Could potassium-ion batteries become a competitive technology?
Abstract
Potassium-ion batteries (PIBs) have attracted significant attention as a complement to lithium-ion and sodium-ion batteries (SIBs). PIBs can theoretically provide higher specific energy and power density than SIBs due to lower standard electrode potential of K/K+ and faster K+ ion diffusion, maintaining the benefits of low-cost and sustainability. However, research on PIBs is in its infancy; therefore, further efforts are necessary to enhance their performance and position them as a competitive technology. In this perspective, the remaining challenges and possible strategies to advance the development of PIBs are presented.
Keywords
STATE-OF-THE-ART
To achieve climate neutrality, the number of renewable energy sources and clean energy carriers should significantly increase, reducing the dependence on fossil fuel consumption. In parallel, novel efficient energy storage solutions must be developed for grid and household applications along with the implementation of sustainable and smart technologies in transportation systems. The electric vehicle industry is growing considerably, as are stationary energy storage systems (EES), which will become a key facet towards the realization of a sustainable society. Environmentally friendly, efficient, and powerful energy storage devices are required. In this context, batteries are a key technology, with lithium-ion batteries (LIBs) being the preferred choice for electromobility due to their high energy density. However, they utilize toxic (i.e., cobalt) or critical raw materials, i.e., cobalt, natural graphite, and lithium[1]. As a consequence, the feasibility of LIBs for application in large-scale renewable EES is uncertain. This necessitates the development of alternative EES composed of earth-abundant elements that meet the cost, sustainability, availability, and good performance metrics.
Sodium-ion batteries (SIBs) are a competitive technology because they are environmentally friendly, sustainable, and cost-effective[2]. Sodium-based electro-active materials are made from non-critical raw materials, such as manganese- and iron-based layered oxides or Prussian Blue analogs (PBAs) as cathodes and biowaste-derived hard carbon anodes[3]. In addition, aluminum can be used as anode current collector because sodium does not alloy with aluminum at low potential[4]. The main challenge of SIBs is to enhance their energy and power density to be more competitive with LIBs. Experimental energy densities of 711 W·h·kg-1 and more than 1,450 W·h·kg-1 have recently been reported for LIBs at the cell level (with practical batteries around 350 W·h·kg-1)[5]. Nevertheless, Contemporary Amperex Technology Co., Limited (CATL) announced the commercialization of SIBs with 160 W·h·kg-1 and more than 2,000 cycles by 2023[6].
In theory, potassium-ion batteries (PIBs) can achieve superior energy and power densities than SIBs, considering their lower standard electrode potential and faster ion diffusion[7]. Austin-based PIB startup Group 1 aims for a large-scale launch of PIB production by 2027 due to comparable energy density to lithium iron phosphate (LFP)-based LIBs[8]. For the research-based development of PIBs, further advances are necessary in electrode and electrolyte design, understanding of the interfacial chemistry, and manufacturing potassium full cells to enhance their viability.
POTASSIUM-ION BATTERY ADVANTAGES
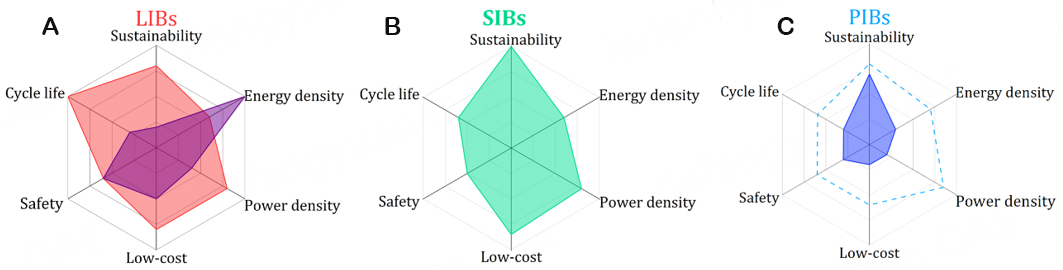
The main expected advantages of PIBs in comparison to other technologies, such as LIBs and SIBs, include affordability and environmental friendliness resulting from the material replacement in PIBs vs. LIBs, along with higher power density, high energy density, and safety of PIBs vs. SIBs due to the more negative voltage of reduction of K metal [Figure 1][9,10]. However, these properties are not supported by experimental data. In fact, the current performance and safety of PIBs are inferior to that of both LIBs and SIBs [Figure 1]. Recent energy density calculations hardly reach 160 W·h·kg-1 for PIBs[11]. Regarding the inferior safety of PIBs, the literature points out the highest reactivity of K metal with respect to Li and Na. While it is understood that PIBs do not contain metallic K, the risk of metal plating on the anode exists for low-voltage anode materials when using high currents, making PIBs less safe. Nevertheless, research on PIBs is still in its infancy; thus, the potential for them to become a feasible technology remains open.
Low-cost and environmentally friendly technology
PIBs can be made with abundant and non-critical raw materials, such as aluminum, iron, manganese, potassium, etc., avoiding the use of critical raw and scarce elements (i.e., lithium, cobalt, copper, nickel, natural graphite) used in LIBs[1]. An aluminum current collector can also be used in both electrodes because potassium does not alloy with aluminum to thermodynamically form intermetallic compounds, thus reducing battery cost and weight[12]. For example, in the case of SIBs, 55% and 3% of battery mass and cost reduction can be obtained by replacing 10 µm thick copper with 15 µm thick aluminum[13]. A similar outcome could be expected for PIBs.
Potential high power and energy density
PIBs could provide high power densities because K+ ions diffuse faster than Li+ and Na+ ions in liquid electrolytes[14]. This is due to weaker Lewis acidity and Coulombic interactions with the anions and solvents. The K+ ion exhibits smaller Stokes radii (3.6 Å) in propylene carbonate (PC) than Na+ and Li+ ions (i.e., 4.6 Å and 4.8 Å, respectively). In addition, the K+ ion shows lower desolvation energy; thus, PIBs might exhibit enhanced rate capability. Nevertheless, the K+ ion diffusion in the electrolyte is not the only factor to be considered. Indeed, the power capability is still poor compared to LIBs and SIBs due to the limited K+ ion kinetics within the electro-active materials.
Moreover, PIBs could also be considered high energy density batteries, mainly due to the low standard electrode potential of K/K+ (-2.93 V vs. SHE), even lower than Li/Li+ in organic electrolytes[14], leading to high operating average voltages.
Potassium metal reactivity
A controversial opinion remains in the research field regarding the safety properties of PIBs due to the higher reactivity of K metal than Li or Na. However, the lower standard electrode potential of K/K+ compared with Li/Li+ and Na/Na+ could reduce the chances of K metal plating due to overpotential at low voltages. In addition, the higher reactivity of K metal (compared to Li and Na metal) may have a positive impact, similar to the way metallic AI reacts when it comes into contact with oxygen[15]. This reaction facilitates easy interaction with the liquid electrolyte, rapidly forming a coherent solid electrolyte interphase (SEI) to protect the electrode surface from further decomposition reactions.
CHALLENGES OF POTASSIUM-ION BATTERIES
Although PIBs possess several advantages, the experimental results are far from the theoretical properties. Consequently, several challenges must be overcome for their practical implementation [Figure 2].
Low K+ ion diffusion and poor K+ ion reaction kinetics
It is expected that PIBs possess a high rate capability due to the weak Lewis acidity and small Stokes radii in solvents. However, poor ion diffusivity and kinetically limited reactions are commonly reported in electro-active materials with small tunnels or interlayer cavities, such as polyanion compounds and layered oxides[16,17]. The main reason is the larger ionic radius of unsolvated potassium in comparison to lithium and sodium. As examples, K+ ions can be poorly inserted in polyanion compounds, i.e., olivine phase
Larger volume changes upon K+ ion (de)insertion/(de)intercalation
The large size of the K+ ion also induces poor long-term stability due to the large volume change upon cycling. Therefore, large cavity electro-active materials must be designed to improve the structural stability and extend the lifetime of PIBs.
The anode of choice for PIBs is graphite due to the possibility of intercalating K+ ions and forming KC8, which delivers a reversible capacity of 279 mAh g-1[18]. However, the large volume expansion of graphite after potassiation (61%), which is six times larger than for lithiation, results in relatively fast capacity decay. Similar behavior is observed for K-based layered oxide cathodes, where the large volume expansion upon cycling induces permanent structural changes, high hysteresis, and poor cycling stability[19].
In-situ investigations of electrode materials during battery operation can provide very useful information with respect to structural transitions or changes in the oxidation state of transition metals and are particularly informative with respect to the crystallographic volumetric expansions[7,20].
Side reactions, electrolyte consumption, SEI formation
The commonly used liquid electrolytes of LIBs and SIBs, i.e., carbonate- or ether-based solvents, are unstable at low potential. Furthermore, the high reactivity of K metal with liquid electrolytes easily induces electrolyte decomposition, delivering low Coulombic efficiencies. In addition, the formed SEI is often unstable, increasing polarization and reducing long-term stability. Many studies have demonstrated the importance of electrode-electrolyte formulation to avoid unfavorable side reactions and achieve good electrochemical performances. For example, potassium bis(fluorosulfonyl)imide (KFSI) salt leads to the formation of stable SEI due to the decomposition products of the anion and the reduction of free solvents due to the stronger solvation[21,22]. In contrast, the potassium hexafluorophosphate (KPF6) decomposition products do not stabilize the SEI.
Solvent selection is also critical; cyclic carbonates, i.e., ethylene carbonate (EC) and PC, form stable SEI and provide higher Coulombic efficiencies. Meanwhile, the linear carbonates (dimethyl carbonate (DMC) or diethyl carbonate (DEC)) are not appropriate for stabilizing the SEI, leading to poor capacity retention as reported for graphite anodes and KVPO4F and KVOPO4 polyanion cathodes[23,24]. Nonetheless, SEI studies should also be performed on full cells since cross-contamination from very unstable SEI on metallic K may lead to misinterpretations.
Battery safety hazards
PIBs are considered to present safety hazards due to the high reactivity and low melting point (63 °C) of K metal. However, it has been reported that the heat generation during thermal runaway in PIBs is lower than in LIBs[25]. In addition, the K metal reactivity towards liquid electrolytes can be decreased by using electrolytes, such as triethyl phosphate (TEP) or trimethyl phosphate (TMP), or ionic liquids due to the formation of a protective layer[26,27]. Another strategy is to substitute liquid electrolytes with solid-state electrolytes, such as ceramics or polymers[28], which are non-flammable and could suppress/reduce dendrite growth.
OVERCOMING THE CHALLENGES
Electrolytes: from liquids to polymers
Research on PIB electrolytes is dominated by fluorinated organic salts dissolved in liquid carbonate or ether solvents, as in LIBs and SIBs. If a fire or explosion occurs, the fluorinated salt can release toxic fluoro-organic compounds, i.e., bis(2-fluoroethyl)-ether[29] and hydrofluoric acid[30]. Moreover, ether solvents limit the upper voltage cut-off to < 3.8 V vs. K/K+, restricting the use of high-voltage cathode materials. The surface modification of active materials or the addition of electrolyte additives can reduce side reactions and stabilize the SEI. The electrolyte additives decompose at a higher potential than solvents, forming a stable SEI and preventing further side reactions. For example, it is known that vinylene carbonate (VC) and fluoroethylene carbonate (FEC) are good additives for lithium- and sodium-ion systems, respectively[31,32]. However, they significantly decrease the capacity and stability of PIBs due to the formation of high concentrations of insoluble KF and K2CO3, which increase the interfacial resistance and hinder K+ ion transport through the SEI[33]. In contrast, it has been demonstrated that sulfur-based additives, such as ethylene sulfate (DTD), can suppress the side reactions and form a stable SEI on graphite anodes and PBA cathodes[34,35].
Replacing flammable carbonate- and ether-based electrolytes could also enhance the stability of PIBs. If non-flammable solvents are used, the PIB safety profile is also ameliorated. TEP and TMP are well-known non-flammable solvents for SIBs and PIBs; they form a robust and stable SEI, enhancing cycling stability[36]. Ionic liquids are another non-flammable alternative. For example, KFSI or potassium bis(trifluoromethanesulfonyl) imide (KTFSI) mixed with pyrrolidinium-based ionic liquid form effective SEI, leading to good electrochemical performance[37].
Finally, polymer electrolytes could overcome the side reactions and safety challenges of PIBs[38]. Incipient research in solid-state PIBs shows that the main challenges are the low ionic conductivity at room temperature and the interfacial charge transfer due to low wetting and compatibility. However, for example, Ni3S2@Ni cathode electrodes exhibit higher capacity retention in PEO-KFSI solid electrolytes than in carbonate-based electrolytes, suggesting further investigations on the use of solid electrolytes for long-term cycling PIBs[39].In addition, the ionic conductivity could be improved by adding inorganic fillers, such as Al2O3, SiO2, etc.[40]. Moreover, mixing with additives such as carbonate solvents or ionic liquids, which result in gel electrolytes, can enhance ionic conductivity at room temperature and reduce the electrolyte-electrode charge transfer resistance[41].
3D structure cathode materials
PBAs are the most promising materials among the families of 3D materials proposed as cathodes for PIBs, i.e., layered oxides, phosphates, and PBAs[42]. Their 3D open framework structure provides suitable channels and interstitial sites for large K+ ion diffusion, allowing faster K+ ion kinetics. One of the main drawbacks of layered oxide and polyanion compounds is the low K+ ion content in the structure. This is related to the strong K+-K+ repulsion in the interlayer and small cavities[43]. Particle morphology engineering could facilitate ionic transport, shortening the ion diffusion distance and allowing faster K+ ion diffusion. Furthermore, this engineering could be suitable for controlling the volume expansion upon cycling, preventing the SEI cracking upon (dis)charge, and delivering superior cycle life.
Among the PBAs, the Fe-/Mn-based Prussian White (PW), K2Mn2[Fe(CN)6][44], is the most promising. It exhibits the reversible insertion of two K+ ions per formula unit at a voltage of 4.0 V vs. K/K+. Cycle life approaches 8,000 cycles when controlling the crystallinity and defect content. However, PBAs have two limitations: low bulk density and low electron conductivity, originating from their structure and chemical bond[43], resulting in lower specific capacity and inferior performance compared to layered oxides. The low bulk density of PBAs is difficult to overcome. Modifying the mixing conditions of electrode components and using large particle sizes and additives can improve the density and homogeneity of the electrode. Meanwhile, metal substitution and/or carbon coating could overcome their poor electronic conductivity. Unfortunately, the latter strategy requires temperatures above the thermal stability of PW. One possible approach is a hybridization or coating of the PW active material with electronically conducting polymers, acting as conducting additives and binders[45].
Alternative anode materials
Graphite reversibly (de)intercalates K+ ions around 250 mAh·g-1 in liquid electrolytes[14]. However, low initial Coulombic efficiencies, severe capacity fading, and moderate rate capabilities are observed, related to the 61% volume change during K+ ion (de)intercalation. In addition, the very low insertion voltage implies a risk of K metal plating at high C-rates, yet it remains the anode of choice for PIBs.
Other carbon-based materials can be used as anodes. For example, hard carbon anodes deliver
As alternative anode materials, metal oxides are interesting for high-power PIBs. In particular, titanium-based oxides exhibit high density and low-cost. Furthermore, the K+ ion insertion typically occurs at higher voltages than in carbon-based anodes, enabling a fast charge without the risk of K metal plating. Nanostructured layered materials prepared through hydrothermal processes followed by high-temperature calcination show some degree of K+ ion insertion, suggesting surface adsorption rather than bulk insertion. Regarding tunneled oxides, the hollandite-type structures (e.g., K0.17TiO2) enable fast bulk diffusion, delivering 60 mAh·g-1 at 5C[53]. Fine-tuning the metal doping in known hollandites and hexagonal bronzes will optimize the ionic and electron transport properties. In addition, particle morphology control could minimize the dimension along which K+ ions and electrons travel, enhancing the power capability.
Alloying materials (Sn, P, Bi, Sb, Pb, Ge, etc.) also represent a large class of anode materials with high reversible capacities and high energy densities[54]. However, they do not stand out as the most sustainable and abundant materials and, therefore, are not candidates for sustainable large-scale batteries. Meanwhile, organic electro-active materials are often produced through low-cost synthesis methods while delivering competitive electrochemical performance[55]. Potassium terephthalates (PETs) deliver reversible capacities of 250 mAh·g-1 at an average voltage of 0.3 V vs. K/K+, making them suitable for use as anodes[56]. Even more sustainable, PETs derived from recycled plastic films show similar capacities at a slightly higher voltage of 0.7 V vs. K/K+[57].
TOWARD SUSTAINABLE POTASSIUM-ION BATTERIES
Electrode material chemistry
PIBs can be considered sustainable and low-cost devices because electrodes can be based on non-critical raw materials. As a negative electrode, although potassium can be intercalated into graphite, more sustainable carbon-based species could be implemented, such as bio-waste-derived hard carbons. For example, Mn-/Fe-based PW and bio-waste-derived hard carbons can be synthesized by low energy routes, i.e., PW by room temperature precipitation and hard carbons at a lower temperature than graphite
Electrode manufacturing
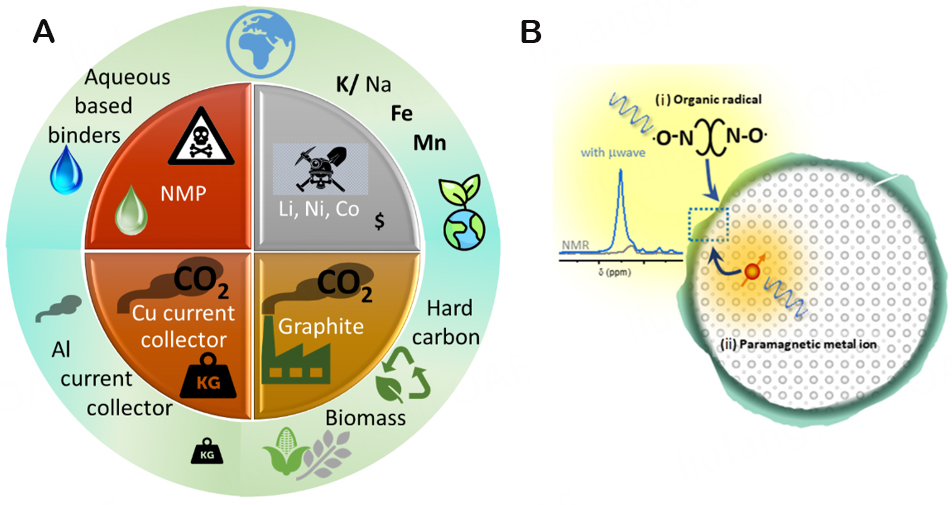
The standard electrode processing is based on a polyvinylidene difluoride (PVDF) binder, which is soluble in hazardous, irritating, and teratogenic N-methyl-2-pyrrolidone (NMP)[58]. Therefore, additional focus on alternative electrode processing using a low-cost and low-carbon footprint system should be performed [Figure 3A]. For example, bio-sourced aqueous binders should be employed to ensure the reuse and recycling of electrode materials[58]. In addition, several reported works demonstrated superior electrochemical performance in terms of Coulombic efficiencies and cycling using water-soluble binders, i.e., carboxymethyl cellulose (CMC) and sodium polyacrylate (PAANa). This enhancement has been attributed to the formation of an effective SEI[59].
Figure 3. (A) Scheme towards more sustainable PIBs. (B) A schematic showing the paths for enhancing NMR signals from chemical environments in the SEI through DNP from different polarizing agents: in (i), organic biradicals are used as an exogenous source of polarization, and in (ii), paramagnetic metal ions are introduced as dopants into the bulk of the material. In both cases, microwave irradiation results in polarization transfer from the unpaired electrons to the nuclei in the sample, thereby enhancing their polarization and making them easily detectable in NMR.
The recycling and separation of the different components found in LIBs have yet to be standardized[60]. The metallurgical routes and processes employed are energy-consuming. In some cases, the waste derived from processes such as lixiviation, e.g., mother liquours and gaseous emissions, is harmful and far from being environmentally friendly. The recycling of PIBs using sustainable materials should enable the realization of such batteries to satisfy the requirements of a (battery) circular economy.
Advanced characterization tools to extend the lifetime of PIBs
The manufacture of reliable long-cycle life PIBs should also be targeted. The study of the stability of the electrode and electrolyte materials, the interfacial chemistry, relevant ion diffusion mechanisms, and the formation and decomposition of the SEI are important for developing high specific energy/power devices with extended lifetime. PIBs are still at an early stage of development; hence, little is known about the formation, composition, and evolution of SEI. Similar to LIBs and SIBs, advanced analytical tools are requisite to probe ion transport across the electrode-electrolyte interfaces. As the SEI is a nanoscale heterogeneous, disordered, and reactive layer, standard characterization tools are limited in their applicability. For example, solid-state nuclear magnetic resonance (ssNMR) spectroscopy might be a versatile technique, providing an atomic-molecular scale insight into the composition and structure of the electrode-electrolyte interface[61]. Leveraging the successful developments of this approach to probe interphases in LIBs, the nuclear magnetic resonance (NMR) methodology could also be adapted and developed to investigate the SEI on PIB anodes. Dynamic nuclear polarization (DNP) could be employed to increase the sensitivity of ssNMR to detect hidden and buried interfaces [Figure 3B][62,63]. In DNP, the large electron spin polarization is transferred to surrounding nuclear spins, increasing the sensitivity of their detection in ssNMR by up to four orders of magnitude. In combination with X-ray photoelectron spectroscopy (XPS) for investigating the SEI chemical composition in PIBs[64] and advanced transmission electron microscopy (TEM) for evaluating changes in the electrode surface structure, detailed chemical composition maps and structural insights into the architecture of the SEI can be gained. The correlation between electrochemical performance and chemical and morphological properties of the SEI will provide valuable information about potential strategies to enhance the lifetime of PIBs. Besides, in-situ and ex-situ ssNMR approaches can be employed to monitor K+ ion transport across the electrode-electrolyte interface. The correlation of ion exchange rates with the SEI composition can guide the choice of beneficial electrode-electrolyte formulations.
Advanced characterization techniques and in-situ and operando experiments will be crucial to understanding the degradation mechanism and the correlations among structures, morphology, K+ ion transport, and performance. This will allow the discovery of advanced anode and cathode materials with enhanced K+ ion kinetics and high energy/power densities. In addition, future research directions must involve designing alternative electrolyte formulations (non-flammable liquid electrolytes or solid-state electrolytes) that enable the formation of a stable SEI and mitigate side reactions, providing extended cycle life. Finally, the practical potential of PIBs should be investigated by manufacturing real cells (i.e., full cells). Resolving these standing problems can realize PIBs as a competitive technology in the near future.
DECLARATIONS
Acknowledgments
The support of M.ERA.NET and national agencies is acknowledged. Zarrabeitia M and Passerini S wish to extend their gratitude to Bundesministerium für Bildung und Forschung - BMBF (03X90508), Grants PCI2022-133005, PCI2022-133010, PID2021-127864OB-I00 funded by MCIN/AEI/10.13039/501100011033 and, by the European Union PRTR funding through projects are acknowledged by Carretero-González J and Castillo-Martínez E, and Leskes M acknowledges and values the backing provided by Israel Ministry of Science (1001536555). Adenusi H recognizes the support of the University of Hong Kong and the Hong Kong Quantum AI Lab Limited, AIR@InnoHK.
Authors’ contributions
Proposed the topic of this review: Zarrabeitia M, Carretero-González J, Castillo-Martínez E
Writing the manuscript: Zarrabeitia M, Carretero-González J
Collectively discussed and revised the manuscript: Zarrabeitia M, Carretero-González J, Leskes M, Adenusi H, Iliev B, Schubert TJS, Passerini S, Castillo-Martínez E
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by M.ERA.net 2021 call and national agencies, including Bundersministerium für Bildung und Forschung (BMBF) - No. 03X90508; Agencia Estatal de investigación - No. PCI2022-133055, No. PCI2022-133010, and No. PID2021-127864OB-I00; and Israel Ministry of Science - No. 1001536555. The basic funding of the Helmholtz Association is kindly acknowledged by the HIU co-authors.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2023.
REFERENCES
1. RMIS - raw materials information system. Available from: https://rmis.jrc.ec.europa.eu/?page=crm-list-2020-e294f6/ [Last accessed on 14 Aug 2023].
2. Goikolea E, Palomares V, Wang S, et al. Na-ion batteries - approaching old and new challenges. Adv Energy Mater 2020;10:2002055.
3. Hasa I, Mariyappan S, Saurel D, et al. Challenges of today for Na-based batteries of the future: from materials to cell metrics. J Power Sources 2021;482:228872.
5. Li Q, Yang Y, Yu X, Li H. A 700 Wh kg-1 rechargeable pouch type lithium battery. Chin Phys Lett 2023;40:048201. Available from: https://iopscience.iop.org/article/10.1088/0256-307X/40/4/048201/meta [Last accessed on 14 Aug 2023].
6. CATL news. The first-generation sodium-ion battery launch event. Available from: https://www.catl.com/en/news/685.html [Last accessed on 14 Aug 2023].
7. Xu Y, Titirici M, Chen J, et al. 2023 roadmap for potassium-ion batteries. J Phys Energy 2023;5:021502.
8. Energy storage news. Available from: www.energy-storage.news/potassium-ion-batterystartup-group1-lfp-is-our-benchmark/ [Last accessed on 14 Aug 2023].
9. Xu Z, Wang J. Toward emerging sodium-based energy storage technologies: from performance to sustainability. Adv Energy Mater 2022;12:2201692.
10. Liu M, Wang Y, Wu F, et al. Advances in carbon materials for sodium and potassium storage. Adv Funct Mater 2022;32:2203117.
11. Sun L, Li G, Zhang S, et al. Practical assessment of the energy density of potassium-ion batteries. Sci China Chem 2022.
13. Vaalma C, Buchholz D, Weil M, Passerini S. A cost and resource analysis of sodium-ion batteries. Nat Rev Mater 2018;3:18013.
14. Komaba S, Hasegawa T, Dahbi M, Kubota K. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem Communn 2015;60:172-5.
15. Greenwood NN, Earnshaw A. Chemistry of the elements. Oxford: Pergamon Pub; First edition 1984, reprinted 1989, p. 253.
16. Madram AR, Daneshtalab R, Sovizi MR. Effect of Na+ and K+ co-doping on the structure and electrochemical behaviors of LiFePO4/C cathode material for lithium-ion batteries. RSC Adv 2016;6:101477-84.
17. Nathan MGT, Yu H, Kim GT, et al. Recent advances in layered metal-oxide cathodes for application in potassium-ion batteries. Adv Sci 2022;9:e2105882.
19. Zhang X, Wei Z, Dinh KN, et al. Layered oxide cathode for potassium-ion battery: recent progress and prospective. Small 2020;16:2002700. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/smll.202002700 [Last accessed on 23 Aug 2023].
20. Yang J, Muhammad S, Jo MR, et al. In situ analyses for ion storage materials. Chem Soc Rev 2016;45:5717-70.
21. Zhang Q, Mao J, Pang WK, et al. Boosting the potassium storage performance of alloy-based anode materials via electrolyte salt chemistry. Adv Energy Mater 2018;8:1703288.
22. Wang H, Wang H, Chen S, et al. A depth-profiling study on the solid electrolyte interface: bis(fluorosulfuryl)imide anion toward improved K+ storage. ACS Appl Energy Mater 2019;2:7942-51.
23. Zhao J, Zou X, Zhu Y, Xu Y, Wang C. Electrochemical intercalation of potassium into graphite. Adv Funct Mater 2016;26:8103-10.
24. Chihara K, Katogi A, Kubota K, Komaba S. KVPO4F and KVOPO4 toward 4 volt-class potassium-ion batteries. Chem Commun 2017;53:5208-11.
25. Zhang W, Liu Y, Guo Z. Approaching high-performance potassium-ion batteries via advanced design strategies and engineering. Sci Adv 2019;5:eaav7412.
26. Zeng G, Xiong S, Qian Y, Ci L, Feng J. Non-flammable phosphate electrolyte with high salt-to-solvent ratios for safe potassium-ion battery. J Electrochem Soc 2019;166:A1217.
27. Silvester DS, Jamil R, Doblinger S, Zhang Y, Atkin R, Li H. Electrical double layer structure in ionic liquids and its importance for supercapacitor, battery, sensing, and lubrication applications. J Phys Chem C 2021;125:13707-20.
28. Zhu X, Ali RN, Song M, Tang Y, Fan Z. Recent advances in polymers for potassium ion batteries. Polymers 2022;14:5538.
29. Hammami A, Raymond N, Armand M. Lithium-ion batteries: runaway risk of forming toxic compounds. Nature 2003;424:635-6.
30. Sloop SE, Pugh JK, Wang S, Kerr JB, Kinoshita K. Chemical reactivity of PF5 and LiPF6 in ethylene carbonate/dimethyl carbonate solutions. Electrochem Solid-State Lett 2001;4:A42.
31. Hekmatfar M, Hasa I, Eghbal R, Carvalho DV, Moretti A, Passerini S. Effect of electrolyte additives on the LiNi0.5Mn0.3Co0.2O2 surface film formation with lithium and graphite negative electrodes. Adv Mater Interfaces 2020;7:1901500.
32. Komaba S, Ishikawa T, Yabuuchi N, Murata W, Ito A, Ohsawa Y. Fluorinated ethylene carbonate as electrolyte additive for rechargeable Na batteries. ACS Appl Mater Interfaces 2011;3:4165-8.
33. Ells AW, May R, Marbella LE. Potassium fluoride and carbonate lead to cell failure in potassium-ion batteries. ACS Appl Mater Interfaces 2021;13:53841-9.
34. Liu G, Cao Z, Zhou L, et al. Additives engineered nonflammable electrolyte for safer potassium ion batteries. Adv Funct Mater 2020;30:2001934.
35. Hosaka T, Fukabori T, Matsuyama T, Tatara R, Kubota K, Komaba S. 1,3,2-dioxathiolane 2,2-dioxide as an electrolyte additive for K-metal cells. ACS Energy Lett 2021;6:3643-9.
36. Liu S, Mao J, Zhang Q, et al. An intrinsically non-flammable electrolyte for high-performance potassium batteries. Angew Chem Int Ed 2020;59:3638-44.
37. Yoshii K, Masese T, Kato M, Kubota K, Senoh H, Shikano M. Sulfonylamide-based ionic liquids for high-voltage potassium-ion batteries with honeycomb layered cathode oxides. ChemElectroChem 2019;6:3901-10.
38. Elmanzalawy M, Sanchez-ahijón E, Kisacik O, Carretero-gonzález J, Castillo-martínez E. High conductivity in a fluorine-free K-ion polymer electrolyte. ACS Appl Energy Mater 2022;5:9009-19.
39. Fei H, Liu Y, An Y. Safe all-solid-state potassium batteries with three dimentional, flexible and binder-free metal sulfide array electrode. J Power Sources 2019;433:226697.
40. Schaefer JL, Morganty SS, Archer LA. Nanoscale organic hybrid electrolytes. Adv Mater 2010;22:3677-80.
41. Zheng J, Li W, Liu X, Zhang J, Feng X, Chen W. Progress in gel polymer electrolytes for sodium-ion batteries. Energy Environ Mater 2022;6:e12422.
42. Kubota K, Dahbi M, Hosaka T, Kumakura S, Komaba S. Towards K-ion and Na-ion batteries as “beyond Li-ion”. Chem Rec 2018;18:459-79.
43. Rajagopalan R, Tang Y, Ji X, Jia C, Wang H. Advancements and challenges in potassium ion batteries: a comprehensive review. Adv Funct Mater 2020;30:1909486.
44. Deng L, Qu J, Niu X, et al. Defect-free potassium manganese hexacyanoferrate cathode material for high-performance potassium-ion batteries. Nat Commun 2021;12:2167.
45. Zhou M, Tian X, Sun Y, et al. Pillar effect boosting the electrochemical stability of Prussian blue-polypyrrole for potassium ion batteries. Nano Res 2023;16:6326-33.
46. Vaalma C, Giffin GA, Buchholz D, Passerini S. Non-aqueous K-ion battery based on layered K0.3MnO2 and hard carbon/carbon black. J Electrochem Soc 2016;163:A1295.
47. Pan J, Sun YY, Yan Y, et al. Revisit electrolyte chemistry of hard carbon in ether for Na storage. JACS Au 2021;1:1208-16.
48. Arnaiz M, Huang P, Ajuria J, Rojo T, Goikolea E, Balducci A. Protic and aprotic ionic liquids in combination with hard carbon for lithium-ion and sodium-ion batteries. Batteries Supercaps 2018;1:204-8.
49. Wu J, He J, Wang M, et al. Electrospun carbon-based nanomaterials for next-generation potassium batteries. Chem Commun 2023;59:2381-98.
50. Qiann Y, Jiang S, Li Y, et al. In situ revealing the electroactivity of P-O and P-C bonds in hard carbon for high-capacity and long-life Li/K-ion batteries. Adv Energy Mater 2019;9:1901676.
51. Wu X, Li Z, Liu J, Luo W, Gaumet J, Mai L. Defect engineering of hierarchical porous carbon microspheres for potassium-ion storage. Rare Met 2022;41:3446-55.
52. Li W, Li Z, Zhang C, et al. Hard carbon derived from rice husk as anode material for high performance potassium-ion batteries. Solid State Ion 2020;351:115319.
53. Jo JH, Kim HJ, Yaqoob N, et al. Hollandite-type potassium titanium oxide with exceptionally stable cycling performance as a new cathode material for potassium-ion batteries. Energy Stor Mater 2023;54:680-8.
54. Imtiaz S, Amiinu IS, Xu Y, Kennedy T, Blackman C, Ryan KM. Progress and perspectives on alloying-type anode materials for advanced potassium-ion batteries. Mater Today 2021;48:241-69.
55. Zhang W, Huang W, Zhang Q. Organic materials as electrodes in potassium-ion batteries. Chemistry 2021;27:6131-44.
56. Lei K, Li F, Mu C, et al. High K-storage performance based on the synergy of dipotassium terephthalate and ether-based electrolytes. Energy Environ Sci 2017;10:552-7.
57. Kang Z, Sun K, Sun CF, Liu Q. A plastics-derived organic anode material for practical and sustainable potassium-ion batteries. Int J Electrochem Sci 2023;18:100222.
58. Bresser D, Buchholz D, Moretti A, Varzi A, Passerini S. Alternative binders for sustainable electrochemical energy storage - the transition to aqueous electrode processing and bio-derived polymers. Energy Environ Sci 2018;11:3096-127.
59. Wu X, Xing Z, Hu Y, et al. Effects of functional binders on electrochemical performance of graphite anode in potassium-ion batteries. Ionics 2019;25:2563-74.
60. Harper G, Sommerville R, Kendrick E, et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019;575:75-86.
61. Haber S, Leskes M. What can we learn from solid state NMR on the electrode-electrolyte interface? Adv Mater 2018;30:e1706496.
62. Leskes M, Kim G, Liu T, et al. Surface-sensitive NMR detection of the solid electrolyte interphase layer on reduced graphene oxide. J Phys Chem Lett 2017;8:1078-85.
63. Haber S, Rosy, Saha A, et al. Structure and functionality of an alkylated LixSiyOz interphase for high-energy cathodes from DNP-ssNMR spectroscopy. J Am Chem Soc 2021;143:4694-704.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Zarrabeitia M, Carretero-González J, Leskes M, Adenusi H, Iliev B, Schubert TJS, Passerini S, Castillo-Martinez E. Could potassium-ion batteries become a competitive technology?. Energy Mater 2023;3:300046. http://dx.doi.org/10.20517/energymater.2023.41
AMA Style
Zarrabeitia M, Carretero-González J, Leskes M, Adenusi H, Iliev B, Schubert TJS, Passerini S, Castillo-Martinez E. Could potassium-ion batteries become a competitive technology?. Energy Materials. 2023; 3(6): 300046. http://dx.doi.org/10.20517/energymater.2023.41
Chicago/Turabian Style
Zarrabeitia, Maider, Javier Carretero-González, Michal Leskes, Henry Adenusi, Boyan Iliev, Thomas J S Schubert, Stefano Passerini, Elizabeth Castillo-Martinez. 2023. "Could potassium-ion batteries become a competitive technology?" Energy Materials. 3, no.6: 300046. http://dx.doi.org/10.20517/energymater.2023.41
ACS Style
Zarrabeitia, M.; Carretero-González J.; Leskes M.; Adenusi H.; Iliev B.; Schubert TJS.; Passerini S.; Castillo-Martinez E. Could potassium-ion batteries become a competitive technology?. Energy Mater. 2023, 3, 300046. http://dx.doi.org/10.20517/energymater.2023.41
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 10 clicks
Cite This Article 10 clicks















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.