Sn23 during the whole discharge process. And then, due to the fact that graphene sheets may successfully prevent Sn4P3 nanoparticle aggregation and enhance electronic conductivity, Du et al. created Sn4P3/multilayer graphene sheets
Figure 13. (A) Schematic illustration of 3D Sn@G-PGNWs. Copyright 2014, ACS Nano[141]. (B) Schematic illustration of the synthesis of Sn4P3 and Sn4P3@C. Copyright 2019, Energy Storage Materials[144]. (C) Schematic illustration of the synthesis of Sn4P3/MGS-80.
For designing ideal high-capacity Sn-based KIBs, effective procedures, such as structural modification, nanomaterialization, combining conductive materials, and interface management, have a major practical value. In spite of several studies on tin-based anodes in recent years, research into the development of
P and its derivative anode for KIBs
P compounds, such as white, red, and BP, have also been investigated as prospective anode materials for KIBs, with a theoretical capacity of up to 843 mAh/g, as evidenced by LIBs and SIBs[148,149]. Among them, white P cannot be utilized as an electrode material for scientific study since it is poisonous, volatile, unstable, and combustible in the air. Non-toxic and comparatively stable, amorphous RP can store ions (Li+, Na+) in an amorphous form. However, its poor conductivity and significant volume growth restrict its practicality[150]. Lastly, during the high-temperature and high-pressure transition of RP into white P, an occurrence of BP was observed. Its exceptional 2D layered structure and superior conductivity render it perfect for the creation of high-performance KIBs. However, the harsh synthetic conditions for obtaining BP are difficult problems that must be solved[151]. Likewise to SIB and LIB, the alloying reaction of manufacturing KxP is thought to be the basic mechanism of RP in KIBs based on the existing three-electron mechanism[152]. Although K3P theoretically holds a very high capacity of 2,596 mAh/g, no empirical findings have proven this claim[153,154]. On the other hand, the potassium method caused the production of KP and
Figure 14. (A) Schematic illustration of the (de-)Potassiation process of red P particles, hollow carbon fibers coated with red P, and nanostructured red P confined in the N-PHCNF matrix in KIBs. (B) In-situ XRD patterns of the red P/N-HCNFs-5 composite when discharged to 0.01 V. Copyright 2019, Nano Letters[92]. (C) P/C electrodes in KIBs at different potentials. Copyright 2017, Journal of the American Chemical Society[159]. (D) Formation energies of possible phase during potassiation for BP. Copyright 2019, Electronic Materials Letters[160].
Despite having a large theoretical specific capacity (2,596 mAh/g), pure P exhibited a very poor specific charge capacity and CE, which severely restricts its broad use in the filed of KIBs. In particular, due to significant volume growth during the charge-discharge operation, the pure RP and BP anodes deliver very low reversible capacity. According to weak electrochemical involvement and strong polarization of RP, active materials for ion storage have limited electrical conductivity[154]. Low redox kinetics can then impair cycle performance, especially when there is a high current density. Higher battery polarization can cause the formation of uneven SEI films and the excessive decomposition of electrolytes, resulting in low ICE and significant capacity degradation as electrode materials[149]. In brief, the development of P anodes for KIBs has advanced significantly, but there are still numerous obstacles to overcome, including poor electronic conductivity[92], significant volume expansion[161], and an unstable SEI. These challenges will also cause capacity decay and poor coulomb efficiency[162]. Researchers are now looking into the optimization of modification techniques for high-performance anode materials in an attempt to eliminate the barrier witnessed by the aforementioned KIB utilizing P anode materials. Recent studies have mainly focused on the combination of carbon-based materials to establish heterostructure, heteroatom doption, and electrolyte composition optimization. In 2019, Chang et al. used a straightforward one-pot wet-ball milling (WBM) technique to effectively create a KIB anode with excellent performance RP[163]. The RP/C electrode has a high reversible specific capacity (750 mAh/g) and excellent rate capacity, which can be attributed to the assistance of the conductive network made of multi-walled CNTs and the equally dispersed nanoscale RP particles, making RP an appealing anode material for K ion storage. In particular, it is hypothesized that the absence of a P-C bond facilitates the efficient reaction of the K+ with the RP, which is based on the examination of electrochemical measurement. However, Wu et al. manufactured phosphorous/carbon composites using a straightforward mechanical ball milling procedure and investigated them as KIB anode materials[157]. The findings indicate that the P/C electrode has a high capacity of 323.5 mAh/g, medium cycle stability, and rate performance. This is primarily attributable to the high theoretical specific capacity of P and the homogeneous distribution of amorphous BP in the carbon matrix by ball milling, which forms a steady P-carbon bond and effectively inhibits the volume expansion in the process of potassium and potassium removal [Figure 15A]. Ball milling is useful for promoting intimate contact between primary particles, conductive carbon matrix and P, and the viability of P/C composites with rich P-C or P-O-C chemical bonds, but it is still unable to further reduce the particle size of P to the nanoscale level. Since there was insufficient electrical contact between the collector and the active material and the limited carbon covering, which was unable to react to the significant volume shift of the P anode, performance eventually suffered. As a result, it needs to be used in conjunction with other synthesis techniques in order to significantly raise the electrochemical performance of P anodes. And then, the growing trend of the P anode is still limited by its intrinsic insulating properties and significant volume fluctuations. To overcome this situation, Liu et al. combined RP with mesoporous carbon (TBMC) and CNTs to create a special P@TBMC Composite material[164]. Multi-walled CNTs can hasten electron transmission because they contain a lot of sp2 carbon, while the mesoporous carbon layer can be used to load the right amount of P and act as a buffer layer to reduce the extreme volume expansion. Thus the P@TBMC composite performs admirably as a KIB anode in terms of reversible specific capacity, cycle performance, and rate capacity. The creation of high-performance electrode materials is facilitated by the design that restricts the active component to hybrid carbon nanostructures combined with highly conductive sp2 carbon and porous carbon.
Figure 15. (A) Schematic diagram of the synthesis of the P/C composite. Copyright 2018, Journal of Power Sources[157]. (B) Schematic illustration of the fabrication process of red P@AC and red P@AC@PPy composite. Copyright 2020, Nano energy[165]. (C) DFT computations of P atoms adsorbed on nondefective or nitrogen-doped carbon surface and corresponding adsorption energy (Ead). The brown, blue, and red balls represent C, N, and P atoms, respectively. Copyright 2019, Nano Letters[92]. (D) The corresponding three-dimensional charge density and two-dimensional charge density maps in V3Se4/CNF system and V3Se4/NPCNF system, respectively. The three-dimensional charge density par value is set at 0.14 e/bohr3. Saturation values are marked on the two-dimensional charge density scale, and high values correspond to electron abundance. Copyright 2021, Chemical Engineering Journal[168].
The introduction of carbon materials into P-based anode materials may significantly increase rate performance and structural stability owing to their larger surface area and powerful conductivity. For instance, Fang et al. synthesized an air-stabilized RP anode that improves electronic conductivity by isolating the active substance from direct contact with the electrolyte [Figure 15B][165]. The composite as a potassium electric anode presents reversible capacity and long cycle life, which is mainly attributed to the RP-activated carbon (P@AC) composite surface coated conductive polypyrrole (PPy) layer can prevent air oxidation, protect the RP material, and improve the structural stability of active materials. Additionally,
In addition, the use of P in electronic equipment is also constrained by various flaws. High carrier mobility and magnetism are vital for spintronic devices, which motivates researchers to keep investigating the electrical structure and magnetic properties of materials. Doping is a dependable and effective way to change the electrical structure of 2D materials and introduce magnetism. Nowadays, a novel 2D material known as a phosphor that possesses higher ranges of magnetic and electron mobility at ambient temperature has been successfully extracted from black phosphor block by mechanical stripping[169]. Currently, Te-doped phosphors have been made by Zhang et al. using chemical vapor transfer, while
Figure 16. (A)The spin-polarized DOS of Ce-Ce, Ti-Ti, Ce-Ti, Ce-up, Ti-up, Ce- dn, and Ti-dn, respectively. Copyright 2019, Nano Structures[172]. (B) Band structures of Y-, Zr-, Nb-, and Mo-doped blue phosphorene by substitutional doping method. The blue lines (red lines) represent the spin-up (spin-down) channels. The Fermi level (EF) represented by a dotted line is set at E = 0 eV. The TDOS and PDOS of (C) Y-, (D) Zr-, (E) Nb-, and (F) Mo-doped blue phosphorene by substitutional doping method. The EF is set at zero energy and indicated by the vertical black dashed line. Copyright 2021, Thin Solid Films[173].
CONCLUSION
This article comprehensively discusses the mechanism and structure-activity relationship of carbon-based and alloy-based anodes with high performance, interface stability, and energy storage mechanisms, and the specific performance is shown in Table 1. Carbon materials are superior to many other materials because of their high conductivity, low cost, and non-toxic properties. In addition, heteroatom doping and nano-engineering can significantly improve the potassium storage performance of carbon-based materials. For example, N doping can improve the adsorption energy and electronic conductivity of K ions, which helps to quickly embed or detach ions or electrons and maintain structural integrity. In addition, carbon-based materials with nanostructures have strong structural stability, which can reduce the diffusion path of ions and ensure excellent electrochemical capability. Alloy-based materials are attributed to their good working voltage and strong theoretical specific capacity, making them one of the most promising candidate materials in KIBs. At present, the problems of scale production of KIBs mainly focus on excessive volume expansion, short cycle life, and low initial coulomb efficiency during charging and discharging. Therefore, future research may gradually shift from structural design to alleviating volume expansion and improving electronic conductivity, further promoting interface stability and capacity reversibility. Several possible research options are listed as follows:
Recent progress on carbon-based and alloy-based anode materials in KIBs
| Materials | Charge capacity (mA/g) | Rate capacity | Capacity retention | Refs. |
| K2TP | 229 (200 mA/g) | - | 94.6% | [26] |
| NOPC@G | 445 | 112 (40 C) | - | [28] |
| MG | 115 (4 A/g) | - | 75% (20,000 cycles) | [31] |
| PODG | 474 (50 mA/g) | - | 160 mAh/g (2,000 mA/g) | [33] |
| HC/CB | 200 (0.1 C) | - | 57% (685 cycles) | [36] |
| HCS | 262 | - | 83% (100 cycles) | [38] |
| HC-HCl | 253 (40 mA/g) | 164 (4 A/g) | 92% (150 cycles) | [39] |
| HHC | 195.3 (0.1 A/g) | - | 67.43% (150 cycles) | [40] |
| LPG | 150 (100 mA/g) | 70 (200 mA/g) | 225 mAh/g (400 cycles) | [45] |
| NCS | 154 (72 C) | - | 180 mAh/g (4,000 mA/g) | [49] |
| CoSe/C | 597.2 (0.2 A/g) | - | 361.9 mAh/g (16 A/g) | [50] |
| HN-CFA | 282 (0.1 A/g) | 175 (5 A/g) | 249 mAh/g (7,000 cycles) | [51] |
| A-KBT-7 | 496.1 (50 mA/g) | - | 102.1 mAh/g (2,300 cycles) | [52] |
| SP-HC | 284 (0.1 C) | 167(1C) | 71% (300 cycles) | [53] |
| CNF | 210 (1 A/g) | - | 100 mAh/g (7.7 A/g) | [59] |
| CNC | 175 (35 C) | - | 79% (35 C) | [60] |
| OCFGs | 370.2 (0.2 A/g) | 137 (4 A/g) | - | [61] |
| MHCNFs | 329.1 (100 mA/g) | 268.5 (5,000 mA/g) | 252.6 mAh/g (2,500 cycles) | [63] |
| PCMs | 226.6 (50 mA/g) | 158.1 (1,000 mA/g) | 108.4 mAh/g (2,000 cycles) | [64] |
| HG-CNFs | 200 | 226 (35 C) | fade 0.008% (400 cycles) | [67] |
| NCNFs | 248 (25 mA/g) | 101 (20 A/g) | 146 mAh/g (4,000 cycles) | [71] |
| HPNCFs | 197 (50 mA/g) | 57 (250 mA/g) | 197 mAh/g (90 cycles) | [72] |
| NPCF | 327 (100 mA/g) | 144 (10 A/g) | 258.9 mAh/g (2,000 cycles) | [73] |
| N-SPC | 288 (0.2 A/g) | 116 (20 A/g) | 296 mAh/g (2,000 cycles) | [75] |
| PN-PCM | 396 (0.1 A/g) | 168 (5 A/g) | 218 mAh/g (3,000 cycles) | [76] |
| PIBN-G | 271 (0.1 C) | - | 86% (300 cycles) | [88] |
| red P@N-PHCNFs | 465 (2 A/g) | 342 (5 A/g) | 465 mAh/g ( 2 A/g, 800 cycles) | [92] |
| Bi0.5Sb0.5@P | 339.1 (1 A/g) | 258.5 (6.5 A/g) | 295.4 mAh/g (500 mA/g, 550 cycles) | [94] |
| 8-Sn@C | 493.6 (200 mA/g) | 349 (4,000 mA/g) | 415 mAh/g (1,000 mA/g, 500 cycles) | [100] |
| S-BC | 339.3 (50 mA/g) | 124.2 (1 A/g) | 203.8 mAh/g (200 mA/g, 300 cycles) | [102] |
| CoS@CNF-CNT/EG | 411 | 184 (2 A/g) | 84.9% (100 cycles) | [107] |
| Sb@rGO | 381 (100 mA/g) | - | 210 mAh/g (500 mA/g, 200 cycles) | [109] |
| Sb4O5Cl2 | 530 (50 mA/g) | 316 (100 mA/g) | - | [111] |
| Co3O4-Fe2O3/C | 220 (50 mA/g) | - | 93% (180 cycles) | [112] |
| Sb@G@C | 474 (100 mA/g) | - | 72.3% (800 cycles) | [113] |
| Sb@NPMC | 384.8 (50 mA/g) | 130 (1 A/g) | - | [114] |
| NP-Sb | 560 (50 mA/g) | 265 (500 mA/g) | - | [116] |
| Sb-C-rGO | 310 (0.5 A/g) | - | 79% (100 cycles) | [117] |
| Sb@C-3DP | 516 (0.05 A/g) | 286 (1 A/g) | 97% (260 cycles) | [119] |
| Sb2Se3@C | 312.8 (100 mA/g) | 223.3 (1 A/g) | 191.4 mAh/g (500 mA/g, 400 cycles) | [123] |
| BiSb@C | 598.2 (100 mA/g) | 152 (2 A/g) | 97.5% (500 mA/g, 600 cycles) | [124] |
| Ti3C2-Sb2S3 | 461 (100 mA/g) | 102 (2 A/g) | 79 % (500 cycles) | [125] |
| SnO2-G-C | - | 114.81 (1 A/g) | 202.06 mAh/g (100 mA/g, 100 cycles) | [139] |
| Sn4P3@C | 473.3 (50 mA/g) | 183.6 (2A /g) | 181.5 mAh/g (500 mA/g, 800 cycles) | [144] |
| PAC-50 | 430 | - | 70% (500 cycles) | [156] |
| P@CN | 655 (100 mA/g) | 323.7 (2 A/g) | - | [158] |
(1) Alloying and heterostructures may become the primary targets of structural design and morphological optimization due to significant studies on basic materials. Unique qualities that set it apart from ordinary additions may come from material design. However, not all unique structural designs are advantageous without any drawbacks, such as the nanoscale method that gives alloy-based materials a lot of specific surface area while also consuming a lot of electrolytes. Therefore, the balance between particle size and specific surface area should be taken into account while designing structures.
(2) Equally important is the choice of anode materials that serve as K+ carriers in the electrochemical process. Apart from the carbon-based and alloy-based anode materials discussed above, they also include other types of anodes, such as intercalation anodes, conversion anodes, organic anode materials, and so on. Potassium metal was originally the chosen anode material for high-performance KIBs due to its large theoretical capacity. However, potassium metal undergoes excessive chemical reactions with the electrolyte components, forming an unstable SEI layer and increasing electrode polarization during the entire charging and discharging process attributed to its high chemical reactivity, leading to a noticeably lower capacity. Additionally, owing to its volume expansion, high air oxidizability, and associated safety considerations, it has gradually been removed from the list of possible anode materials for KIBs. Moreover, it has a reduced energy density as an electrode material because of its low specificity and high operating voltage. Nevertheless, due to their high theoretical specific capacity and distinctive pseudo capacitance phenomenon, transition metal oxides, transition metal sulfides, transition metal selenide, and other conversion anode materials generated great concern among researchers. But innate flaws, such as poor conductivity and significant volume expansion, have a negative impact on the performance of electrode materials. To enhance its electrochemical performance, it is typically coupled with carbon-based materials or constructed with suitable nanostructures. Additionally, organic molecules can provide more buffer space between one another, which enhances the ability of electrode materials to store potassium. However, its practical application is restricted by the lower energy density (low material density) and poor electronic conductivity. In order to address this issue, the electrode preparation procedure can include the proper addition of conductive carbon and active materials.
(3) To build an industrial chain for realistic large-scale production, it will be crucial to investigate the performance of carbon- and alloy-based anode materials of full-cell KIBs. The matching of full-cell KIBs necessitates a thorough investigation of the electrochemical reaction processes between the cathode, anode, and various electrolytes in order to obtain high capacity and energy density. The performance of PIB full batteries has received relatively few research reports to date. For instance, Zhao et al. utilized Prussian blue potassium (KPB) and ZnSe/CoSe2@N-CNTs /rGO assembled full battery, exhibiting a capacity of
(4) Safety has always been the focus of attention for KIBs. The primary concern raised here is the propensity for explosive reactions of potassium metal anodes with highly flammable organic electrolytes and potential safety risks, such as short circuits, overheating, and explosions. This can be accomplished by creating potassium batteries that are entirely solid-state, creating potassium metals with complex structures and stable interfaces, or employing liquid alloys based on alkali metals. Additionally, employing flame retardant electrolytes, ionic liquid electrolytes, and all solid electrolytes can reduce the flammability of electrolytes and their combinations, which can enhance safety to some extent.
(5) Within this overview, the potassium storage mechanism of KIBs developed for carbon- and alloy-based anode materials was thoroughly investigated via DFT theory calculations, and positive evidence is given for multiple modification strategies to enhance the potassium storage capacities of the materials. Meanwhile, the worth of material calculation simulation extends beyond this and involves the following: (1) separate the influence parameters in experimental conditions as a single factor to explore their contribution to experimental results and then find internal laws to reasonably explain experimental phenomena; (2) predict the characteristics of novel materials; (3) simulate experimental processes under extreme conditions or super ideal states and; (4) establish a new structural model based on comprehensive experimental conditions, analyze and establish a new theoretical system, etc. The primary DFT algorithms contain the molecular dynamics algorithm, the forward (reverse) Monte Carlo method, and the first-principles algorithm. In-depth investigation and exploration are the activities we need to complete next while our comprehension and implementation of DFT theoretical calculations are still in stages of development.
DECLARATIONS
Authors’ contributions
Searched a large amount of literature, wrote a complete manuscript, and obtained copyright licenses for all cited images in the manuscript: Chen Y, Sun H
Provide the research direction and funding support: Zhang Z
Helped to guide the review, including language checking and polishing: Li WJ, Chou S, Jiang Y
Image organization and layout: Guo J, Zhao Y, Yang H, Li H
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was financially supported by the National Natural Science Foundation of China (No. 52271011).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2023
REFERENCES
1. Yuan F, Zhang W, Zhang D, et al. Recent progress in electrochemical performance of binder-free anodes for potassium-ion batteries. Nanoscale 2021;13:5965-84.
2. Wu Y, Zhao H, Wu Z, et al. Rational design of carbon materials as anodes for potassium-ion batteries. Energy Stor Mater 2021;34:483-507.
3. Yang M, Kong Q, Feng W, Yao W. N/O double-doped biomass hard carbon material realizes fast and stable potassium ion storage. Carbon 2021;176:71-82.
4. Yang M, Kong Q, Feng W, Yao W, Wang Q. Hierarchical porous nitrogen, oxygen, and phosphorus ternary doped hollow biomass carbon spheres for high-speed and long-life potassium storage. Carbon Energy 2022;4:45-59.
5. McCulloch WD, Ren X, Yu M, Huang Z, Wu Y. Potassium-ion oxygen battery based on a high capacity antimony anode. ACS Appl Mater Inter 2015;7:26158-66.
6. Han Y, Li T, Li Y, et al. Stabilizing antimony nanocrystals within ultrathin carbon nanosheets for high-performance K-ion storage. Energy Stor Mater 2019;20:46-54.
7. Ma L, Lv Y, Wu J, et al. Recent advances in anode materials for potassium-ion batteries: a review. Nano Res 2021;14:4442-70.
8. An Y, Fei H, Zeng G, et al. Commercial expanded graphite as a low-cost, long-cycling life anode for potassium-ion batteries with conventional carbonate electrolyte. J Power Sources 2018;378:66-72.
10. Han C, Han K, Wang X, et al. Three-dimensional carbon network confined antimony nanoparticle anodes for high-capacity K-ion batteries. Nanoscale 2018;10:6820-6.
11. Zeng S, Chen X, Xu R, et al. Boosting the potassium storage performance of carbon anode via integration of adsorption-intercalation hybrid mechanisms. Nano Energy 2020;73:104807.
12. Tao S, Xu W, Zheng J, et al. Soybean roots-derived N, P Co-doped mesoporous hard carbon for boosting sodium and potassium-ion batteries. Carbon 2021;178:233-42.
13. Wu X, Chen Y, Xing Z, et al. Advanced carbon-based anodes for potassium-ion batteries. Adv Energy Mater 2019;9:1900343.
14. Xu H, Rosa AL, Frauenheim T, Zhang RQ. N-doped ZnO nanowires: surface segregation, the effect of hydrogen passivation and applications in spintronics. Phys Stat Sol 2010;247:2195-201.
15. Zhu Y, Wang Y, Wang Y, Xu T, Chang P. Research progress on carbon materials as negative electrodes in sodium- and potassium-ion batteries. Carbon Energy 2022;4:1182-213.
16. Ma C, Shao X, Cao D. Nitrogen-doped graphenenanosheets as anode materials for lithium ion batteries: a first-principles study. J Mater Chem 2012;22:8911-15.
17. Liu W, Deng X, Cai S. Transport properties for carbon chain sandwiched between heteroatom-doped carbon nanotubes with different doping sites. AIP Adv 2016;6:075103.
18. Wang Z, Selbach SM, Grande T. Van der Waals density functional study of the energetics of alkali metal intercalation in graphite. RSC Adv 2014;4:4069-79.
19. Luo P, Zheng C, He J, et al. Structural engineering in graphite-based metal-ion batteries. Adv Funct Mater 2022;32:2107277.
20. Li X, Li J, Ma L, et al. Graphite anode for potassium ion batteries: current status and perspective. Energy Environ Mater 2022;5:458-69.
21. Wang D, Zhang J, Dong Y, et al. Progress on graphitic carbon materials for potassium- based energy storage. New Carbon Mater 2021;36:435-48.
22. Costard E, Bois P, Marcadet X, Nedelcu A. QWIP and 3rd generation IR imagers. Infrared Technol Appl 2005 ;5783:728.
23. Dimiev AM, Shukhina K, Behabtu N, Pasquali M, Tour JM. Stage transitions in graphite intercalation compounds: role of the graphite structure. J Phys Chem C 2019;123:19246-53.
24. Xing Z, Qi Y, Jian Z, Ji X. Polynanocrystalline graphite: a new carbon anode with superior cycling performance for K-ion batteries. ACS Appl Mater Inter 2017;9:4343-51.
25. Zhang W, Liu Y, Guo Z. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci Adv 2019;5:eaav7412.
26. Lei K, Li F, Mu C, et al. High K-storage performance based on the synergy of dipotassium terephthalate and ether-based electrolytes. Energy Environ Sci 2017;10:552-7.
27. Komaba S, Hasegawa T, Dahbi M, Kubota K. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem Commun 2015;60:172-5.
28. Sun Y, Xiao H, Li H, et al. Nitrogen/Oxygen co-doped hierarchically porous carbon for high-performance potassium storage. Chemistry 2019;25:7359-65.
29. Luo W, Wan J, Ozdemir B, et al. Potassium ion batteries with graphitic materials. Nano Lett 2015;15:7671-7.
30. Li J, Yi Y, Zuo X, et al. Graphdiyne/Graphene/Graphdiyne sandwiched carbonaceous anode for potassium-ion batteries. ACS Nano 2022;16:3163-72.
31. Yuan F, Lei Y, Wang H, et al. Pseudo-capacitance reinforced modified graphite for fast-charging potassium-ion batteries. Carbon 2021;185:48-56.
32. Capone I, Aspinall J, Lee HJ, Xiao AW, Ihli J, Pasta M. A red phosphorus-graphite composite as anode material for potassium-ion batteries. Mater Today Energy 2021;21:100840.
33. Ma G, Huang K, Ma J, Ju Z, Xing Z, Zhuang Q. Phosphorus and oxygen dual-doped graphene as superior anode material for room-temperature potassium-ion batteries. J Mater Chem A 2017;5:7854-61.
34. Lee B, Kim M, Kim S, et al. High capacity adsorption - dominated potassium and sodium ion storage in activated crumpled graphene. Adv Energy Mater 2020;10:1903280.
35. Li L, Liu L, Hu Z, et al. Understanding high-rate K+-solvent Co-intercalation in natural graphite for potassium-ion batteries. Angew Chem Int Ed 2020;59:12917-24.
36. Vaalma C, Giffin GA, Buchholz D, Passerini S. Non-aqueous K-ion battery based on layered K0.3MnO2 and hard carbon/carbon black. J Electrochem Soc 2016;163:A1295-9.
37. Liu Y, Dai H, Wu L, et al. A large scalable and low-cost sulfur/nitrogen dual-doped hard carbon as the negative electrode material for high-performance potassium-ion batteries. Adv Energy Mater 2019;9:1901379.
38. Jian Z, Xing Z, Bommier C, Li Z, Ji X. Hard carbon microspheres: potassium-ion anode versus sodium-ion anode. Adv Energy Mater 2016;6:1501874.
39. He X, Liao J, Tang Z, et al. Highly disordered hard carbon derived from skimmed cotton as a high-performance anode material for potassium-ion batteries. J Power Sources 2018;396:533-41.
40. Zhang Y, Yang L, Tian Y, et al. Honeycomb hard carbon derived from carbon quantum dots as anode material for K-ion batteries. Mater Chem Phys 2019;229:303-9.
41. Stevens DA, Dahn JR. An in situ small-angle X-ray scattering study of sodium insertion into a nanoporous carbon anode material within an operating electrochemical cell. J Electrochem Soc 2000;147:4428.
42. Kim J, Lee G, Lau VW, et al. A microstructural investigation into Na-ion storage behaviors of cellulose-based hard carbons for Na-ion batteries. J Phys Chem C 2021;125:14559-66.
43. Bommier C, Surta TW, Dolgos M, Ji X. New mechanistic insights on Na-ion storage in nongraphitizable carbon. Nano Lett 2015;15:5888-92.
44. Li W, Zhang R, Chen Z, et al. Microstructure-dependent K+ storage in porous hard carbon. Small 2021;17:e2100397.
45. Wu Z, Wang L, Huang J, et al. Loofah-derived carbon as an anode material for potassium ion and lithium ion batteries. Electrochimica Acta 2019;306:446-53.
46. Yuan F, Zhang D, Li Z, et al. Unraveling the intercorrelation between micro/mesopores and K migration behavior in hard carbon. Small 2022;18:e2107113.
47. Wang X, Han K, Qin D, et al. Polycrystalline soft carbon semi-hollow microrods as anode for advanced K-ion full batteries. Nanoscale 2017;9:18216-22.
48. Bin DS, Chi ZX, Li Y, et al. Controlling the compositional chemistry in single nanoparticles for functional hollow carbon nanospheres. J Am Chem Soc 2017;139:13492-8.
49. Chen C, Wang Z, Zhang B, et al. Nitrogen-rich hard carbon as a highly durable anode for high-power potassium-ion batteries. Energy Stor Mater 2017;8:161-8.
50. Zhang Y, Pan A, Ding L, et al. Nitrogen-doped yolk-shell-structured CoSe/C dodecahedra for high-performance sodium ion batteries. ACS Appl Mater Inter 2017;9:3624-33.
51. Lu Y, Li D, Lyu C, et al. High nitrogen doped carbon nanofiber aerogels for sodium ion batteries: synergy of vacancy defects to boost sodium ion storage. Appl Surf Sci 2019;496:143717.
52. Gao Y, Ru Q, Zheng M, et al. Recovery of kitchen bio-waste from spent black tea as hierarchical biomorphic carbon electrodes for ultra-long lifespan potassium-ion storage. Appl Surf Sci 2021;555:149675.
53. Chen C, Wu M, Wang Y, Zaghib K. Insights into pseudographite-structured hard carbon with stabilized performance for high energy K-ion storage. J Power Sources 2019;444:227310.
54. Li J, Kaur AP, Meier MS, Cheng Y. Stacked-cup-type MWCNTs as highly stable lithium-ion battery anodes. J Appl Electrochem 2014;44:179-87.
55. Tang Y, Zhao Y, Burkert SC, Ding M, Ellis JE, Star A. Efficient separation of nitrogen-doped carbon nanotube cups. Carbon 2014;80:583-90.
56. Tang Y, Burkert SC, Zhao Y, Saidi WA, Star A. The effect of metal catalyst on the electrocatalytic activity of nitrogen-doped carbon nanotubes. J Phys Chem C 2013;117:25213-21.
57. Gabaudan V, Monconduit L, Stievano L, Berthelot R. Snapshot on negative electrode materials for potassium-ion batteries. Front Energy Res 2019;7:46.
59. Zhao X, Xiong P, Meng J, Liang Y, Wang J, Xu Y. High rate and long cycle life porous carbon nanofiber paper anodes for potassium-ion batteries. J Mater Chem A 2017;5:19237-44.
60. Cao B, Zhang Q, Liu H, et al. Graphitic carbon nanocage as a stable and high power anode for potassium-ion batteries. Adv Energy Mater 2018;8:1801149.
61. Wang G, Xiong X, Xie D, et al. Chemically activated hollow carbon nanospheres as a high-performance anode material for potassium ion batteries. J Mater Chem A 2018;6:24317-23.
62. Sun J, Ma L, Sun H, et al. Design of free-standing porous carbon fibers anode with high-efficiency potassium-ion storage. Chem Eng J 2023;455:140902.
63. Wu Y, Wu Z, Yue L, et al. Directionally Tailoring Macroporous Honeycomb-Like Structured Carbon Nanofibers toward High-Capacitive Potassium Storage. ACS Appl Mater Inter 2021;13:30693-702.
64. Chen M, Wang W, Liang X, et al. Sulfur/Oxygen codoped porous hard carbon microspheres for high-performance potassium-ion batteries. Adv Energy Mater 2018;8:1800171.
65. Hu X, Zhong G, Li J, et al. Hierarchical porous carbon nanofibers for compatible anode and cathode of potassium-ion hybrid capacitor. Energy Environ Sci 2020;13:2431-40.
66. Wen J, Xu L, Wang J, et al. Lithium and potassium storage behavior comparison for porous nanoflaked Co3O4 anode in lithium-ion and potassium-ion batteries. J Power Sources 2020;474:228491.
67. Tian S, Jiang Q, Cai T, et al. Graphitized electrospun carbon fibers with superior cyclability as a free-standing anode of potassium-ion batteries. J Power Sources 2020;474:228479.
68. Ma H, Qi X, Peng D, et al. Novel fabrication of N/S Co-doped hierarchically porous carbon for potassium-ion batteries. ChemistrySelect 2019;4:11488-95.
69. Tao L, Yang Y, Wang H, et al. Sulfur-nitrogen rich carbon as stable high capacity potassium ion battery anode: performance and storage mechanisms. Energy Stor Mater 2020;27:212-25.
70. Wang Y, Yuan F, Li Z, Zhang D, Yu Q, Wang B. Heteroatom-doped carbon anode materials for potassium-ion batteries: from mechanism, synthesis to electrochemical performance. APL Mater 2022;10:030902.
71. Xu Y, Zhang C, Zhou M, et al. Highly nitrogen doped carbon nanofibers with superior rate capability and cyclability for potassium ion batteries. Nat Commun 2018;9:1720.
72. Zhang M, Shoaib M, Fei H, et al. Hierarchically porous N-doped carbon fibers as a free-standing anode for high-capacity potassium-based dual-ion battery. Adv Energy Mater 2019;9:1901663.
73. Tong H, Wang C, Lu J, et al. Energetic metal-organic frameworks derived highly nitrogen-doped porous carbon for superior potassium storage. Small 2020;16:e2002771.
74. Chen S, Feng Y, Wang J, Zhang E, Yu X, Lu B. Free-standing N-doped hollow carbon fibers as high-performance anode for potassium ion batteries. Sci China Mater 2021;64:547-56.
75. Zhao L, Yang H, He F, et al. Biomimetic N-doped sea-urchin-structured porous carbon for the anode material of high-energy-density potassium-ion batteries. Electrochim Acta 2021;388:138565.
76. Gong J, Zhao G, Feng J, et al. Controllable phosphorylation strategy for free-standing phosphorus/nitrogen cofunctionalized porous carbon monoliths as high-performance potassium ion battery anodes. ACS Nano 2020;14:14057-69.
77. Yang J, Ju Z, Jiang Y, et al. Enhanced capacity and rate capability of nitrogen/oxygen dual-doped hard carbon in capacitive potassium-ion storage. Adv Mater 2018;30:1700104.
78. Lin X, Huang J, Zhang B. Correlation between the microstructure of carbon materials and their potassium ion storage performance. Carbon 2019;143:138-46.
79. Share K, Cohn AP, Carter R, Rogers B, Pint CL. Role of nitrogen-doped graphene for improved high-capacity potassium ion battery anodes. ACS Nano 2016;10:9738-44.
80. Shang Y, Duan X, Wang S, Yue Q, Gao B, Xu X. Carbon-based single atom catalyst: synthesis, characterization, DFT calculations. Chin Chem Lett 2022;33:663-73.
81. Adekoya D, Qian S, Gu X, et al. DFT-guided design and fabrication of carbon-nitride-based materials for energy storage devices: a review. Nanomicro Lett 2020;13:13.
82. Liu Q, Hu Z, Chen M, et al. The cathode choice for commercialization of sodium-ion batteries: layered transition metal oxides versus prussian blue analogs. Adv Funct Mater 2020;30:1909530.
83. Xiao Z, Meng J, Xia F, et al. K+ modulated K+/vacancy disordered layered oxide for high-rate and high-capacity potassium-ion batteries. Energy Environ Sci 2020;13:3129-37.
84. Zhao L, Zhang T, Zhao H, Hou Y. Polyanion-type electrode materials for advanced sodium-ion batteries. Mater Today Nano 2020;10:100072.
85. Chen M, Liu Q, Zhang Y, Xing G, Chou SL, Tang Y. Emerging polyanionic and organic compounds for high energy density, non-aqueous potassium-ion batteries. J Mater Chem A 2020;8:16061-80.
86. Liang Y, Luo C, Wang F, et al. An organic anode for high temperature potassium-ion batteries. Adv Energy Mater 2019;9:1802986.
87. Deng Q, Pei J, Fan C, et al. Potassium salts of para-aromatic dicarboxylates as the highly efficient organic anodes for low-cost K-ion batteries. Nano Energy 2017;33:350-5.
88. Luo Z, Liu L, Ning J, et al. A microporous covalent-organic framework with abundant accessible carbonyl groups for lithium-ion batteries. Angew Chem Int Ed 2018;57:9443-6.
89. Wu Y, Jiang Y, Shi J, Gu L, Yu Y. Multichannel porous TiO2 hollow nanofibers with rich oxygen vacancies and high grain boundary density enabling superior sodium storage performance. Small 2017;13:1700129.
90. Li Y, Wang H, Wang L, et al. Mesopore-induced ultrafast Na+-storage in T-Nb2O5/carbon nanofiber films toward flexible high-power Na-ion capacitors. Small 2019;15:e1804539.
91. Cui Y, Liu W, Feng W, et al. Controlled design of well-dispersed ultrathin MoS2 nanosheets inside hollow carbon skeleton: toward fast potassium storage by constructing spacious “houses” for K ions. Adv Funct Mater 2020;30:1908755.
92. Wu Y, Hu S, Xu R, et al. Boosting potassium-ion battery performance by encapsulating red phosphorus in free-standing nitrogen-doped porous hollow carbon nanofibers. Nano Lett 2019;19:1351-8.
93. Wang Q, Zhao X, Ni C, et al. Reaction and capacity-fading mechanisms of tin nanoparticles in potassium-ion batteries. J Phys Chem C 2017;121:12652-7.
94. Chen KT, Tuan HY. Bi-Sb nanocrystals embedded in phosphorus as high-performance potassium ion battery electrodes. ACS Nano 2020;14:11648-61.
95. Xia M, Chen B, Gu F, et al. Ti3C2Tx MXene nanosheets as a robust and conductive tight on Si anodes significantly enhance electrochemical lithium storage performance. ACS Nano 2020;14:5111-20.
96. Yang Q, Wang Z, Xi W, He G. Tailoring nanoporous structures of Ge anodes for stable potassium-ion batteries. Electrochem Commun 2019;101:68-72.
97. Zhang L, Li Y, Zhang S, et al. Non-newtonian fluid state K-Na alloy for a stretchable energy storage device. Small Methods 2019;3:1900383.
98. Eftekhari A. Low voltage anode materials for lithium-ion batteries. Energy Stor Mater 2017;7:157-80.
99. Kim H, Kim JC, Bianchini M, Seo D, Rodriguez-garcia J, Ceder G. Recent progress and perspective in electrode materials for K-ion batteries. Adv Energy Mater 2018;8:1702384.
100. Liu Y, Zhang N, Jiao L, Tao Z, Chen J. Ultrasmall Sn nanoparticles embedded in carbon as high-performance anode for sodium-ion batteries. Adv Funct Mater 2015;25:214-20.
101. Yao Q, Zhu C. Advanced post-potassium-ion batteries as emerging potassium-based alternatives for energy storage. Adv Funct Mater 2020;30:2005209.
102. Tian S, Guan D, Lu J, et al. Synthesis of the electrochemically stable sulfur-doped bamboo charcoal as the anode material of potassium-ion batteries. J Power Sources 2020;448:227572.
103. Yang G, Ilango PR, Wang S, et al. Carbon-based alloy-type composite anode materials toward sodium-ion batteries. Small 2019;15:e1900628.
104. Liang S, Cheng Y, Zhu J, Xia Y, Müller-buschbaum P. A chronicle review of nonsilicon (Sn, Sb, Ge)-based lithium/sodium-ion battery alloying anodes. Small Methods 2020;4:2000218.
105. Sultana I, Rahman MM, Chen Y, Glushenkov AM. Potassium-ion battery anode materials operating through the alloying-dealloying reaction mechanism. Adv Funct Mater 2018;28:1703857.
106. Yang GZ, Chen YF, Feng BQ, et al. Surface-dominated potassium storage enabled by single-atomic sulfur for high-performance K-ion battery anodes. Energy Environ Sci 2023;16:1540-7.
107. Wang L, Zhao Y, Cao L, et al. CoS nanoparticle encapsulated S-doped carbon nanofiber/nanotube hybrid grown on exfoliated graphite for long-lifespan and high-rate potassium ion batteries. Appl Surf Sci 2022;603:154370.
108. Song K, Liu C, Mi L, Chou S, Chen W, Shen C. Recent progress on the alloy-based anode for sodium-ion batteries and potassium-ion batteries. Small 2021;17:e1903194.
109. Yi Z, Lin N, Zhang W, Wang W, Zhu Y, Qian Y. Preparation of Sb nanoparticles in molten salt and their potassium storage performance and mechanism. Nanoscale 2018;10:13236-41.
110. Gabaudan V, Berthelot R, Stievano L, Monconduit L. Inside the alloy mechanism of Sb and Bi electrodes for K-ion batteries. J Phys Chem C 2018;122:18266-73.
111. Shi Y, Wang L, Zhou D, Wu T, Xiao Z. A flower-like Sb4O5Cl2 cluster-based material as anode for potassium ion batteries. Appl Surf Sci 2022;583:152509.
112. Sultana I, Rahman MM, Mateti S, Ahmadabadi VG, Glushenkov AM, Chen Y. K-ion and Na-ion storage performances of
113. Liu Q, Fan L, Ma R, et al. Super long-life potassium-ion batteries based on an antimony@carbon composite anode. Chem Commun 2018;54:11773-6.
114. Zhang W, Miao W, Liu X, Li L, Yu Z, Zhang Q. High-rate and ultralong-stable potassium-ion batteries based on antimony-nanoparticles encapsulated in nitrogen and phosphorus Co-doped mesoporous carbon nanofibers as an anode material. J Alloys Compd 2018;769:141-8.
115. Gabaudan V, Touja J, Cot D, Flahaut E, Stievano L, Monconduit L. Double-walled carbon nanotubes, a performing additive to enhance capacity retention of antimony anode in potassium-ion batteries. Electrochem Commun 2019;105:106493.
116. An Y, Tian Y, Ci L, Xiong S, Feng J, Qian Y. Micron-sized nanoporous antimony with tunable porosity for high-performance potassium-ion batteries. ACS Nano 2018;12:12932-40.
117. Ko YN, Choi SH, Kim H, Kim HJ. One-pot formation of Sb-carbon microspheres with graphene sheets: potassium-ion storage properties and discharge mechanisms. ACS Appl Mater Inter 2019;11:27973-81.
118. Ge X, Liu S, Qiao M, et al. Enabling superior electrochemical properties for highly efficient potassium storage by impregnating ultrafine Sb nanocrystals within nanochannel-containing carbon nanofibers. Angew Chem Int Ed 2019;58:14578-83.
119. He XD, Liu ZH, Liao JY, et al. A three-dimensional macroporous antimony@carbon composite as a high-performance anode material for potassium-ion batteries. J Mater Chem A 2019;7:9629-37.
120. Sultana I, Rahman MM, Liu J, et al. Antimony-carbon nanocomposites for potassium-ion batteries: insight into the failure mechanism in electrodes and possible avenues to improve cyclic stability. J Power Sources 2019;413:476-84.
121. Luo W, Li F, Gaumet J, et al. Bottom-up confined synthesis of nanorod-in-nanotube structured Sb@N-C for durable lithium and sodium storage. Adv Energy Mater 2018;8:1703237.
122. Huang Y, Wang Z, Jiang Y, et al. Conductivity and pseudocapacitance optimization of bimetallic antimony-indium sulfide anodes for sodium-ion batteries with favorable kinetics. Adv Sci 2018;5:1800613.
123. Yi Z, Qian Y, Tian J, Shen K, Lin N, Qian Y. Self-templating growth of Sb2Se3@C microtube: a convention-alloying-type anode material for enhanced K-ion batteries. J Mater Chem A 2019;7:12283-91.
124. Xiong P, Wu J, Zhou M, Xu Y. Bismuth-antimony alloy nanoparticle@porous carbon nanosheet composite anode for high-performance potassium-ion batteries. ACS Nano 2020;14:1018-26.
125. Wang T, Shen D, Liu H, Chen H, Liu Q, Lu B. A Sb2S3 nanoflower/MXene composite as an anode for potassium-ion batteries. ACS Appl Mater Inter 2020;12:57907-15.
126. Yi Z, Jiang S, Du Y, et al. Coordinatively and spatially coconfining high-loading atomic Sb in sulfur-rich 2D carbon matrix for fast K+ diffusion and storage. ACS Mater Lett 2021;3:790-8.
127. Nguyen TH, Man MT, Do HM, Nguyen VV. Magnetic properties and electronic structure of the Sb-doped MnBi from DFT calculations. Solid State Commun 2021;336:114385.
128. Zhao M, Zhao Q, Qiu J, Xue H, Pang H. Tin-based nanomaterials for electrochemical energy storage. RSC Adv 2016;6:95449-68.
129. Huang B, Pan Z, Su X, An L. Tin-based materials as versatile anodes for alkali (earth)-ion batteries. J Power Sources 2018;395:41-59.
130. Nita C, Fullenwarth J, Monconduit L, et al. Understanding the Sn loading impact on the performance of mesoporous carbon/Sn-based nanocomposites in Li-ion batteries. ChemElectroChem 2018;5:3249-57.
131. Liu Y, Zhang N, Jiao L, Chen J. Tin nanodots encapsulated in porous nitrogen-doped carbon nanofibers as a free-standing anode for advanced sodium-ion batteries. Adv Mater 2015;27:6702-7.
132. Sultana I, Ramireddy T, Rahman MM, Chen Y, Glushenkov AM. Tin-based composite anodes for potassium-ion batteries. Chem Commun 2016;52:9279-82.
133. Ji B, Zhang F, Song X, Tang Y. A novel potassium-ion-based dual-ion battery. Adv Mater 2017;29:1700519.
134. Li Z, Ding J, Mitlin D. Tin and Tin compounds for sodium ion battery anodes: phase transformations and performance. ACC Chem Res 2015;48:1657-65.
135. Qiu D, Guan J, Li M, et al. Kinetics enhanced nitrogen-doped hierarchical porous hollow carbon spheres boosting advanced potassium-ion hybrid capacitors. Adv Funct Mater 2019;29:1903496.
136. Han X, Liu Y, Jia Z, et al. Atomic-layer-deposition oxide nanoglue for sodium ion batteries. Nano Lett 2014;14:139-47.
137. Ramireddy T, Kali R, Jangid MK, Srihari V, Poswal HK, Mukhopadhyay A. Insights into electrochemical behavior, phase evolution and stability of Sn upon K-alloying/de-alloying via in situ studies. J Electrochem Soc 2017;164:A2360-7.
138. Gabaudan V, Berthelot R, Sougrati MT, Lippens P, Monconduit L, Stievano L. SnSb vs. Sn: improving the performance of Sn-based anodes for K-ion batteries by synergetic alloying with Sb. J Mater Chem A 2019;7:15262-70.
139. Huang Z, Chen Z, Ding S, Chen C, Zhang M. Enhanced conductivity and properties of SnO2-graphene-carbon nanofibers for potassium-ion batteries by graphene modification. Mater Lett 2018;219:19-22.
140. Ge X, Liu S, Qiao M, et al. Enabling superior electrochemical properties for highly efficient potassium storage by impregnating ultrafine Sb nanocrystals within nanochannel-containing carbon nanofibers. Angew Chem Int Ed 2019;131:14720-5.
141. Qin J, He C, Zhao N, et al. Graphene networks anchored with sn@graphene as lithium ion battery anode. ACS Nano 2014;8:1728-38.
142. Huang K, Xing Z, Wang L, et al. Direct synthesis of 3D hierarchically porous carbon/Sn composites via in situ generated NaCl crystals as templates for potassium-ion batteries anode. J Mater Chem A 2018;6:434-42.
143. Yang Y, Li D, Zhang J, et al. Sn nanoparticles anchored on N doped porous carbon as an anode for potassium ion batteries. Mater Lett 2019;256:126613.
144. Li D, Zhang Y, Sun Q, et al. Hierarchically porous carbon supported Sn4P3 as a superior anode material for potassium-ion batteries. Energy Stor Mater 2019;23:367-74.
145. Du Y, Yi Z, Chen B, et al. Sn4P3 nanoparticles confined in multilayer graphene sheets as a high-performance anode material for potassium-ion batteries. J Energy Chem 2022;66:413-21.
146. Li C, Bi AT, Chen HL, et al. Rational design of porous Sn nanospheres/N-doped carbon nanofibers as an ultra-stable potassium-ion battery anode material. J Mater Chem A 2021;9:5740-50.
147. Yamamoto T, Nohira T. Tin negative electrodes using an FSA-based ionic liquid electrolyte: improved performance of potassium secondary batteries. Chem Commun 2020;56:2538-41.
148. Liu C, Han X, Cao Y, Zhang S, Zhang Y, Sun J. Topological construction of phosphorus and carbon composite and its application in energy storage. Energy Stor Mater 2019;20:343-72.
149. Sun J, Zheng G, Lee HW, et al. Formation of stable phosphorus-carbon bond for enhanced performance in black phosphorus nanoparticle-graphite composite battery anodes. Nano Lett 2014;14:4573-80.
150. Sun J, Lee HW, Pasta M, et al. A phosphorene-graphene hybrid material as a high-capacity anode for sodium-ion batteries. Nat Nanotechnol 2015;10:980-5.
151. Li W, Yang Z, Li M, et al. Amorphous red phosphorus embedded in highly ordered mesoporous carbon with superior lithium and sodium storage capacity. Nano Lett 2016;16:1546-53.
153. Park C, Sohn H. Black phosphorus and its composite for lithium rechargeable batteries. Adv Mater 2007;19:2465-8.
154. Zhu Y, Wen Y, Fan X, et al. Red phosphorus-single-walled carbon nanotube composite as a superior anode for sodium ion batteries. ACS Nano 2015;9:3254-64.
155. Sultana I, Rahman MM, Ramireddy T, Chen Y, Glushenkov AM. High capacity potassium-ion battery anodes based on black phosphorus. J Mater Chem A 2017;5:23506-12.
156. Huang X, Liu D, Guo X, Sui X, Qu D, Chen J. Phosphorus/Carbon composite anode for potassium-ion batteries: insights into high initial coulombic efficiency and superior cyclic performance. ACS Sustain Chem Eng 2018;6:16308-14.
157. Wu X, Zhao W, Wang H, et al. Enhanced capacity of chemically bonded phosphorus/carbon composite as an anode material for potassium-ion batteries. J Power Sources 2018;378:460-7.
158. Xiong P, Bai P, Tu S, et al. Red phosphorus nanoparticle@3D interconnected carbon nanosheet framework composite for potassium-ion battery anodes. Small 2018;14:e1802140.
159. Zhang W, Mao J, Li S, Chen Z, Guo Z. Phosphorus-based alloy materials for advanced potassium-ion battery anode. J Am Chem Soc 2017;139:3316-9.
160. Yang W, Lu Y, Zhao C, Liu H. First-principles study of black phosphorus as anode material for rechargeable potassium-ion batteries. Electron Mater Lett 2020;16:89-98.
161. Li WJ, Chou SL, Wang JZ, Liu HK, Dou SX. Simply mixed commercial red phosphorus and carbon nanotube composite with exceptionally reversible sodium-ion storage. Nano Lett 2013;13:5480-4.
162. Sun J, Lee H, Pasta M, et al. Carbothermic reduction synthesis of red phosphorus-filled 3D carbon material as a high-capacity anode for sodium ion batteries. Energy Stor Mater 2016;4:130-6.
163. Chang WC, Wu JH, Chen KT, Tuan HY. Red phosphorus potassium-ion battery anodes. Adv Sci 2019;6:1801354.
164. Liu D, Huang X, Qu D, et al. Confined phosphorus in carbon nanotube-backboned mesoporous carbon as superior anode material for sodium/potassium-ion batteries. Nano Energy 2018;52:1-10.
165. Fang K, Liu D, Xiang X, et al. Air-stable red phosphorus anode for potassium/sodium-ion batteries enabled through dual-protection design. Nano Energy 2020;69:104451.
166. Bai J, Xi B, Mao H, et al. One-step construction of N,P-codoped porous carbon sheets/CoP hybrids with enhanced lithium and potassium storage. Adv Mater 2018;30:e1802310.
167. Wang H, Wang L, Wang L, et al. Phosphorus particles embedded in reduced graphene oxide matrix to enhance capacity and rate capability for capacitive potassium-ion storage. Chemistry 2018;24:13897-902.
168. Xu L, Guo W, Zeng L, et al. V3Se4 embedded within N/P Co-doped carbon fibers for sodium/potassium ion batteries. Chem Eng J 2021;419:129607.
169. Zhao X, Huang R, Wang T, Dai X, Wei S, Ma Y. Steady semiconducting properties of monolayer PtSe2 with non-metal atom and transition metal atom doping. Phys Chem Chem Phys 2020;22:5765-73.
170. Zhang Z, Khurram M, Sun Z, Yan Q. Uniform tellurium doping in black phosphorus single crystals by chemical vapor transport. Inorg Chem 2018;57:4098-103.
171. Viti L, Politano A, Zhang K, Vitiello MS. Thermoelectric terahertz photodetectors based on selenium-doped black phosphorus flakes. Nanoscale 2019;11:1995-2002.
172. Hussain F, Imran M, Rana AM, et al. Tailoring magnetic characteristics of phosphorene by the doping of Ce and Ti: a DFT study. Phys E Low Dimens Syst Nanostruct 2019;106:352-6.
173. Duan JP, Zhang JM, Wei XM, Huang YH. Electronic, magnetic and optical properties of blue phosphorene doped with Y, Zr, Nb and Mo: a first-principles study. Thin Solid Films 2021;720:138523.
174. Zhao W, Xu X, Wang L, et al. Boosting lifespan of conversion-reaction anodes for full/half potassium-ion batteries via multi-dimensional carbon nano-architectures confinement effect. J Energy Chem 2022;75:55-65.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Chen Y, Sun H, Guo J, Zhao Y, Yang H, Li H, Li WJ, Chou S, Jiang Y, Zhang Z. Research on carbon-based and metal-based negative electrode materials via DFT calculation for high potassium storage performance: a review. Energy Mater 2023;3:300045. http://dx.doi.org/10.20517/energymater.2023.35
AMA Style
Chen Y, Sun H, Guo J, Zhao Y, Yang H, Li H, Li WJ, Chou S, Jiang Y, Zhang Z. Research on carbon-based and metal-based negative electrode materials via DFT calculation for high potassium storage performance: a review. Energy Materials. 2023; 3(5): 300045. http://dx.doi.org/10.20517/energymater.2023.35
Chicago/Turabian Style
Chen, Yuefang, Heyi Sun, Junpeng Guo, Yuwen Zhao, Huan Yang, Hongwei Li, Wei-Jie Li, Shulei Chou, Yong Jiang, Zhijia Zhang. 2023. "Research on carbon-based and metal-based negative electrode materials via DFT calculation for high potassium storage performance: a review" Energy Materials. 3, no.5: 300045. http://dx.doi.org/10.20517/energymater.2023.35
ACS Style
Chen, Y.; Sun H.; Guo J.; Zhao Y.; Yang H.; Li H.; Li W.J.; Chou S.; Jiang Y.; Zhang Z. Research on carbon-based and metal-based negative electrode materials via DFT calculation for high potassium storage performance: a review. Energy Mater. 2023, 3, 300045. http://dx.doi.org/10.20517/energymater.2023.35
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 9 clicks
Cite This Article 9 clicks




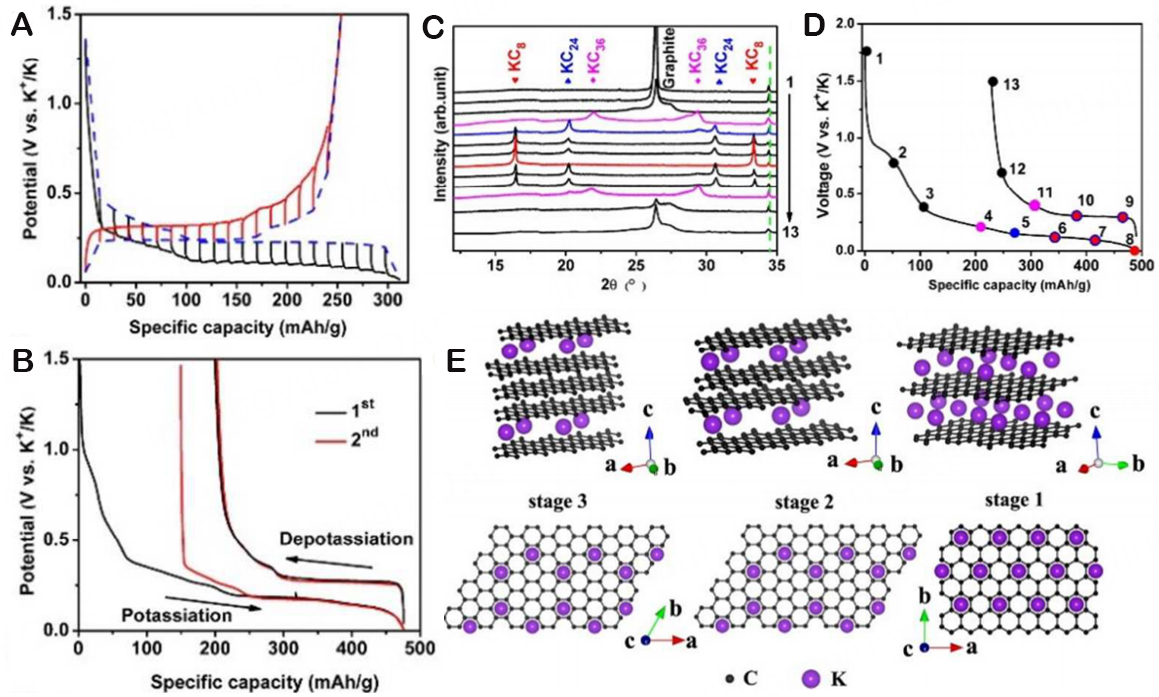
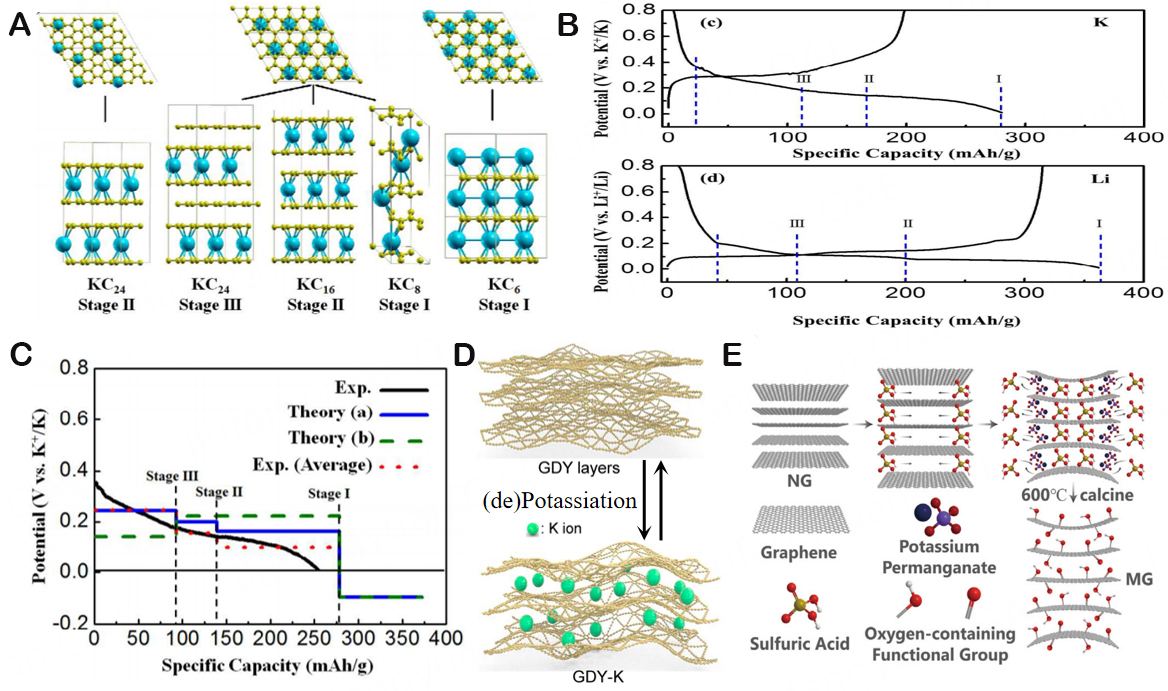
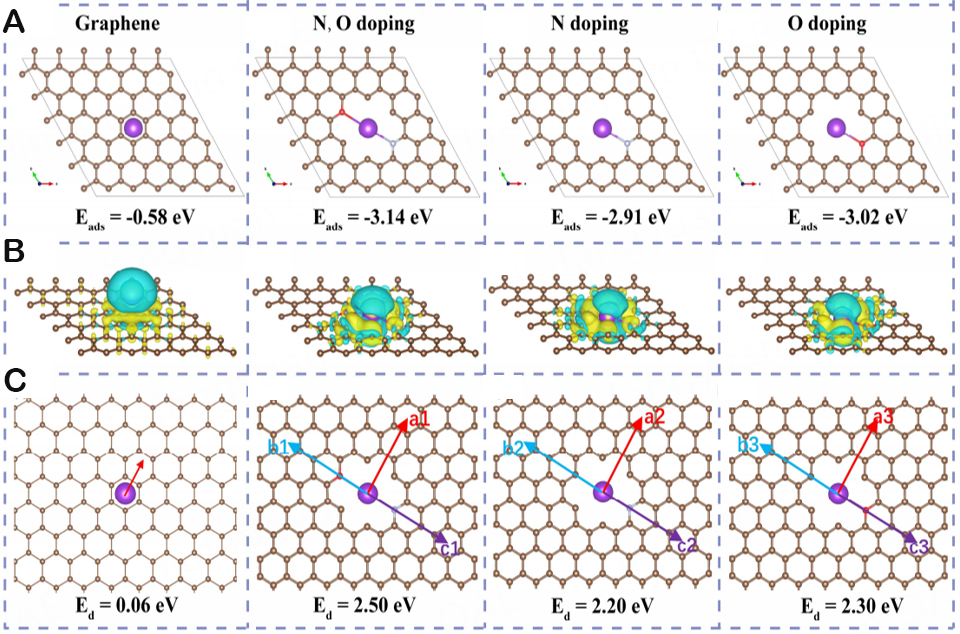


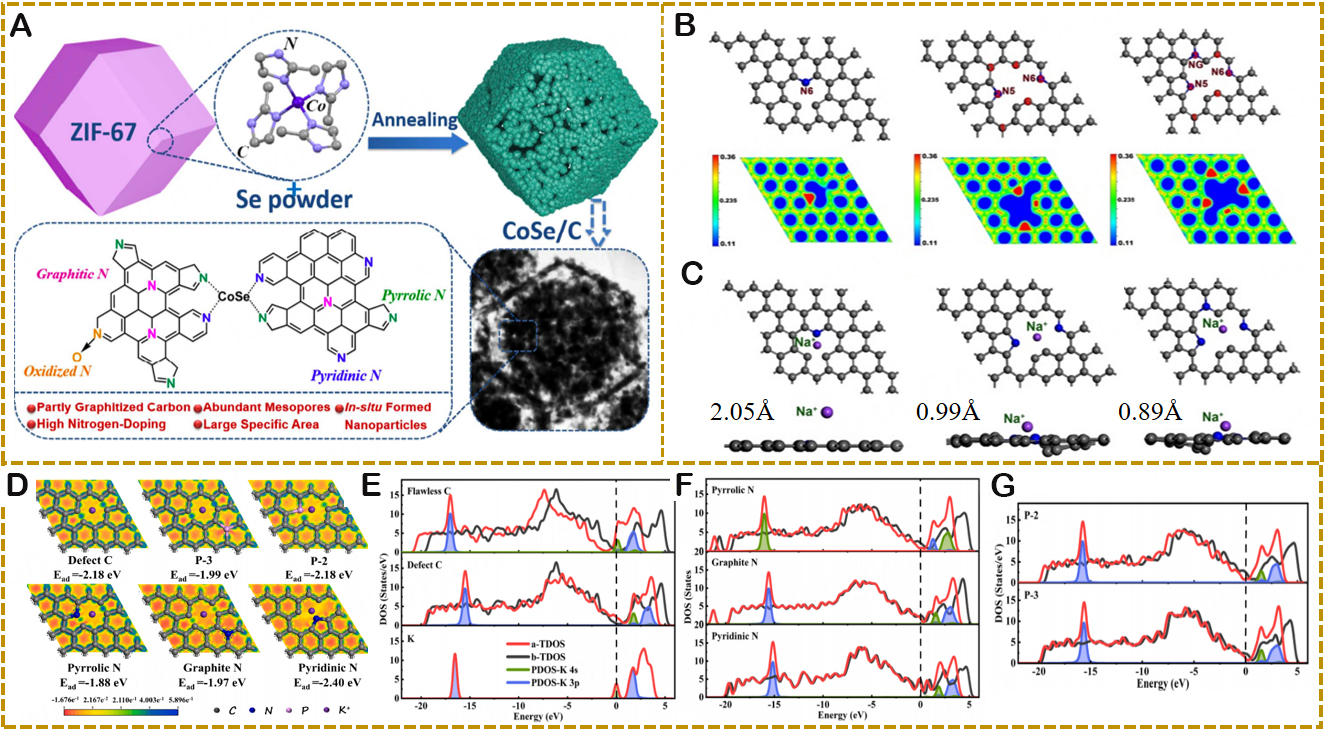
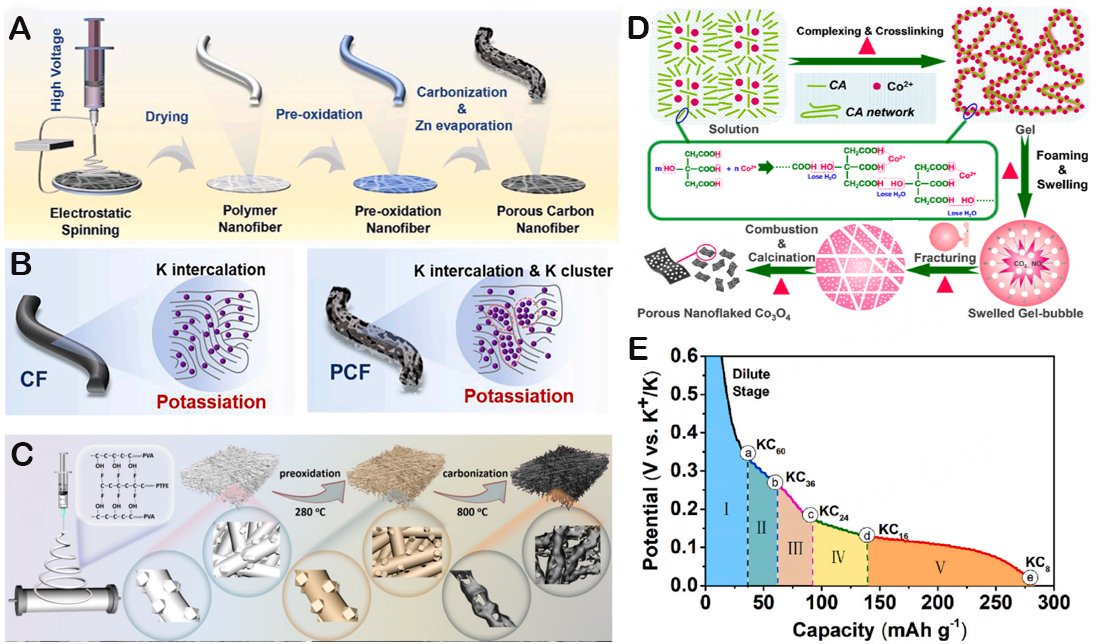

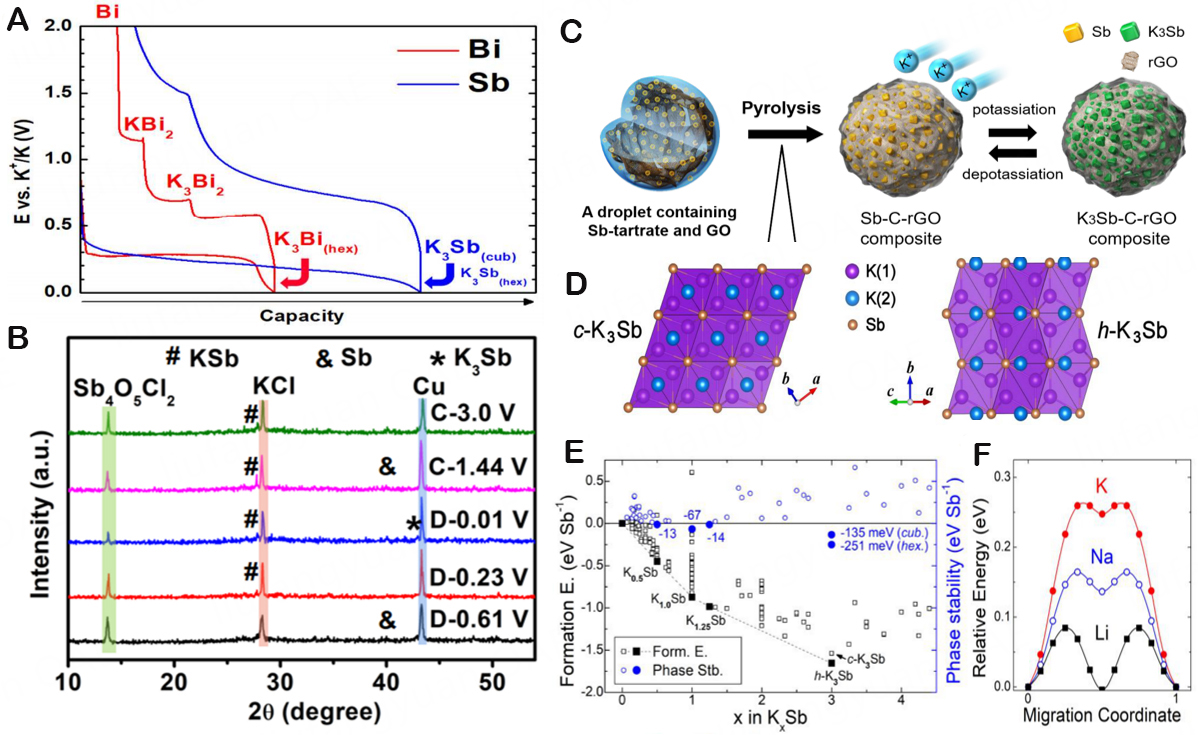
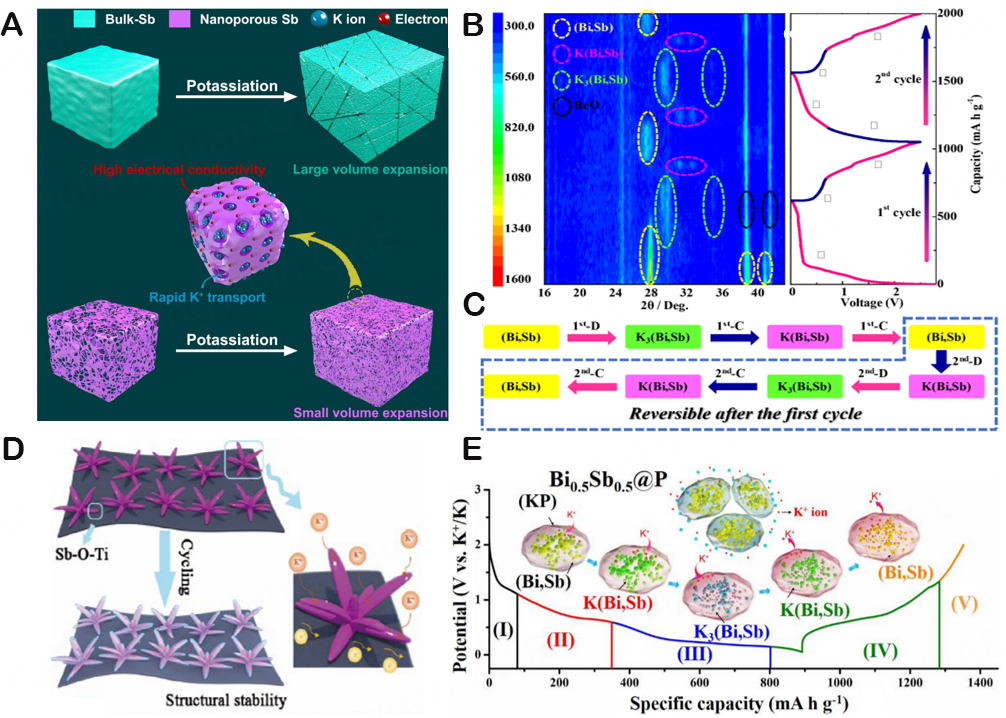
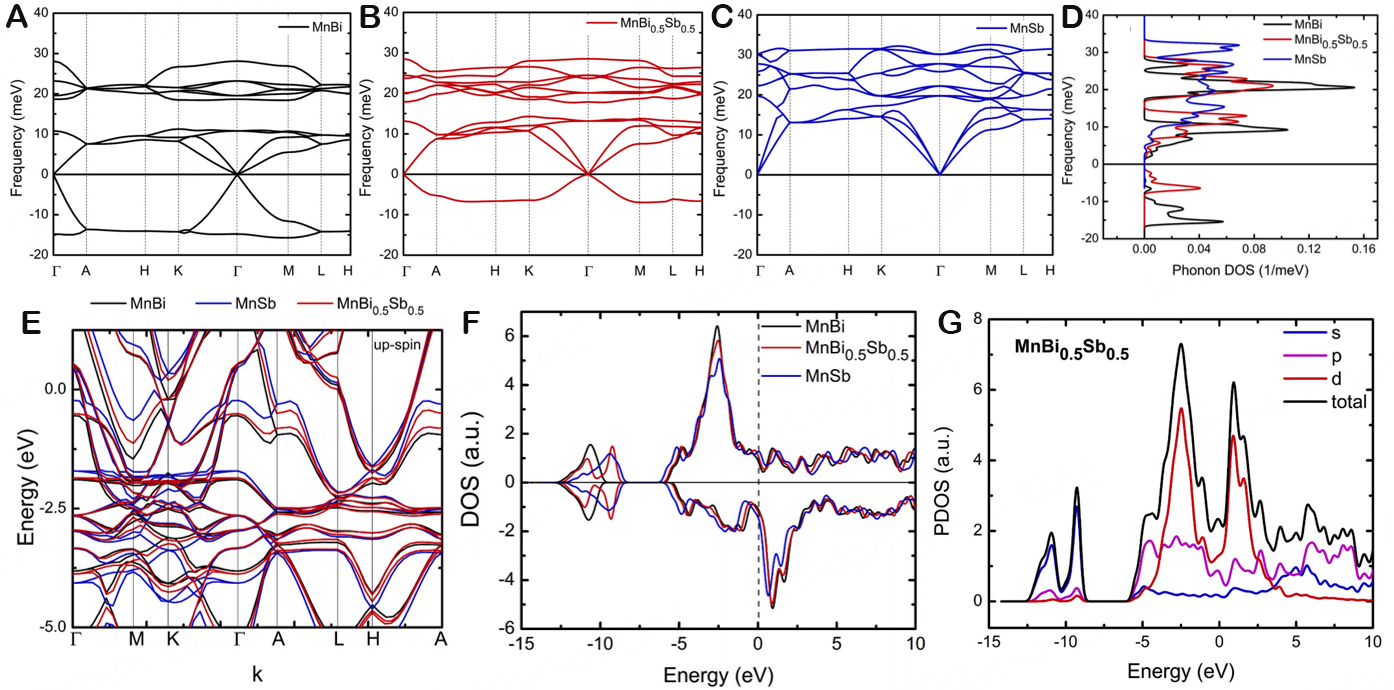
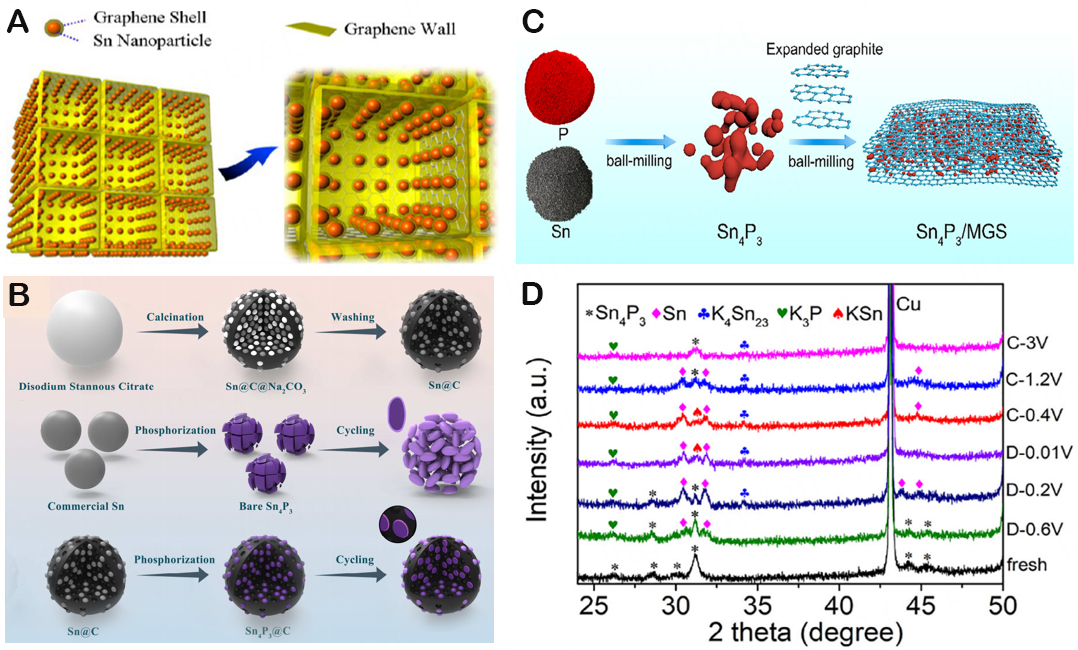

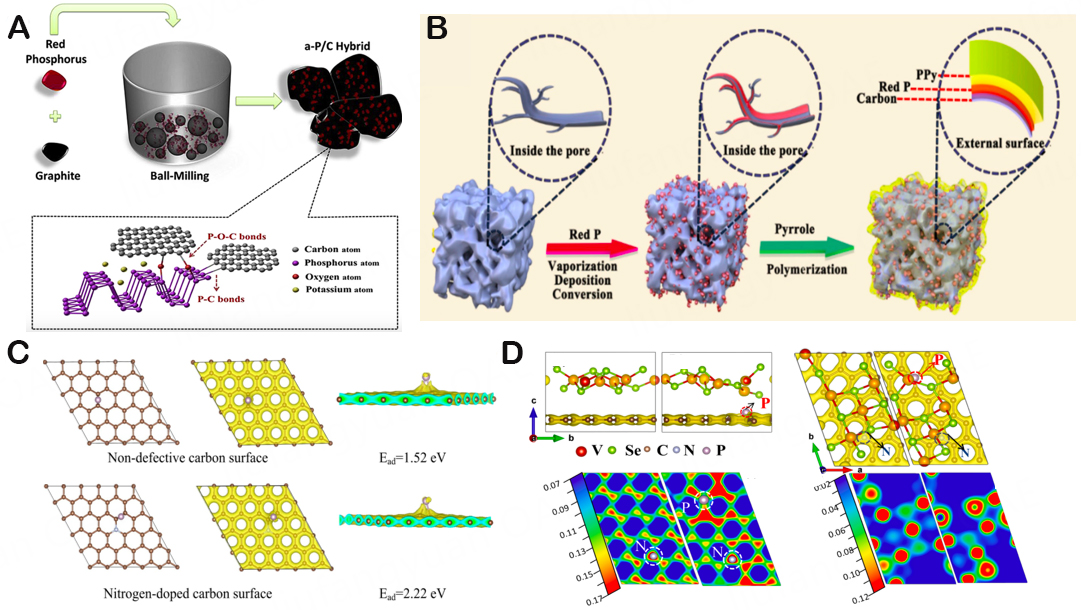
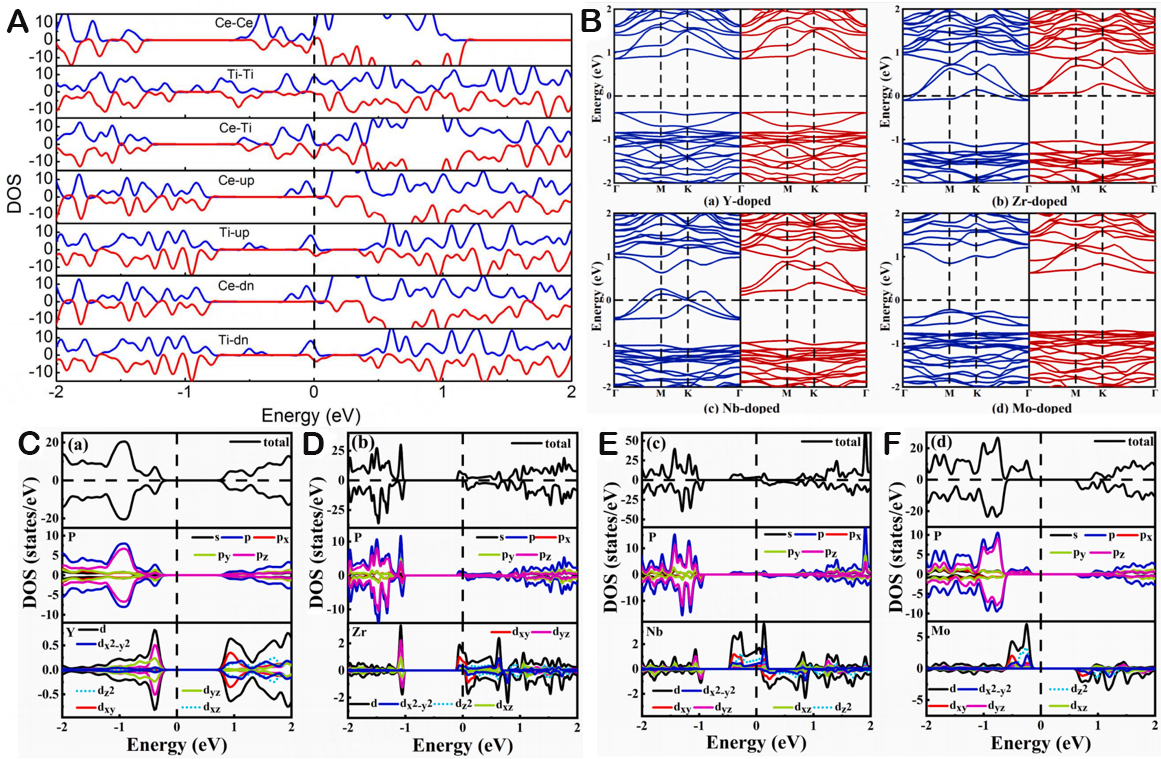











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.