Ionic liquids and their derivatives for lithium batteries: role, design strategy, and perspectives
Abstract
Lithium-ion batteries (LIBs) are the predominant power source for portable electronic devices, and in recent years, their use has extended to higher-energy and larger devices. However, to satisfy the stringent requirements of safety and energy density, further material advancements are required. Due to the inherent flammability and incompatibility of organic solvent-based liquid electrolytes with materials utilized in high energy devices, it is necessary to transition to alternative conductive mediums. The focus is shifting from molecular materials to a class of materials based on ions, including ionic liquids (ILs) and their derivatives such as zwitterionic ILs, polymerized ILs, and solvated ILs, which possess high levels of safety, stability, compatibility, and the ability to rationally design ILs for specific applications. Ion design is crucial to achieve superior control of electrode/electrolyte interphases (EEIs) both on anode and cathode surfaces to realize safer and higher-energy lithium-metal batteries (LMBs). This review summarizes the different uses of ILs in electrolytes (both liquid and solids) for LMBs, reporting the most promising results obtained during the last years and highlighting their role in the formation of suitable EEIs. Furthermore, a discussion on the use of deep-eutectic solvents is also provided, which is a class of material with similar properties to ILs and an important alternative from the viewpoint of sustainability. Lastly, future prospects for the optimization of IL-based electrolytes are summarized, ranging from the functional design of ionic structures to the realization of nanophases with specific features.
Keywords
INTRODUCTION
Lithium-ion batteries (LIBs), launched by Sony in 1991, have quickly outperformed their rivals and become the standard choice for electronic devices[1]. After more than 30 years, LIBs remain a vital part of our everyday life, and their use is spreading to new sectors, such as hybrid/electric vehicles (H/EVs)[2,3] and stationary energy storage systems from renewable sources[4,5]. However, to develop batteries suitable for such applications, advancements are necessary to improve their energy density and cycle life, in addition to their safety and eco-compatibility.
The energy density can be enhanced by replacing graphite anodes with lithium metal anodes (theoretical specific capacities of 372 mAh g-1 and 3,860 mAh g-1, respectively), i.e., shifting from LIBs to lithium-metal batteries (LMBs)[6-9]. Nonetheless, such attempts have been hampered by the incompatibility of lithium metal with commonly used electrolytes, which causes formation of dead lithium and growth of dendrites, inducing battery short-circuits and a consequential risk of fire or explosion[7,9,10]. Another important approach for a higher energy density is the use of high-capacity cathode materials, including lithium nickel manganese cobalt oxides, LiNixMnyCozO2 (NMC), where x ≥ 0.5 is preferred for a higher specific capacity beyond 200 mAh g-1[11], eco-friendly and cobalt-free lithium-rich layered oxides such as Li1.2Ni0.2Mn0.6O2 (LRNM) with a specific capacity over 250 mAh g-1[12,13], or alternatively cathode materials capable of working at a higher potential such as spinel LiNi0.5Mn1.5O4 (LNMO) with a working potential of 4.7 V vs. Li+/Li and a capacity of 147 mAh g-1[14,15]. Nevertheless, the development of these materials is still in progress due to detrimental phenomena such as the release of oxygen related to structural degradation of the electrode, transition-metal dissolution upon cycling, and degradation of the electrolyte at high potentials[15-19].
Although the electrolytes do not directly affect the energy density of batteries, they are crucial components since they are the medium governing ion migration between the anode and cathode. To maximize the performance of electrodes, the electrolyte should possess a series of properties: (i) high ionic conductivity and low viscosity to ensure a fast Li+ diffusion; (ii) high electrochemical stability and a wide electrochemical stability window (ESW) to avoid its degradation; (iii) thermal stability to guarantee the safety of devices; (iv) chemical inertness against other components of the battery, including separators, current collectors, and the electrodes, to avoid their corrosion; and (v) an ability to form a protective, Li+-conductive, and electron-insulating layer on the surface of anode materials, the so-called a solid electrolyte interphase (SEI)[20]. This heterogeneous nanoscale layer is of great importance to avoid reduction of the electrolyte during prolonged cycling and ensure an efficient and homogeneous lithium deposition, avoiding the formation of lithium dendrites and dead lithium[21]. Additionally, from the viewpoint of sustainability, other desired features of the electrolyte include low cost, non-toxicity, and eco-compatibility. To date, the most common types of electrolytes used in LIBs are mixtures of alkyl carbonates, such as ethylene carbonate (EC), propylene carbonate (PC), diethyl carbonate (DEC), ethyl methyl carbonate (EMC), or dimethyl carbonate (DMC), dissolving a lithium salt (usually lithium hexafluorophosphate, LiPF6)[22]. These organic solvent-based liquid electrolytes (OLEs) are suitable in terms of their performance but raise safety concerns due to (i) low thermal stability, characterized by high vapor pressure and flammability at high temperatures[22] and crystallization phenomena at low temperatures; (ii) anodic stability limited to 4.2-4.3 V vs. Li+/Li, not sufficient for combination with a high-voltage cathode[23]; and (iii) low chemical stability of LiPF6, which is hydrolyzed easily in the presence of even a few ppm of water resulting in the formation of HF[13,23]. Furthermore, while the organic component-rich SEI produced by the OLEs has been found to be suitable for graphite anodes, it is not robust enough for lithium electrodes to prevent crack formation due to a notable morphology change of the lithium upon cycling, leading to the formation of dendrites and dead lithium[21,24,25].
Given these issues, considerable effort is necessary to develop safer alternatives for next-generation lithium batteries. Due to the intrinsic volatility of molecular solvents, ionic materials with high thermal stability owing to the presence of Coulombic interactions represent a viable conductive medium. Ionic liquids (ILs) are expected to play an integral role in the progress of modern electrolytes because of their well-balanced ionic interactions and dissociation, resulting in both high thermal stability and high ionic conductivity. The proposed approaches range from the realization of new, safer, and widely compatible liquid electrolytes based on lithium salts with different solvents and additives to the complete replacement of the liquid electrolyte with innovative solid-state electrolytes (SSEs). In this review, we discuss the use of ILs in lithium batteries, presenting the amelioration of this technology by ILs and detailing impactful results obtained in recent years. The discussion will be extended to their derivatives, such as zwitterionic ILs (ZILs), polymerized ILs (PILs), and solvated ILs (SILs). Furthermore, we examine the use of deep-eutectic solvents (DESs), which are a neoteric class of materials with similarities to ILs[26] and are recently under trial as electrolytes in lithium batteries.
IONIC LIQUIDS AND THEIR DERIVATIVES FOR BATTERY APPLICATIONS
The first IL, ethyl ammonium nitrate, was introduced in literature by Walden in 1914[27,28]. Years later, at the NATO Advanced Research Workshop on Green Industrial Applications of Ionic Liquids in 2000, ILs were defined as salts that possess a melting point (Tm) below 100 °C, and those having Tm at room temperature or well below 0 °C can be defined as “room-temperature ILs (RTILs)”. To yield RTILs, it is necessary to assess their ionic structure; they are usually composed of a large and asymmetric organic cation and an organic/inorganic anion with a charge delocalization to reduce the Coulombic interactions.
Being composed only of ions, ILs possess unique physicochemical properties that cannot be attained by molecular solvents, such as exceptional thermal stability, low vapor pressure and flammability, good ionic conductivity, and an ability to dissolve inorganic salts. However, ILs still suffer from several disadvantages compared to organic solvents, which limits their use as a direct replacement. First, ILs are highly viscous owing to their Coulombic interactions and the steric hindrance caused by the large ions[29], and their Li+ transference number (tLi+) is lower than that of the OLEs because ions composing ILs also migrate according to a gradient potential, comparatively reducing Li+ conductivity. Second, the synthesis procedures of ILs present an obstacle to their industrial use since these involve the utilization of environmentally unfriendly organic solvents and expensive precursors and energy and time-consuming steps generally consisting of quaternization, anion exchange reaction, and purifications. Many efforts have been devoted to the development of economical, greener, and faster synthesis methods[30,31].
Although several issues remain, ILs are still recognized as potential materials for battery applications because of their design flexibility. A thorough understanding of structure-property relationships revealed that even a small change in the structure of ions, such as the alkyl chain length of cations or the presence of functional groups, leads to a great change in their physical and chemical properties. For instance, in the case of 1-alkyl-3-methylimidazolium tetrafluoroborate, by changing the alkyl side chain from methyl to butyl, its Tm decreases from 100 to -71 °C[32]. Also, the choice and modification of the anion influence the properties of the resulting IL, enabling the possibility of ‘fine-tuning’ for desired applications[28,33]. It is estimated that the number of possible ILs is in the order of 1018[34], despite around 600 organic solvents used widely in research and industry[35]. In recent years, this advantage has attracted considerable attention for their use in batteries and diverse task-specific ILs, such as one exerting a notable control for interphase formation on electrode surfaces (electrolyte-electrode interphases, EEIs) by forming a SEI and CEI (cathode-electrolyte interphase), or one behaving as redox active sites[36]. Regarding EEIs, ILs play an important role as raw materials for the formation of robust interphases through their decomposition. It has been highlighted that both the anion and cation composing an IL can participate in the SEI formation on lithium metal anodes. The anions, being part of the Li+ solvating shell, can reach the anode and decompose, producing inorganic species such as Li3N and LiF. Whereas the cations can be electrostatically absorbed on the surface of lithium, avoiding the deposition of new Li+ and hindering the formation of dendrites due to their large volume and positive charge[37]. Regarding the CEI, the decomposition products, due to the oxidation of the anions, produce inorganic species of notable interest[37]. Therefore, a well-designed IL leads to the formation of EEIs containing desired species, including F-, N-, P-, or B-based ones, with notable stability, low ionic resistance, and electron-insulating properties.
In particular, the formation of a protective CEI due to the use of a well-designed IL-based electrolyte can minimize the effect of issues related to the use of high-capacity cathode materials: suppression of the transition to rock-salt phase, which occurs starting from the surface of bare particles[13], the reduced exposure of lattice oxygen due to the beneficial effects of species in the CEI avoiding oxygen evolution[8], and prevention of direct contact between the electrolyte and the cathode, minimizing the dissolution of transition metals and blocking the attack by HF produced upon cycling[38]. The design strategy of ILs toward battery electrolyte applications is summarized in the next section.
Beyond these conventional ILs (composed of an anion and a cation), it is also possible to design ionic materials with peculiar structures that have inter-ionic chemical or physical bonds, as shown in Figure 1. Through the development of these peculiar structures, ILs and their derivatives attained new functions while maintaining their previously mentioned advantages. For example, by fixing a pair of cation and anion or several cation structures with covalent bonds, new groups of materials such as ZILs[39-41] and PILs[42-45] are obtained. As charges are fixed via covalent bonds, ZILs and PILs tend to have Tm and glass transition temperature (Tg) higher than those of ILs[39-43,45]. However, these materials are also considered as promising electrolyte components enabling selective Li+ conduction[40-44]. By tethering anions and cations in the case of ZILs, the material becomes neutral, and thus, the movement of ZILs in an electric field is suppressed. In the case of PILs, by polymerizing the anion structure, it is possible to obtain Li+ single ion conductors, which are generally considered as SPEs rather than PILs. Although polymerization of the cationic structure reduces Li+ conduction selectivity, PILs are, nevertheless, a promising material to avoid the drawbacks of liquid electrolytes[46] (see next sections for specific examples).
In contrast to ZILs and PILs with inter-ionic chemical bonds, there are other classes of materials with properties akin to those of ILs but with charged structures maintained via inter-ionic physical bonds, known as SILs[47,48] and DESs[35,49]. SILs are composed of a lithium salt in which Li+ is coordinated by an oligomeric ether (glyme), forming [Li(glyme)]+ complexes and noticeably lowering the Tm of the salt[47,48]. DESs are typically composed of a metal salt solvated by an amide that acts as a hydrogen bond donor (HBD) through N-H groups, interacting with the anion and lowering the Tm of the salt[35,49]. However, in the case of DESs specifically designed for battery applications, it has been recently determined that the solvation of Li+ by the C=O groups of amides [which acts as a hydrogen bond acceptor (HBA) in this case] plays an even more important role[50]. Although several components for SILs and DESs have moderate flammability, the resulting SILs and DESs have high thermal stability, low volatility, and low vapor pressures because of strong physical interactions between the constituents; hence, they are a suitable alternative conductive medium[35,47-49]. In particular, SILs are considered as promising electrolytes for Li-S batteries due to the reduced dissolution of polysulfides[51,52]. DESs are appealing due to their ease of preparation: usually, it is sufficient to mix two reagents in the right molar ratio under heating and stirring[35,49]. As a result, no additional solvents or purification steps are needed, ensuring low cost[35,49].
This review focuses on the role of ILs and their derivatives as the electrolytes in LMBs; however, it is important to state that these classes of materials can also be used as solvents for the recovery of precious materials from spent LIBs. In addition, not all of the IL derivatives are suitable for use as electrolytes due to their viscosity, but they can fulfill different roles in LMB technologies. The use of lithium, nickel, manganese, and cobalt is requisite for these batteries, even though they are all classified as critical raw materials (CRMs). Considering their ever-increasing cost and supply risk, it is fundamental to retrieve these metals from spent batteries to prevent the rising cost of lithium batteries and to achieve a sustainable society. Most of the processes for battery recycling involve the use of highly volatile and highly flammable organic solvents, causing safety and environmental issues[53,54]. Together with ILs, DESs are considered an appealing, eco-friendly, and economic substitute[53-56]. In addition, studies on the recovery and reuse of ILs are being conducted[53].
LIQUID ELECTROLYTES
In this section, liquid electrolytes employing traditional ILs as a component are summarized and discussed. The electrolytes are divided into two main categories: the electrolytes in which an IL is the only solvent dissolving a lithium salt (i.e., IL + Li-salt system) and the electrolytes in which an OLE is the main component and ILs are used as an additive (i.e., OLE + IL system). Table 1 summarizes the structures, names, and abbreviations of typical cations and anions compromising ILs, while Supplementary Table 1 summarizes specific results obtained during the last years from the application of ILs in liquid electrolytes for LMBs.
Common cations and anions comprising ILs
| Cations | ||
| Structure | Name | Abbreviation |
 | 1-Ri-3-methylimidazolium | [RMI]/[Rmim] R=initial of Ri [Cimim] |
 | N-Ri-N-methylpiperidinium | [Pip1,i] |
 | N-Ri-N-methylpyrrolidinium | [Pyr1,i] |
 | RiRjRkRl-ammonium | [Ni,j,k,l] |
 | RiRjRk-sulfonium | [Si,j,k] |
 | RiRjRkRl-phosphonium | [Pi,j,k,l] |
| Anions | ||
| Structure | Name | Abbreviation |
 | Tetrafluoroborate | BF4- |
 | Hexafluorophosphate | PF6- |
 | Bis(trifluoromethanesulfonyl)imide | [TFSI] |
 | Bis(fluorosulfonyl)imide | [FSI] |
 | Bis(pentafluoroethansulfonyl)imide | [BETI] |
 | (Nonafluorobutanesulfonyl)(trifluoromethanesulfonyl)imide | [IM14] |
 | (Fluorosulfonyl)(trifluoromethanesulfonyl)imide | [FTFSI] |
 | Difluoro(oxalate)borate | [DFOB] |
 | Bis(oxalate)borate | [BOB] |
IL and Li-salt system
The replacement of OLEs with new electrolytes based on ILs aims to realize safer electrolytes with enhanced thermal and electrochemical stabilities while maintaining an acceptable ionic conductivity and tLi+.
To design suitable IL-based mixtures for use as electrolytes, it is paramount that the employed ILs have a low melting point (or preferably a total absence of a crystallization behavior even at sub-zero temperatures) and a low viscosity, in addition to the ability to dissolve lithium salts and a wide ESW. Given these conditions, a combination of a bulky and asymmetric cation (for a lower Tm) without unsaturated bonds and acidic protons (for better cathodic stability) coupled with a fluorinated anion (for a lower Tg, better ionic conductivity[57] and anodic stability[58]) is usually employed.
The cation is usually an imidazolium or an aliphatic quaternary ammonium. Imidazolium is the most common due to its association with high conductivity and low viscosity due to the planarity of the imidazolium ring[31]; yet, the presence of double bonds and the acidity of the proton at the C2 position results in ILs that are unstable at low potential. The issue of deprotonation can be mitigated by substituting this proton with a methyl group, increasing the cathodic stability of the IL, as reported by Bonhôte et al.[59]. On the other hand, aliphatic quaternary ammonium cations, such as tetraalkylammonium, pyrrolidinium, and piperidinium, are slightly less conductive compared to imidazolium ILs but are stable at lower potentials[60]. In addition, the use of phosphonium and sulphonium ILs is currently under investigation as they display a wide ESW and high thermal stability[61,62]. From a comparative study, the cathodic stability of these cations is found to increase in the following order: sulfonium < ammonium < phosphonium[63]. The superior stability of phosphonium cations arises from the more delocalized positive charge on the P atom center. However, phosphonium ILs are less common, which may be due to the air/moisture-sensitive and spontaneously flammable nature of trialkylphosphines and their successive toxic gas formation[64]. It is also possible to enhance the cathodic stability of ILs with alkyl side chains through a shielding effect of positive charge, which can be maximized by alteration of the alkyl chains with a moderate length such as a butyl group[65], because a shorter chain has a weak shielding effect whereas a longer chain is a good leaving group and not tolerant against reduction[66]. A common approach to reduce Tm is the introduction of an ether group in the side chain of the cation. This causes an increment of the degrees of freedom of the side chain, reducing the Tm or even inhibiting the crystallization behavior of ILs[31,38,67]. In particular, an ethoxyethyl group has been reported to contribute to the reduction of the Tm than the shorter methoxyethyl group.
With respect to the anions, BF4- and PF6- are among the first choices as counter ions (especially the latter corresponding to LiPF6, the most common lithium salt found in commercial electrolytes[69]). However, due to their perfect symmetry causing an increase in the Tm (e.g., Tm are 15 °C and 60 °C for [EMI]BF4 and [EMI]PF6, respectively[70,71]) and their tendency to be hydrolyzed in the presence of even a small amount of water resulting in the formation of HF, the use of these anions has declined, and imide-based anions have become the preferred choice for IL-based electrolytes. Bis(trifluoromethanesulfonyl)imide ([TFSI]) and bis(fluorosulfonyl)imide ([FSI]) are the main representatives of this family. ILs with [TFSI] anions exhibit a notable ESW, thermal stability, and good ionic conductivity (8.8 mS cm-1 for [EMI][TFSI][59]) and tend to have a reduced melting point (-3 °C for [EMI][TFSI][59]). ILs with [FSI] anions can exhibit enhanced ionic conductivity (15.6 mS cm-1 for [EMI][FSI][72]) due to the reduced steric hindrance of the anion in comparison to TFSI[31], while they suffer from a reduced electrochemical and thermal stability because of the low stability of S-F bonds compared to S-CF3 bonds[31]. Other (less common) imide-based anions are bis(pentafluoroethanesulfonyl)imide ([BETI]) and the asymmetrical ones ([IM14] and [FTFSI],
The current choice of lithium salt is Li[FSI] or Li[TFSI] because the same anions are used for the ILs, which act as solvents. IL electrolytes (ILEs) can also utilize different anions. For example, the use of a TFSI salt in an FSI-based IL has been reported to exhibit positive synergistic effects, such as higher anodic stability, a wider liquid range[74], an efficient formation of favorable EEIs on the surface of electrodes[75,76], an improved wettability[77], and a higher ionic conductivity/reduced viscosity[78]. Another important point regarding the additive salt is its concentration. Usually, with the aim of maintaining low viscosity of the mixtures, the molar ratio of IL:lithium salts varies between 9:1 and 8:2. However, addition of a large amount of salts in the electrolyte (more than 2 M) is known to reduce the gradient of Li+ concentration in the electrolyte, favoring a more uniform lithium deposition on lithium metal and the formation of a more robust SEI[7,8,34,77,79,80].
A mixture that has shown excellent properties in recent years is 0.8[PYR1,4][FSI]-0.2Li[TFSI]. This dual anion mixture possesses all the previously mentioned synergistic effects, especially good conductivity[13,78] coupled with a wide ESW[13,76]. Furthermore, the combination of these two anions permits the formation of suitable inorganic EEIs based on LiF, Li3N, LiNSO, and LixSOy, both on the surfaces of a lithium-metal anode and cathode, demonstrating a promising feature for the realization of high-energy LMBs. Employing this ILE in a high-voltage Li||LRNM system, Wu et al. obtained a discharge capacity of ~155 mAh g-1 and a capacity retention rate of 56.0% after 2,000 cycles at 1 C[13], which is an outstanding result among different ILEs from the viewpoints of C-rates and the number of cycles [Supplementary Table 1][31,61,73]. The enhanced cycle life can be explained by the formation of a protective CEI on the surface of the cathode, which suppressed the structural transformation of the LRNM to a rock-salt phase[13]. The same ILE was also used in a Li||NM88 battery, which achieved a discharge capacity of ~200 mAh g-1 with 88% capacity retention over 1,000 cycles at 0.3 C, while a similar system where an OLE (instead of ILE) was used, attained only a capacity retention rate of 36.9%[76]. Figure 2 shows the morphology of the cathode surface after cycling. The crack formation was evident when OLE, specifically 1M LiPF6 in EC/DMC 1:1 v/v (LP30), was used. XPS measurement on the same cathodes showed that the CEI present on the surface was rich with LiF and S-based species when the ILE was used, which is beneficial for a high mechanical strength[21,24,25] and electrochemical stability coupled with high Li+-conducting and electron insulating properties[21,24,25]. In addition, these CEI products are considered favorable to suppress electrolyte decomposition when in contact with highly charged and highly reactive metal species, i.e., Ni4+. Furthermore, due to the absence of LiPF6, HF was not produced upon cycling, avoiding the formation of rock-salt phases[76].
Figure 2. Top-view and cross-sectional SEM micrographs of pristine (fresh) NCM88 electrodes (A, D, G, and J) and NCM88 electrodes after 200 cycles at 0.3 C-rate in LP30 (B, E, H, and K) and ILE (C, F, I, and L). The electrodes shown in (A-I) were pressed at 8 t cm2, while those shown in (J-L) are unpressed electrodes. This figure is quoted with permission from Wu et al.[76]. Copyright (2021) The Author(s). Published by Elsevier Inc.
For super-concentrated ILEs, Heist and Lee showed that 4.2 M Li[FSI] in [PYR1,3][FSI] allowed the cycle of a Li||NMC811 battery for 1,000 times at 1 C maintaining ~77% of the initial capacity retention, in contrast to the cell with an OLE resulted in far lower capacity retention (~17%)[79]. With the increase in the salt concentration, the ionic conductivity decreased as expected. However, tLi+of the electrolyte increased, and SEI resistance decreased[79]. Sun et al. further developed this approach using a high-concentration ILE composed of 5M Li[FSI] in [EMI][FSI] with and without 0.16M Na[TFSI][8]. Using this electrolyte, a
Figure 3. (A) Cyclic stability of
ILs as co-solvent/additive
ILEs possess strong Coulombic interactions, which, in some cases, hinder their use as electrolytes, especially when a high concentration of lithium salts is added, the viscosity of ILEs increases, causing a reduced ionic conductivity and sluggish Li+ transport[77,81,82]. A possible approach to overcome this challenge is the use of the conventional organic solvents employed in the traditional OLEs as a co-solvent[77,82].
The combination of ILs and organic solvents achieves a medium with high ionic conductivity, which is mainly governed by the ions solubilized in the organic solvent. The role of the IL is to provide two functions in this type of system: first, it ensures enhanced safety, maintaining the thermal stability at the same level as ILEs. Secondly, a well-designed IL allows the formation of stable and protective EEIs on both electrodes. Since the former has been discussed extensively in the literature, the focus of this section is the formation of robust EEIs.
When ILs are added in OLEs, inorganic species in EEIs are present, and CF3- derived from [TFSI], N- due to ammonium cations[13], and B- from borate anions[38] are particularly important for CEI. In addition, P- species from phosphonium cations are reported to enhance the thermal stability of the CEI[61], and S-based ones are known to prevent lattice oxygen exposure[8].
On the anodic side, it is well known that the addition of an IL tends to produce a stable and inorganic SEI on the surface of lithium metal[7-9,75,77,83-85]. The most important component of this inorganic SEI is LiF, and it is usually coupled with other species produced by the decomposition of both the anion and the cation, depending on the structure of the employed IL. Recently, it has been reported that the cations play another significant role in the improvement of the performance of lithium metal because of the formation of a lithiophobic protective layer on Li protuberant tips[75,83]. Jang et al. reported that the addition of
Figure 4. Cycling performance of Li-metal batteries and Li-metal morphology after three initial cycles at a different C rate (E/C: electrolyte/cathode ratio and N/P: negative/positive ratio). (A) Li||LiFePO4 cell performance at 1 C (Li thickness: 300 μm and LiFePO4 loading: 3-4 mg cm-2). (B) Li||NCM622 cell performance at 1 C (Li thickness: 300 μm and NCM622 loading: 4-5 mg cm-2). (C) Li||NCM622 cell performance at 0.5 C (Li thickness: 40 μm and NCM622 loading: ≈12 mg cm-2). Cross-section SEM images of Li-metal anodes retrieved from the 40 μm Li||NCM622 cells with (D) [Pyr6,6][FSI, (E) [Pyr1,12][FSI], and (F) [Pyr3,3][FSI] containing electrolytes after 100 cycles at 0.5 C. (G) Schematic illustration of the Li-metal stabilization by lithiophobic protective layers on Li tips formed by three different IL cations [Pyr1,12]+, [Pyr6,6]+, and [Pyr3,3]+ (from left to right). This figure is reprinted (adapted) with permission from Jang et al.[75]. Copyright (2022) Wiley-VCH GmbH.
This layer produces a positive shielding effect that repels Li+ and hinders inhomogeneous lithium deposits, maintaining a smooth lithium surface, as shown in Figure 4G[75]. The effect was less pronounced when ILs with an asymmetric structure or with shorter alkyl chains were added. From SEM analysis of NMC622 recovered from the cycled cells [Figure 4D-F], the thickness of the SEI formed in the presence of [PYR6,6]+ was thinner and more compact than with other ILs.
Wang et al. assembled Li||LFP batteries by adding 20 wt% [BzMI][TFSI]/[EMI][TFSI] (9:1 in mol) in a
On the cathodic side, the addition of ILs leads to the formation of a CEI based on inorganic species similar to the ones found on the SEI. The CEI is a nanoscale layer that has demonstrated numerous beneficial features[13,38,61,69,76,86], including protection from corrosion, reduction of oxygen evolution, and prevention of the dissolution of transition metals. Typical anions that form CEI are oxalatoborate-based ones, such as
Although an effective reduction of the viscosity of the electrolyte is possible by using OLEs in addition to ILs, as a tradeoff, this approach potentially leads to an increase of the flammability of the electrolyte[77]. Furthermore, due to strong coordination of Li+ by alkyl-carbonates, these organic solvents change the Li+-solvation structure and affect the formation of SEI on the lithium metal surface, increasing its organic content and reducing its stability[77,82]. To solve this problem, another approach emerged in recent years using hydrofluoroethers (HFEs) instead of alkyl-carbonates as a co-solvent in ILEs, realizing the so-called “locally highly concentrated electrolytes, LHCEs”[9,77,80,84]. HFEs possess a low viscosity, are non-flammable, and due to their dielectric constant, they are not able to strongly coordinate Li+[9,77,84]. Because of these properties, their presence does not alter the coordination environment of Li+, allowing the formation of the inorganic-based SEI with faster Li+ transport [Figure 5A-C][84]. Using TTE as a HFE, Wang et al. realized an LHCE with a composition of Li[FSI]/[Pip1,3][FSI]/TTE (1:2:4 in mol) that allowed a Li||LFP battery to achieve a capacity retention of 87% after 1,000 cycles at a rate as high as 5 C. This cycling performance is superior to the same electrolyte without the addition of the HFE [Figure 5D][84].
Figure 5. Schematic diagrams of Li plating on Cu current collectors in (A) commercial electrolyte, (B) ILE, and (C) LHCE. (D) Cycling performance of Li||LFP cells at room temperature with different electrolytes at 5 C. This figure is reprinted (adapted) with permission from Wang et al.[84]. Copyright (2021) Wiley-VCH GmbH.
Other fluorinated additives, namely fluorinated aromatic compounds (FACs), are also of interest. Liu et al. demonstrated that dFBn added to Li[FSI]/[EMI][FSI] do not strongly interact with Li+ but instead preferentially interact with [EMI]+ through π-π interactions[82]. Using a mixture of Li[FSI]/[EMI][FSI]/dFBn (in a molar ratio of 1:2:2), they obtained a highly stable cycling of Li||NMC811 cells at C/3 charge and 1 C discharge for 500 cycles with a discharge capacity of 192 mAh g-1 and a capacity retention of 93%
Figure 6. (A) The evolution of the discharge specific capacity and Coulombic efficiency (CE) during long-term cycling of the cells at C/3 charge and 1C discharge after two formation cycles at C/10. XPS detail spectra in the C 1s, F 1s, and N 1s regions for the lithium cycled in (B) Li[FSI]/[EMI][FSI] (FE) and in (C) Li[FSI]/[EMI][FSI]/dFBn (FEdF) and for cathodes in (D) FE and (E) FEdF. This figure is reprinted (adapted) with permission from Liu et al.[82]. Copyright (2022) The Authors. Advanced Energy Materials published by Wiley-VCH GmbH.
The use of co-solvents has other roles besides the reduction in viscosity. For example, the addition of co-solvents forming a protective SEI on the Li-metal anodes enables the use of ILs with an acidic proton (so-called “protic ILs”), which generally reacts with lithium. Protic ILs are beneficial in terms of an easier and cheaper preparation method in comparison to common ILs because they can be synthesized merely by mixing a Brønsted base and acid[89-91]. In addition, they possess a high Li+ mobility when they are mixed with lithium salts due to the less shielded cation in comparison to aprotic ILs, leading to a “competition” between Li+ and the cation to coordinate the anion that results in a loosely coordinated Li+[89-91]. Lingua et al. recently showed that the addition of vinylene carbonate (VC) to 0.8[PYRH4][FSI]-0.2Li[FSI] allows the use of this electrolyte in a Li||NMC622 battery, obtaining a discharge capacity of 155 mAh g-1 and maintaining a capacity retention of 95% after 60 cycles[89]. This excellent result is ascribed to the formation of a protective layer on the lithium surface due to the decomposition of VC during the first cycle, which protects the electrolyte from further degradation upon cycling[89]. This preliminary result demonstrates that with further optimization, protic ILs can play an important role in the future development of high-voltage LMBs.
Besides the use of the co-solvent, ILs have been used as an additive with OLEs to improve their safety and to optimize the formation of both the SEI and CEI. Zhang et al. reported the addition of only a 1 wt% of
IONIC LIQUIDS IN POLYMER ELECTROLYTES
Another approach to overcome the limitations of OLEs is the replacement of liquid materials with solid materials to eliminate volatile and flammable components, hence reducing the risk of electrolyte leakage in case of abuse[92-98]. This can be achieved through two different approaches: first is the use of ILs in the form of polymer (PILs), and second is polymeric materials in which ILs are used as additives and plasticizers. Both materials can be further classified into two systems based on their form: gel polymer electrolytes (GPE) swollen by liquid components and solid polymer electrolytes (SPE) in a dry state.
Polymerized ionic liquids
The first polymerized ionic liquids (PIL) was reported in 1998 by Ohno and Ito[99]. In the past decades, many different PILs, such as (i) linear polycationic and polyanionic types; (ii) copolymer type (co-PIL); and (iii) cross-linked types (xl-PIL), including star-shaped types, have been reported[43]. Most PILs for LMB applications have polycationic structures, in which single or multiple cations are included either in the main chain, side chains, or bridges between cross-links, and ion species are mainly limited to imidazolium, quaternary ammonium (including pyrrolidinium structure), and rarely pyridinium.
Polymerization of [Vmim]Y, in which Y is the type of anion structure, with water-soluble azo-initiator yields linear PILs having imidazolium structure in the side chain. By selecting diallyldimethylammonium Y ([DADMA]Y) or imidazolium cation with two allyl groups, it is possible to include pyrrolidinium and imidazolium structures in the polymer backbone, respectively. There are numerous reports of poly([DADMA]Y)-derivatives containing different anion structures Y[100] and modified alkyl side chains[101]. Among them, the most conventional one is poly([DADMA][TFSI]), and its nanofiber prepared by electrospinning was found to show good wettability with ILEs, with an uptake almost five times more than that of polypropylene separators[102]. It was also demonstrated that the replacement of [TFSI] with [FSI] enables higher ionic conductivity of Li+, specifically poly([DADMA][FSI]-[Pyr1,3][FSI]-Li[FSI] (1:1:1, by molar ratio) possessed σLi of 0.22 mS cm-1 at 50 °C because of a lower amount of ion aggregates[103]. By employing poly[DADMA][FSI] mixed with 1M Li[FSI] in [Pyr1,3][FSI] (6:4 by weight), Fu et al. developed high-voltage LMBs[46]. The cell with NMC811 exhibited an initial capacity of 162 mAh g-1 (cut-off voltage of 4.4 V), and the one with LNMO displayed an initial capacity of 132 mAh g-1[46]. Both were cycled at
In a similar manner, there is also a growing trend to develop polyanions, which are generally classified as SPEs rather than PILs because the cation for polyanions is Li+ and not organic cations. Since anion structures are fixed in the polymer main chain, the tLi+ of unity can be achieved. Recently, fixation of sulfonylimide anion moieties on SPEs has been dominating material development because their delocalized negative charge is favorable for ion dissociation[106]. By enlarging the conjugation structure adjacent to the anion structure, the ionic interactions can be further reduced, and several poly-styrene derivatives with sulfonylimide structures have been designed as Li+ conductive SPEs[106].
There are also reports of polymer blends or copolymers of PIL to compensate for the weakness of each component or to improve the properties by their synergic effect. Hu et al. reported tensile strength and toughness enhanced through a composite formation based on poly(vinylidene fluoride) (PVdF) and PIL owing to ion-dipole interactions among the components[107]. For the design of suitable composite materials, compatibility between polymers and ILs should be a useful parameter to control microphase separation and properties of resulting couples[108,109]. In contrast, by forming a copolymer of PIL and other polymers, it is possible to control their phase separation. Zhu et al. prepared a copolymer based on PILs and poly(ethylene oxide) (PEO) that exhibited reduced crystallinity in PEO and microphase separation with enhanced tLi+[110]. Other examples of copolymer-type PILs are through integration with task-specific moieties, such as a flexible ether chain structure, to reduce glass transition temperature, or boron as an anion receptor[44,111].
Cross-linking has also been attempted, which is advantageous in terms of improved mechanical stability and ionic conductivity. The latter is because structural flexibility of the polymer chain allows faster ion conduction; thus, PILs with cross-links connecting short molecular chains are favorable for the ion conduction[112]. As summarized in Supplementary Table 2, most PILs contain a considerable amount of ILs as plasticizers that allow them to function as the electrolyte, while some copolymer and cross-linked PILs can operate as the electrolyte by merely adding lithium salts due to their intrinsically low Tg and presence of coordinating sites that allow the dissociation of the salt. Although it is difficult to systematically summarize all series of PILs because of their complicated structures, including unidentified molecular weight between cross-linking points and cross-link density, materials can be classified into two groups, one comprising an IL structure in the main polymer (in backbone or side chain structures) and one consisting of an IL structure as the bridge between polymer backbones. In the first case, cross-linkers such as Bisphenol A ethoxylate dimethacrylate[88], trimethylolpropane triacrylate[113], epoxy polyhedral oligomeric silsesquioxanes (POSS)[114], poly(ethylene glycol) diacrylate[115,116], and isocyanoethyl ethacrylate[117] have been used. In contrast, in the second case, cross-links are formed between ILs modified in two or more places with epoxy, vinyl, or allyl, and cross-link acceptors such as sulfur[118], pentaerythritol tetrakis(3-mercaptopropionate)[119], azobis(isobutyronitrile)[120], and poly(ethyleneimine)[121].
In the polymer-based electrolytes, their ion conduction property is governed by the segmental motions of polymers, which strongly correlate to Tg. The molecular weight is one of the most critical factors in determining the Tg, together with cross-linking density and the presence of plasticizer and functional groups. Therefore, it greatly affects their performance in batteries; thus, it is necessary to assess these factors by reliable methods. Yin et al. suggested that the polymerization degree is affected by the synthesis procedure, but determination of accurate molecular weight by means of chromatography is difficult because of polymer aggregation and column interaction[122]. To address this problem, high-performance IL chromatography (HPILC) is a promising technique in which ILs are used as an eluent[123].
As summarized in this section, there are different classes of PILs. However, their performance as electrolytes for LMBs is strongly dependent on the additives, such as liquid electrolytes and ILs, especially in the case of linear or copolymer type PILs. Therefore, the development of PILs should focus on increasing the ionic conductivity while incorporating other additional functionalities, such as improved mechanical stability, anti-folding, and self-healing properties. As a new type of PIL, toward greener materials, PIL based on biomass has also been reported[124]. Since the material can be synthesized through the reaction between chitosan and glycidyl trimethylammonium chloride, complicated polymerization steps can be avoided[125].
Ionic liquids in solid-polymer electrolytes
Regarding solid-polymer electrolytes (SPEs), as suggested by Armand at the Second Meeting on Solid Electrolytes in 1978, the first studied system is PEO with the addition of a lithium salt, namely Li[TFSI], which is still a relevant system even today[94,97,126-128]. This system works as electrolytes because of the Lewis acid-base interactions between Li+ (due to its high positive charge density) and the oxygen atoms in PEO chains (due to their lone pairs of electrons) that permit the dissociation of the lithium salt when Li+ and the anion are not strongly associated, as in the case of Li[TFSI][94,97,126-128]. This system is particularly appealing due to the absence of liquid electrolytes, avoiding any risk of leakage and thus improving the safety profile of devices[129]. Furthermore, the polymer can partly suppress the growth of dendrites on the surface of the lithium metal anode due to its high elastic modulus[129,130]. However, the main disadvantage of this system is related to its low ionic conductivity at room temperature[94,97,126-128]. This is primarily owing to Li+-conduction occurring along the chains of PEO, where it breaks the coordination with one oxygen atom and establishes coordination with the next one. This process is promoted by the segmental motion of PEO in the amorphous phase[94,97,127]. Due to the high Tg of PEO, the crystalline phase is predominant at room temperature, reducing the amount of amorphous phase available for Li+-conduction[94,97,127]. To overcome this limitation, the use of ILs as additives with PEO, resulting in GPE, is a desirable approach[126-128]. ILs in PEO act as plasticizing agents, reducing the crystallinity of the polymer and its Tg while simultaneously supporting the solvation of the lithium salt[128] and increasing the ionic conductivity. Atik et al. recently reported a promising PEO-based electrolyte with ionic conductivity of 0.66 mS cm-1, achieved by adding Li[TFSI]:[Pyr1,(2O)7][TFSI] ([Pyr1,(2O)7][TFSI] is an oligoether-functionalized IL with seven ether groups)[126] to the polymer matrix. This improvement is related to a “molecular-shuttle” effect: the oligoether chains of the cation solvate Li+ and unlock a new Li+ transport path, decoupling the movement from the usual chain-related transport characteristic of PEO-based electrolytes [Figure 7][126]. This efficient and faster Li+ transport reduces the formation of lithium dendrites, attaining a 99.3% capacity retention after 200 cycles in a Li||LFP cell[126], with an average discharge capacity of ~150 mAh g-1.
Figure 7. Illustration of the accelerated Li+ conduction modes in PEO-based electrolytes by state-of-the-art oligoether-functionalized IL plasticizer [Pyr1,(2O)7][TFSI], which enables new conduction modes. This figure is quoted with permission from Atik et al.[126]. Copyright (2021) The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Other polymeric materials frequently combined with ILs are fluorinated polymers such as PVdF and PVdF-HFP. These polymers have higher Tm (165 °C for the former, 142 °C for the latter) than PEO, which is advantageous for high temperature applications. PVdF-HFP has ionic conductivities between 10-6 and
Poiana et al. obtained a non-flammable GPE with ionic conduction of 1.46 mS cm-1 and a tLi+of 0.4 at 20 °C by soaking a PVdF-HFP membrane in a mixture of LP30/[Pyr14]PF6 (70:30 w/w)[129]. The GPE was found to be suitable for high-voltage applications, and in a
To further improve the ionic conductivity of GPEs, they have also been combined with inorganic solid electrolytes due to their many complementary features[92-98,131,135-139].Several GPEs, such as PEO with [Pyr14][TFSI]+Li[TFSI][93] and PVdF-HFP with [EMIM][TFSI] + Li[TFSI][131], have been combined with ceramic-type solid electrolytes [e.g.,
BEYOND IONIC LIQUIDS
Solvated ionic liquids and zwitterionic ionic liquids
Beyond traditional ionic liquids (ILs), other ionic materials have also been developed for the realization of task-specific liquid electrolytes, such as solvated ionic liquids (SILs) and zwitterionic ionic liquids (ZILs). SILs, extensively studied by Prof. Watanabe and coll[48,140-143], are composed of a lithium salt and an oligomeric ether such as triglyme or tetraglyme, predominantly solvating the cation[48,140]. Due to the strong Lewis basicity given by the lone pairs on the oxygen atoms, the ethers coordinate the lithium ion, forming the complex cations [Li(Glyme)]+, and dissociate the salt[48,143,144]. This phenomenon reduces the Tm of the lithium salt, producing a molten salt with properties similar to traditional ILs. In an equimolar mixture of Li[TFSI] and triglyme or tetraglyme, only a negligible amount of free-glyme molecules are left[140,144,145], as shown in Figure 8[145]. Thus, high thermal stabilities, negligible volatility, and non-flammability can be attained[48,143,144]. Furthermore, they can possess a high tLi+ due to their high concentration of Li+ and the presence of a reduced number of different charged species in comparison to binary IL-lithium salt mixtures, as well as a notable stability toward oxidation because of the scarce presence of free-glyme molecules and their HOMO energy level lowered by a donation of lone pairs[144]. Due to this improvement of the oxidative stability, Yoshida et al. realized a
Figure 8. Estimated percentages of free glyme (cf) over total glyme amount (cG) in equimolar molten mixtures [Li(glyme)1]Y at 30 °C. Reproduced from Ref.[145] with permission from the PCCP Owner Societies. Copyright (2015) The PCCP Owner Societies.
Unlike ILs, whose properties can be altered by inserting functional groups via covalent bonds, the properties of SILs are designed by tuning the physical bonds, which are influenced by the combination and composition of lithium salts and glyme. For the selection of lithium salts, their Lewis basicity is a critical factor and determines whether the resulting material will be a SIL or simply a highly concentrated salt solution in glyme[147]. This is because the interaction of glyme-Li+ and counter anion-Li+ is competitive. Glyme forms superior SIL, behaving as typical ILs with lithium salts with a weak Lewis basicity.
ZILs are another promising material for batteries, pioneered by Prof. Ohno et al., and have organic ion structures that can be found in ILs, but the cation and the anion are tethered together by covalent bonds[39-41,148-151]. Due to the presence of positive and negative charges in one molecule enhancing intermolecular electrostatic interaction, ZILs usually have a high Tm and are solid at room temperature[41]. Similar to ILs, by choosing ion structures with charge delocalization, such as imidazolium cations and sulfonamide anions, as well as flexible alkyl side chains, it is possible to realize ZILs that are liquid at room temperature[41].
Another interesting approach for liquefaction, which is specific to ZIL, is the addition of Li[TFSI]. Although Li[TFSI] is solid having Tm at 234-238 °C, specific combinations with ZIL, such as 1-(1-ethyl-3-imidazolio)butane-4-sulfonate and Li[TFSI] equimolar mixture, do not show crystallization behavior[152]. This is because of the formation of an IL-like phase between imidazolium cation in ZIL and added TFSI anions. However, even carefully designed room temperature ZILs are more viscous compared to RTILs[41]. As a consequence, it is difficult to realize ZIL-based electrolytes with satisfying ionic conductivity[41], and it is common to couple them with OLEs. The main advantage of ZILs is that the molecules do not move according to the electric field because of their neutrality, increasing the ionic conductivity of Li+[39,40,148-151]. Furthermore, due to their large dipole moment, they can easily dissolve lithium salts[148], enhancing the Li+-transport[148,150,153]. In addition, it is also reported that their presence is beneficial in terms of anti-freeze properties[153], the formation of favorable SEI morphologies with reduced resistances[151,154], and the ability to hinder the degradation of organic electrolytes[148,153]. Guan et al. reported on the use of a liquid ZIL as the electrolyte, which is based on quaternary ammonium structures modified with ether oxygen and sulfonate anions, containing Li+ and ClO4-, with the addition of only 7 wt% of EC[149]. The realized electrolyte achieved conductivity of 1 mS cm-1 at 20 °C and allowed a Li||LFP cell to operate stably for 100 cycles, achieving a discharge capacity of 120 mAh g-1. This was possible by forming a thin and stable SEI on lithium metal[149].
Deep eutectic solvents
For large-scale applications, complicated synthesis and purification of ILs must be simplified[30]. Furthermore, for these syntheses, a notable amount of organic solvents, such as acetonitrile, dichloromethane, and acetone, are consumed[30]. Thus, the implementation of ILs on the industrial scale is hindered by these aspects. Recently, deep eutectic solvents (DESs), which possess similar properties to ILs, and a facile synthesis method have been proposed as alternatives[35,49].
In general, DESs are defined as a mixture of an HBD and an HBA that, due to the charge delocalization caused by the hydrogen bonds formed between them, results in a Tm far below that of the employed components[35,49]. Some of the HBDs used for the realization of DESs are amides, such as urea, N-methylacetamide (NMA), 2,2,2-trifluoroacetamide (TFA), methanesulfonamide (MSA), and N-dimethyl MSA (DMMSA), while the most famous HBA is choline chloride ([Ch]Cl), which is not RTIL but can be regarded as ILs in a broader sense. The mixture between urea and [Ch]Cl in a molar ratio 1:2 (called “reline”) is the most famous DES, which has been extensively studied over the years for applications such as electrodeposition of metals[35,155-157], CO2 capture[35,158], redox-flow battery testing[35,159], biocatalysis[35,160], biomass pretreatment[35,161], and synthesis of nanoparticles[35,162-166]. However, this type of DES is not adequate for LMB applications because of its high viscosity[35,167] and its low cathodic stability due to the presence of N-H groups in the HBDs. As in the case of ILs, the structure of DESs can also be modulated[49] (the estimated number of possible structures of DESs composed from two materials is between 106 and 108[35]). This enables the formation of DESs displaying high ionic conductivity and exerting control on the EEIs.
A type of DESs studied for applications in lithium batteries as the electrolyte is the mixture of an amide and Li[TFSI] or LiPF6[50,168-170]. In this case, the driving forces for lowering the Tm are both the interaction between the N-H group and the anion and the interaction between the Li+ and the lone pair on the C=O group[50]. These two interactions weaken the hydrogen bonding among the amide molecules, lowering its melting point and producing a DES with (usually) a high tLi+ due to the high content of lithium. While the first HBD used for the realization of DES-based electrolytes is acetamide[170], its use has quickly become obsolete due to the instability of N-H bonds with lithium metal anodes. As a result, NMA is the main choice as the HBD to realize DES-based electrolytes for LMBs[171-176].
Ogawa and Mori recently suggested that the interaction between Li+ and C=O is more intense and influential than that between the N-H group and the anion since the mixture between tetramethylurea and Li[TFSI] can also produce a DES, even though the N-H group is absent in tetramethylurea[50]. This result is critical for future DES development because the presence of the N-H group generally causes low cathodic stability. Recent results on the use of DESs in electrolytes are reported in Supplementary Table 3.
Wu et al. produced a mixture of Li[TFSI]:Li[DFOB]:succinonitrile (SN) (0.8:0.2:10 in mol), in which the CN groups of SN are the HBD[177]. In this case, SN has a high polarity and can dissociate the lithium salts[177]. The mixture was found to have a tLi+ of 0.75, higher than the typical range for common OLEs, which is usually 0.3-0.4[177,178]. The use of Li[DFOB] is advantageous in forming a stable and efficient SEI on the lithium surface, preventing the reduction of SN, and creating a protective CEI on the cathode surface. A
Similarly, Liang et al. demonstrated that Li[TFSI]:NMA (1:4 in mol) is a prospective DES, and by adding
Figure 9. (A) Cycling performance of NCM622||Li cells with DES-0 (without LiNO3 addition), DES-2 (with a 2 wt% LiNO3 addition), and commercial electrolytes at 25 °C and 0.5 C. The inserted images present the surface of separators with the electrolytes DES-0 (left) and DES-2 (right) after 100 cycles. (B) Rate performance of NCM622||Li cells with DES-2 and commercial electrolytes at 25 °C. (C) Cycling performance of NCM811||Li cells with different electrolytes at 25 °C and 0.5 C. (D) Voltage profile of NCM811||Li cells. This figure is reprinted (adapted) with permission from Liang et al.[171]. Copyright (2022) Wiley-VCH GmbH.
Conversely, Li et al. used the DES composed of Li[TFSI]:Urea (1:3 in mol) as an additive to a solution of
In the use of DESs as additives for OLEs, the hydrogen bonds maintaining the structure of DES can be destroyed by the addition of other solvents. In the case of DESs based on [Ch]Cl and urea, the maximum concentration of water in which the structure of DESs is maintained is reported to be ca. 42 wt% H2O. Above this concentration, the solution is just an aqueous solution containing [Ch]Cl and urea separately[180].
Similarly to GPEs and ionogels, the inclusion of DESs in polymers is possible, which are known as “eutectogels”. Joos et al. added a Li[TFSI]:NMA (1:4 in mol) to a polymer based on AcMo, and EGDMA[172]. The eutectogel allowed a Li||LFP cell to provide a specific discharge capacity of 105 mAh g-1 after 250 cycles. Due to hydrogen bonds formed between NMA and AcMo, the amount of free-NMA is reduced, preventing its decomposition, and the contribution of TFSI to form a more stable SEI is increased. A similar beneficial interaction has been reported by Wang et al. between NML and the oxygen in the chains of PEGDA[181]. The DES Li[TFSI]:NML (1:6 in mol) was added to a polymer based on PEGDA and UPyMA[181]. This composition improved both the mechanical strength and the cathodic stability of the electrolyte[181]. This electrolyte has exhibited a tLi+ of 0.66 and allows a
Furthermore, Wang et al. reported the use of an eutectogel based on Li[TFSI]:NMA (1:4 in mol) in a PEO matrix, which also contains metal-organic-framework (MOF) UiO66-NH2[175]. The reactivity of NMA with lithium metal was reduced due to the interaction between the NH2 group of the MOF and the C=O group of NMA. As a result, the SEI formed in a Li||LFP cell was rich in LiF and Li3N and allowed to retain 93.3% of the initial discharge capacity (159 mAh g-1) after 350 cycles at 0.2 C, along with achieving a stable stripping-deposition for over 3,000 h in a Li||Li symmetric battery[175].
Jaumeaux et al. added a Li[TFSI]:NMA (1:4 in mol) + 10 wt% FEC mixture to UPyMA and PETEA, obtaining a polymer electrolyte with ionic conductivity of 1.79 mS cm-1 and tLi+ 0.76[173]. Using this electrolyte, a Li||LMO cell was capable of maintaining a capacity retention of 86.1% after 200 cycles at 0.1 C. This excellent result is due to the addition of FEC, which provides the formation of stable, uniform, thin, and LiF-rich SEI and CEI. The former avoids the formation of lithium dendrites, while the latter avoids the dissolution of Mn2+ into the electrolyte [Figure 10][173].
Figure 10. (A) DES and (B) DES-based self-healing polymer electrolytes in Li||LMO cells. This figure is quoted with permission from Jaumeaux et al.[173]. Copyright (2020) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Toward the development of economical sustainable materials, Zhang et al. added Li[TFSI]:Li[DFOB]:SN (0.8:0.02:1 wt/wt/wt) (a DES similar to the one used in[177]) in cyanoethyl cellulose obtaining an electrolyte with tLi+ 0.64 to attain a
Figure 11. Schematic illustration of the multifunctional mechanism of eutectogel electrolytes in a 4.45 V
CONCLUSIONS AND OUTLOOK
LIBs are integral energy storage devices, yet their safety and energy density remain focal issues to be resolved. The utilization of ILs as the electrolyte will be at the forefront of the transition from LIB to LMB technology, whereby the lithium metal anode is fundamental to realizing high energy density lithium batteries. ILs are prospective conductive materials because they possess two key properties. First, their well-known and intrinsic safety is supported by decades of extensive research. Second, the ability to rationally design ILs through the modification of the constituent ions offers the route to realize superior EEI. This entails creating a well-designed SEI on the lithium surface, allowing a uniform lithium deposition, preventing the formation of dendrites or dead lithium, and establishing a CEI on the cathode surface to prevent corrosion, transition-metal dissolution, and oxygen development. Several strategies exist to design ILs suitable for battery applications. Presently, the most promising ILE is the binary anion system based on TFSI-IL and FSI-salts, which can generate uniform SEI on the lithium metal anode and a protective CEI on cathode materials, in addition to achieving excellent stability, high ionic conductivity, and low viscosity.
Toward the advancement of ILs, it is necessary to carry out molecular engineering to create “IL-based composite materials” or “IL-derivatives”. Thus, IL mixtures with OLEs or other ionic materials, including PIL, SIL, ZIL, and DES, have been developed. When ILs are used as the main component for the electrolyte, the ILs should display good liquid properties, i.e., low viscosity and high ionic conductivity over a wide range of temperatures. In contrast, when ILs are used as the additive for the OLEs, other functions, such as the formation of stable EEIs, take precedence over the IL being in a liquid state with a low viscosity. Thus, more diverse ion structures can be employed when ILs are used as an additive. Among the emerging possibilities of IL-derived materials, namely DES, PIL, SIL, and ZIL, DESs are the most appealing in terms of feasibility and properties similar to those of ILs. Nevertheless, DES research is still in an early stage, and the reactivity of the N-H group with lithium metal is a serious threat, limiting its application. On the other hand, the use of this reactive material to control the formation of EEIs has already been attempted with success. Also, the use of additives or amides without the N-H group (replaced with N-CH3) has the propensity to ameliorate their low cathodic stability.
In addition to molecular design, future focus is necessary on the conformational design between ILs and other materials to control ion segregation and allocate special functions to the segregated phases, e.g., a highly concentrated electrolyte system. The presence of a high amount of lithium salts is unfavorable because it increases the viscosity while providing the formation of the Li+-conduction path. Whereas for the transference number of lithium, a higher amount of lithium salts is favorable. With a maturation of IL development at the molecular scale, the focus will shift to the design of the complete electrolyte, considering functional interactions and related phase formation.
The composite materials of ILs and inorganic SSEs to yield quasi-SSEs are another class of prospective materials under investigation[183]. Inorganic SSEs are critical materials for the next-generation batteries displaying excellent thermal and electrochemical stabilities, but their use is hindered by their poor contact with the electrode surfaces, which causes high interfacial resistances[93,96-98,184-187]. ILs are proposed as interlayers to achieve the formation of solid-solid interphases with high Li+-conducting properties and reduced interfacial resistances. The interlayer can also protect the electrolyte from parasitic reactions with lithium[184-186]. Compositional engineering using a minimal number of liquid components can eliminate interfacial resistance and side reactions of SSEs. Although there are only a few studies on the interaction between SSEs and ILs[188], further examination will be required to provide insights for material design. The same strategy should be undertaken for the recently proposed composite materials with ILs and MOFs[189-191]. Computational modeling, such as density functional theory and molecular dynamics simulations, will be required to understand the structure, dynamics, and complex interactions in ILs and with battery components. Furthermore, given the complexity of the systems and the number of parameters to be considered, it is foreseeable that automation of processes, such as electrolyte screening and design, can be aided by machine learning approaches. The synergy of computational modeling, machine learning, and experiments is essential to realize the development of the next-generation batteries.
DECLARATIONS
Acknowledgments
Passerini S acknowledges the basic funding of the Helmholtz Association. Adenusi H acknowledges the University of Hong Kong and the Hong Kong Quantum AI Lab Limited for supporting his fellowship.
Authors’ contributions
Proposed the topic of this review: Navarra MA, Passerini S
Prepared the manuscript: Palluzzi M, Tsurumaki A
Collectively discussed and revised the manuscript: Palluzzi M, Tsurumaki A, Adenusi H, Navarra MA, Passerini S
Availability of data and materials
Not applicable.
Financial support and sponsorship
Navarra MA, Tsurumaki A, and Palluzzi M acknowledge the Sapienza University of Rome for the financial support within the ILES project, prot. RM120172B3B81486 (Navarra MA and Tsurumaki A), and the “Avvio alla Ricerca” 2022, prot. AR1221816B56A67B (Palluzzi M).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable
Copyright
© The Author(s) 2023.
Supplementary Materials
REFERENCES
1. Reddy MV, Mauger A, Julien CM, Paolella A, Zaghib K. Brief history of early lithium-battery development. Materials 2020;13:1884.
2. Miao Y, Hynan P, von Jouanne A, Yokochi A. Current Li-ion battery technologies in electric vehicles and opportunities for advancements. Energies 2019;12:1074.
3. Lai X, Chen Q, Tang X, et al. Critical review of life cycle assessment of lithium-ion batteries for electric vehicles: a lifespan perspective. eTransportation 2022;12:100169.
4. Kebede AA, Coosemans T, Messagie M, et al. Techno-economic analysis of lithium-ion and lead-acid batteries in stationary energy storage application. J Energy Stor 2021;40:102748.
5. Kebede AA, Kalogiannis T, Van Mierlo J, Berecibar M. A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew Sustain Energy Rev 2022;159:112213.
6. Sadd M, Xiong S, Bowen JR, Marone F, Matic A. Investigating microstructure evolution of lithium metal during plating and stripping via operando X-ray tomographic microscopy. Nat Commun 2023;14:854.
7. Liu X, Zarrabeitia M, Mariani A, et al. Enhanced Li+ transport in ionic liquid-based electrolytes aided by fluorinated ethers for highly efficient lithium metal batteries with improved rate capability. Small Methods 2021;5:e2100168.
8. Sun H, Zhu G, Zhu Y, et al. High-safety and high-energy-density lithium metal batteries in a novel ionic-liquid electrolyte. Adv Mater 2020;32:e2001741.
9. Wang Z, Zhang H, Xu J, et al. Advanced ultralow-concentration electrolyte for wide-temperature and high-voltage Li-metal batteries. Adv Funct Mater 2022;32:2112598.
10. Ren W, Zheng Y, Cui Z, Tao Y, Li B, Wang W. Recent progress of functional separators in dendrite inhibition for lithium metal batteries. Energy Stor Mater 2021;35:157-68.
11. Liu J, Bao Z, Cui Y, et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat Energy 2019;4:180-6.
12. Hua W, Wang S, Knapp M, et al. Structural insights into the formation and voltage degradation of lithium- and manganese-rich layered oxides. Nat Commun 2019;10:5365.
13. Wu F, Kim GT, Diemant T, et al. Reducing capacity and voltage decay of Co-free Li1.2Ni0.2Mn0.6O2 as positive electrode material for lithium batteries employing an ionic liquid-based electrolyte. Adv Energy Mater 2020;10:2001830.
14. Liang G, Peterson VK, See KW, Guo Z, Pang WK. Developing high-voltage spinel LiNi0.5Mn1.5O4 cathodes for high-energy-density lithium-ion batteries: current achievements and future prospects. J Mater Chem A 2020;8:15373-98.
15. Yu X, Yu WA, Manthiram A. Advances and prospects of high-voltage spinel cathodes for lithium-based batteries. Small Methods 2021;5:e2001196.
16. Li T, Yuan XZ, Zhang L, Song D, Shi K, Bock C. Degradation mechanisms and mitigation strategies of nickel-rich NMC-based lithium-ion batteries. Electrochem Energy Rev 2020;3:43-80.
17. Jung R, Linsenmann F, Thomas R, et al. Nickel, manganese, and cobalt dissolution from Ni-rich NMC and their effects on NMC622-graphite cells. J Electrochem Soc 2019;166:A378.
18. Zhang SS. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Stor Mater 2020;24:247-54.
19. Hu S, Pillai AS, Liang G, et al. Li-rich layered oxides and their practical challenges: recent progress and perspectives. Electrochem Energy Rev 2019;2:277-311.
20. Adenusi H, Chass GA, Passerini S, Tian KV, Chen G. Lithium batteries and the solid electrolyte interphase (SEI) - progress and outlook. Adv Energy Mater 2023;13:2203307.
21. Wu H, Jia H, Wang C, Zhang JG, Xu W. Recent progress in understanding solid electrolyte interphase on lithium metal anodes. Adv Energy Mater 2021;11:2003092.
22. Deng K, Zeng Q, Wang D, et al. Nonflammable organic electrolytes for high-safety lithium-ion batteries. Energy Stor Mater 2020;32:425-47.
23. Chandra Rath P, Wu CJ, Patra J, et al. Hybrid electrolyte enables safe and practical 5V LiNi0.5Mn1.5O4 batteries. J Mater Chem A 2019;7:16516-25.
24. Zhai P, Liu L, Gu X, Wang T, Gong Y. Interface engineering for lithium metal anodes in liquid electrolyte. Adv Energy Mater 2020;10:2001257.
25. Yoon I, Jurng S, Abraham DP, Lucht BL, Guduru PR. Measurement of mechanical and fracture properties of solid electrolyte interphase on lithium metal anodes in lithium ion batteries. Energy Stor Mater 2020;25:296-304.
26. Płotka-wasylka J, de la Guardia M, Andruch V, Vilková M. Deep eutectic solvents vs ionic liquids: similarities and differences. Microchem J 2020;159:105539.
28. Singh SK, Savoy AW. Ionic liquids synthesis and applications: an overview. J Mol Liq 2020;297:112038.
29. Jeong S, Li S, Appetecchi GB, Passerini S. Asymmetric ammonium-based ionic liquids as electrolyte components for safer, high-energy, electrochemical storage devices. Energy Stor Mater 2019;18:1-9.
30. Simonetti E, De Francesco M, Bellusci M, et al. A more sustainable and cheaper one-pot route for the synthesis of hydrophobic ionic liquids for electrolyte applications. ChemSusChem 2019;12:4946-52.
31. Brutti S, Simonetti E, De Francesco M, et al. Ionic liquid electrolytes for high-voltage, lithium-ion batteries. J Power Sources 2020;479:228791.
32. Philippi F, Welton T. Targeted modifications in ionic liquids - from understanding to design. Phys Chem Chem Phys 2021;23:6993-7021.
33. Palumbo O, Sarra A, Brubach JB, et al. So similar, yet so different: the case of the ionic liquids N-trimethyl-N (2-methoxyethyl)ammonium Bis (trifluoromethanesulfonyl)imide and N,N-Diethyl-N-methyl-N(2-methoxyethyl)ammonium bis(trifluoromethanesulfonyl)imide. Front Phys 2022;10:851279.
34. Tong J, Wu S, von Solms N, et al. The effect of concentration of lithium salt on the structural and transport properties of ionic liquid-based electrolytes. Front Chem 2019;7:945.
35. Hansen BB, Spittle S, Chen B, et al. Deep eutectic solvents: a review of fundamentals and applications. Chem Rev 2021;121:1232-85.
36. Mourad E, Coustan L, Lannelongue P, et al. Biredox ionic liquids with solid-like redox density in the liquid state for high-energy supercapacitors. Nat Mater 2017;16:446-53.
37. Qi S, Liu J, He J, et al. Structurally tunable characteristics of ionic liquids for optimizing lithium plating/stripping via electrolyte engineering. J Energy Chem 2021;63:270-7.
38. Tsurumaki A, Branchi M, Rigano A, Poiana R, Panero S, Navarra MA. Bis(oxalato)borate and difluoro(oxalato)borate-based ionic liquids as electrolyte additives to improve the capacity retention in high voltage lithium batteries. Electrochim Acta 2019;315:17-23.
39. Yoshizawa M, Hirao M, Ito-akita K, Ohno H. Ion conduction in zwitterionic-type molten salts and their polymers. J Mater Chem 2001;11:1057-62.
40. Ohno H, Yoshizawa M, Ogihara W. A new type of polymer gel electrolyte: zwitterionic liquid/polar polymer mixture. Electrochim Acta 2003;48:2079-83.
41. Yoshizawa-Fujita M, Ohno H. Applications of zwitterions and zwitterionic polymers for Li-ion batteries. Chem Rec 2023;23:e202200287.
42. Ohno H. Design of ion conductive polymers based on ionic liquids. Macromol Symp 2007;249-50:551-6.
44. Matsumi N, Sugai K, Miyake M, Ohno H. Polymerized ionic liquids via hydroboration polymerization as single ion conductive polymer electrolytes. Macromolecules 2006;39:6924-7.
45. Eshetu GG, Mecerreyes D, Forsyth M, Zhang H, Armand M. Polymeric ionic liquids for lithium-based rechargeable batteries. Mol Syst Des Eng 2019;4:294-309.
46. Fu C, Homann G, Grissa R, et al. A Polymerized-ionic-liquid-based polymer electrolyte with high oxidative stability for 4 and 5 V class solid-state lithium metal batteries. Adv Energy Mater 2022;12:2200412.
47. Watanabe M, Dokko K, Ueno K, Thomas ML. From ionic liquids to solvate ionic liquids: challenges and opportunities for next generation battery electrolytes. Bull Chem Soc Jpn 2018;91:1660-82.
48. Mandai T, Dokko K, Watanabe M. Solvate ionic liquids for Li, Na, K, and Mg batteries. Chem Rec 2019;19:708-22.
49. Cappelluti F, Mariani A, Bonomo M, et al. Stepping away from serendipity in deep eutectic solvent formation: prediction from precursors ratio. J Mol Liq 2022;367:120443.
50. Ogawa H, Mori H. Lithium salt/amide-based deep eutectic electrolytes for lithium-ion batteries: electrochemical, thermal and computational study. Phys Chem Chem Phys 2020;22:8853-63.
51. Ueno K, Park JW, Yamazaki A, et al. Anionic effects on solvate ionic liquid electrolytes in rechargeable lithium-sulfur batteries. J Phys Chem C 2013;117:20509-16.
52. Dokko K, Tachikawa N, Yamauchi K, et al. Solvate ionic liquid electrolyte for Li-S batteries. J Electrochem Soc 2013;160:A1304.
53. Inman G, Nlebedim IC, Prodius D. Application of ionic liquids for the recycling and recovery of technologically critical and valuable metals. Energies 2022;15:628.
54. Zante G, Braun A, Masmoudi A, Barillon R, Trébouet D, Boltoeva M. Solvent extraction fractionation of manganese, cobalt, nickel and lithium using ionic liquids and deep eutectic solvents. Miner Eng 2020;156:106512.
55. Zheng H, Huang J, Dong T, et al. A novel strategy of lithium recycling from spent lithium-ion batteries using imidazolium ionic liquid. Chin J Chem Eng 2022;41:246-51.
56. Botelho Junior AB, Stopic S, Friedrich B, Tenório JAS, Espinosa DCR. Cobalt recovery from Li-ion battery recycling: a critical review. Metals 2021;11:1999.
57. Pringle JM, Golding J, Baranyai K, et al. The effect of anion fluorination in ionic liquids - physical properties of a range of bis(methanesulfonyl)amide salts. New J Chem 2003;27:1504-10.
58. Park J, Jung Y, Kusumah P, Lee J, Kwon K, Lee CK. Application of ionic liquids in hydrometallurgy. Int J Mol Sci 2014;15:15320-43.
59. Bonhôte P, Dias AP, Papageorgiou N, Kalyanasundaram K, Grätzel M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg Chem 1996;35:1168-78.
60. Liu K, Wang Z, Shi L, Jungsuttiwong S, Yuan S. Ionic liquids for high performance lithium metal batteries. J Energy Chem 2021;59:320-33.
61. Wu F, Schür AR, Kim GT, et al. A novel phosphonium ionic liquid electrolyte enabling high-voltage and high-energy positive electrode materials in lithium-metal batteries. Energy Stor Mater 2021;42:826-35.
62. Rangasamy VS, Thayumanasundaram S, Locquet JP. Ionic liquid electrolytes based on sulfonium cation for lithium rechargeable batteries. Electrochim Acta 2019;328:135133.
63. Pandian S, Raju SG, Hariharan KS, Kolake SM, Park DH, Lee MJ. Functionalized ionic liquids as electrolytes for lithium-ion batteries. J Power Sources 2015;286:204-9.
64. Blundell RK, Licence P. Quaternary ammonium and phosphonium based ionic liquids: a comparison of common anions. Phys Chem Chem Phys 2014;16:15278-88.
65. Tsurumaki A, Tajima M, Abe M, Sato D, Ohno H. Effect of the cation structure on cellulose dissolution in aqueous solutions of organic onium hydroxides. Phys Chem Chem Phys 2020;22:22602-8.
66. Montanino M, Carewska M, Alessandrini F, Passerini S, Appetecchi GB. The role of the cation aliphatic side chain length in piperidinium bis(trifluoromethansulfonyl)imide ionic liquids. Electrochim Acta 2011;57:153-9.
67. Zhang S, Li J, Jiang N, et al. Rational design of an ionic liquid-based electrolyte with high ionic conductivity towards safe lithium/lithium-ion batteries. Chem Asian J 2019;14:2810-4.
68. Jin Y, Fang S, Chai M, Yang L, Hirano S. Ether-functionalized trialkylimidazolium ionic liquids: synthesis, characterization, and properties. Ind Eng Chem Res 2012;51:11011-20.
69. Tsurumaki A, Agostini M, Poiana R, et al. Enhanced safety and galvanostatic performance of high voltage lithium batteries by using ionic liquids. Electrochim Acta 2019;316:1-7.
70. Wilkes JS, Zaworotko MJ. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J Chem Soc Chem Commun 1992;13:965-67.
71. Fuller J, Carlin RT, De Long HC, Haworth D. Structure of 1-ethyl-3-methylimidazolium hexafluorophosphate: model for room temperature molten salts. J Chem Soc Chem Commun 1994;3:299-300.
72. Sayah S, Ghamouss F, Tran-van F, Santos-peña J, Lemordant D. A bis(fluorosulfonyl)imide based ionic liquid as safe and efficient electrolyte for Si/Sn-Ni/C/Al composite anode. Electrochim Acta 2017;243:197-206.
73. Gao X, Wu F, Mariani A, Passerini S. Concentrated ionic-liquid-based electrolytes for high-voltage lithium batteries with improved performance at room temperature. ChemSusChem 2019;12:4185-93.
74. Kerner M, Plylahan N, Scheers J, Johansson P. Ionic liquid based lithium battery electrolytes: fundamental benefits of utilising both TFSI and FSI anions? Phys Chem Chem Phys 2015;17:19569-81.
75. Jang J, Shin JS, Ko S, et al. Self-assembled protective layer by symmetric ionic liquid for long-cycling lithium-metal batteries. Adv Energy Mater 2022;12:2103955.
76. Wu F, Fang S, Kuenzel M, et al. Dual-anion ionic liquid electrolyte enables stable Ni-rich cathodes in lithium-metal batteries. Joule 2021;5:2177-94.
77. Lee S, Park K, Koo B, et al. Safe, stable cycling of lithium metal batteries with low-viscosity, fire-retardant locally concentrated ionic liquid electrolytes. Adv Funct Mater 2020;30:2003132.
78. Elia GA, Ulissi U, Jeong S, Passerini S, Hassoun J. Exceptional long-life performance of lithium-ion batteries using ionic liquid-based electrolytes. Energy Environ Sci 2016;9:3210-20.
79. Heist A, Lee SH. Improved stability and rate capability of ionic liquid electrolyte with high concentration of LiFSI. J Electrochem Soc 2019;166:A1860-6.
80. Liu X, Mariani A, Adenusi H, Passerini S. Locally concentrated ionic liquid electrolytes for lithium-metal batteries. Angew Chem Int Ed 2023;62:e202219318.
81. Liu X, Mariani A, Zarrabeitia M, et al. Effect of organic cations in locally concentrated ionic liquid electrolytes on the electrochemical performance of lithium metal batteries. Energy Stor Mater 2022;44:370-8.
82. Liu X, Mariani A, Diemant T, et al. Difluorobenzene-based locally concentrated ionic liquid electrolyte enabling stable cycling of lithium metal batteries with nickel-rich cathode. Adv Energy Mater 2022;12:2200862.
83. Zhang S, Cheng B, Fang Y, et al. Inhibition of lithium dendrites and dead lithium by an ionic liquid additive toward safe and stable lithium metal anodes. Chin Chem Lett 2022;33:3951-4.
84. Wang Z, Zhang F, Sun Y, et al. Intrinsically nonflammable ionic liquid-based localized highly concentrated electrolytes enable high-performance Li-metal batteries. Adv Energy Mater 2021;11:2003752.
85. Wang TH, Chen C, Li NW, et al. Cations and anions regulation through hybrid ionic liquid electrolytes towards stable lithium metal anode. Chem Eng J 2022;439:135780.
86. Heist A, Hafner S, Lee SH. High-energy nickel-rich layered cathode stabilized by ionic liquid electrolyte. J Electrochem Soc 2019;166:A873-9.
87. Nagarajan S, Weiland C, Hwang S, Balasubramanian M, Arava LMR. Depth-dependent understanding of cathode electrolyte interphase (CEI) on the layered Li-ion cathodes operated at extreme high temperature. Chem Mater 2022;34:4587-601.
88. Nair JR, Colò F, Kazzazi A, et al. Room temperature ionic liquid (RTIL)-based electrolyte cocktails for safe, high working potential Li-based polymer batteries. J Power Sources 2019;412:398-407.
89. Lingua G, Falco M, Stettner T, Gerbaldi C, Balducci A. Enabling safe and stable Li metal batteries with protic ionic liquid electrolytes and high voltage cathodes. J Power Sources 2021;481:228979.
90. Menne S, Pires J, Anouti M, Balducci A. Protic ionic liquids as electrolytes for lithium-ion batteries. Electrochem Commun 2013;31:39-41.
91. Vogl T, Menne S, Kühnel RS, Balducci A. The beneficial effect of protic ionic liquids on the lithium environment in electrolytes for battery applications. J Mater Chem A 2014;2:8258-65.
92. Wu W, Wei Z, Wang J, et al. Enabling high-energy flexible solid-state lithium ion batteries at room temperature. Chem Eng J 2021;424:130335.
93. Zhang D, Xu X, Huang X, et al. A flexible composite solid electrolyte with a highly stable interphase for dendrite-free and durable all-solid-state lithium metal batteries. J Mater Chem A 2020;8:18043-54.
94. Ye T, Li L, Zhang Y. Recent Progress in solid electrolytes for energy storage devices. Adv Funct Mater 2020;30:2000077.
95. Hou M, Liang F, Chen K, Dai Y, Xue D. Challenges and perspectives of NASICON-type solid electrolytes for all-solid-state lithium batteries. Nanotechnology 2020;31:132003.
96. Li S, Zhang SQ, Shen L, et al. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv Sci 2020;7:1903088.
97. Xi G, Xiao M, Wang S, Han D, Li Y, Meng Y. Polymer-based solid electrolytes: material selection, design, and application. Adv Funct Mater 2021;31:2007598.
98. Dirican M, Yan C, Zhu P, Zhang X. Composite solid electrolytes for all-solid-state lithium batteries. Mater Sci Eng R Rep 2019;136:27-46.
99. Ohno H, Ito K. Room-temperature molten salt polymers as a matrix for fast ion conduction. Chem Lett 1998;27:751-2.
100. Pont AL, Marcilla R, De Meatza I, Grande H, Mecerreyes D. Pyrrolidinium-based polymeric ionic liquids as mechanically and electrochemically stable polymer electrolytes. J Power Sources 2009;188:558-63.
101. Döbbelin M, Azcune I, Bedu M, et al. Synthesis of pyrrolidinium-based poly(ionic liquid) electrolytes with poly(ethylene glycol) side chains. Chem Mater 2012;24:1583-90.
102. Yu L, Yu L, Peng Y, Lan X, Hu X. Electrospun poly(ionic liquid) nanofiber separators with high lithium-ion transference number for safe ionic-liquid-based lithium batteries in wide temperature range. Mater Today Phys 2022;25:100716.
103. Martinez-ibañez M, Boaretto N, Meabe L, et al. Revealing the anion chemistry effect on transport properties of ternary Gel polymer electrolytes. Chem Mater 2022;34:7493-502.
104. Tian X, Yang P, Yi Y, et al. Self-healing and high stretchable polymer electrolytes based on ionic bonds with high conductivity for lithium batteries. J Power Sources 2020;450:227629.
105. Yin K, Zhang Z, Li X, Yang L, Tachibana K, Hirano S. Polymer electrolytes based on dicationic polymeric ionic liquids: application in lithium metal batteries. J Mater Chem A 2015;3:170-8.
106. Zhu J, Zhang Z, Zhao S, Westover AS, Belharouak I, Cao PF. Single-ion conducting polymer electrolytes for solid-state lithium-metal batteries: design, performance, and challenges. Adv Energy Mater 2021;11:2003836.
107. Hu Z, Chen J, Guo Y, et al. Fire-resistant, high-performance gel polymer electrolytes derived from poly(ionic liquid)/P(VDF-HFP) composite membranes for lithium ion batteries. J Memb Sci 2020;599:117827.
108. Xing C, Zhao M, Zhao L, You J, Cao X, Li Y. Ionic liquid modified poly(vinylidene fluoride): crystalline structures, miscibility, and physical properties. Polym Chem 2013;4:5726-34.
109. Tsurumaki A, Kagimoto J, Ohno H. Properties of polymer electrolytes composed of poly(ethylene oxide) and ionic liquids according to hard and soft acids and bases theory. Polym Adv Technol 2011;22:1223-8.
110. Zhu X, Fang Z, Deng Q, et al. Poly(ionic liquid)@PEGMA block polymer initiated microphase separation architecture in poly(ethylene oxide)-based solid-state polymer electrolyte for flexible and self-healing lithium batteries. ACS Sustaina Chem Eng 2022;10:4173-85.
111. Fu D, Sun Y, Zhang F, et al. Enabling polymeric ionic liquid electrolytes with high ambient ionic conductivity by polymer chain regulation. Chem Eng J 2022;431:133278.
112. Liang L, Yuan W, Chen X, Liao H. Flexible, nonflammable, highly conductive and high-safety double cross-linked poly(ionic liquid) as quasi-solid electrolyte for high performance lithium-ion batteries. Chem Eng J 2021;421:130000.
113. Wang D, Jin B, Ren Y, et al. Bifunctional solid-state copolymer electrolyte with stabilized interphase for high-performance lithium metal battery in a wide temperature range. ChemSusChem 2022;15:e202200993.
114. Chen X, Liang L, Hu W, Liao H, Zhang Y. POSS hybrid poly(ionic liquid) ionogel solid electrolyte for flexible lithium batteries. J Power Sources 2022;542:231766.
115. Zhang F, Sun Y, Wang Z, et al. Highly conductive polymeric ionic liquid electrolytes for ambient-temperature solid-state lithium batteries. ACS Appl Mater Interfaces 2020;12:23774-80.
116. Sha Y, Yu T, Dong T, Wu X, Tao H, Zhang H. In Situ network electrolyte based on a functional polymerized ionic liquid with high conductivity toward lithium metal batteries. ACS Appl Energy Mater 2021;4:14755-65.
117. Dong L, Zeng X, Fu J, et al. Cross-linked ionic copolymer solid electrolytes with loose Coordination-assisted lithium transport for lithium batteries. Chem Eng J 2021;423:130209.
118. Shi Y, Yang N, Niu J, Yang S, Wang F. A highly durable rubber-derived lithium-conducting elastomer for lithium metal batteries. Adv Sci 2022;9:e2200553.
119. Tseng YC, Hsiang SH, Lee TY, Teng H, Jan JS, Kyu T. In situ polymerized electrolytes with fully cross-linked networks boosting high ionic conductivity and capacity retention for lithium ion batteries. ACS Appl Energy Mater 2021;4:14309-22.
120. Tseng YC, Ramdhani FI, Hsiang SH, Lee TY, Teng H, Jan JS. Lithium battery enhanced by the combination of in-situ generated poly(ionic liquid) systems and TiO2 nanoparticles. J Membr Sci 2022;641:119891.
121. Liang L, Chen X, Yuan W, Chen H, Liao H, Zhang Y. Highly conductive, flexible, and nonflammable double-network poly(ionic liquid)-based ionogel electrolyte for flexible lithium-ion batteries. ACS Appl Mater Interfaces 2021;13:25410-20.
122. Yin K, Zhang Z, Yang L, Hirano SI. An imidazolium-based polymerized ionic liquid via novel synthetic strategy as polymer electrolytes for lithium ion batteries. J Power Sources 2014;258:150-4.
123. Kuroda K, Ohno H. Ionic liquids enable accurate chromatographic analysis of polyelectrolytes. Chem Commun 2015;51:10551-3.
124. Tan J, Ao X, Dai A, et al. Polycation ionic liquid tailored PEO-based solid polymer electrolytes for high temperature lithium metal batteries. Energy Stor Mater 2020;33:173-80.
125. Ding L, Liu Z, Song S, et al. Structural characteristics and rheological properties of hydroxypropyl trimethyl ammonium chloride chitosan. Int J Biol Macromol 2022;216:312-21.
126. Atik J, Diddens D, Thienenkamp JH, Brunklaus G, Winter M, Paillard E. Cation-assisted lithium-ion transport for high-performance PEO-based ternary solid polymer electrolytes. Angew Chem Int Ed 2021;60:11919-27.
127. Shin JH, Henderson WA, Passerini S. Ionic liquids to the rescue? Overcoming the ionic conductivity limitations of polymer electrolytes. Electrochem Commun 2003;5:1016-20.
128. Shin JH, Henderson WA, Passerini S. PEO-based polymer electrolytes with ionic liquids and their use in lithium metal-polymer electrolyte batteries. J Electrochem Soc 2005;152:A978.
129. Poiana R, Lufrano E, Tsurumaki A, Simari C, Nicotera I, Navarra MA. Safe gel polymer electrolytes for high voltage Li-batteries. Electrochim Acta 2022;401:139470.
130. Barai P, Higa K, Srinivasan V. Lithium dendrite growth mechanisms in polymer electrolytes and prevention strategies. Phys Chem Chem Phys 2017;19:20493-505.
131. Zhang W, Wang X, Zhang Q, et al. Li7La3Zr2O12 ceramic nanofiber-incorporated solid polymer electrolytes for flexible lithium batteries. ACS Appl Energy Mater 2020;3:5238-46.
132. Tseng YC, Wu Y, Tsao CH, Teng H, Hou SS, Jan JS. Polymer electrolytes based on Poly(VdF-co-HFP)/ionic liquid/carbonate membranes for high-performance lithium-ion batteries. Polymer 2019;173:110-8.
133. Rao M, Geng X, Liao Y, Hu S, Li W. Preparation and performance of gel polymer electrolyte based on electrospun polymer membrane and ionic liquid for lithium ion battery. J Membr Sci 2012;399-400:37-42.
134. Zhai W, Zhu H, Wang L, Liu X, Yang H. Study of PVDF-HFP/PMMA blended micro-porous gel polymer electrolyte incorporating ionic liquid [BMIM]BF4 for Lithium ion batteries. Electrochim Acta 2014;133:623-30.
135. Yang Y, Wu Q, Wang D, et al. Ionic liquid enhanced composite solid electrolyte for high-temperature/long-life/dendrite-free lithium metal batteries. J Membr Sci 2020;612:118424.
136. Wang B, Wang G, He P, Fan LZ. Rational design of ultrathin composite solid-state electrolyte for high-performance lithium metal batteries. J Membr Sci 2022;642:119952.
137. Li Z, Guo X. Integrated interface between composite electrolyte and cathode with low resistance enables ultra-long cycle-lifetime in solid-state lithium-metal batteries. Sci China Chem 2021;64:673-80.
138. Lin X, Chu C, Li Z, et al. A high-performance, solution-processable polymer/ceramic/ionic liquid electrolyte for room temperature solid-state Li metal batteries. Nano Energy 2021;89:106351.
139. Liu M, Zhang S, van Eck ERH, Wang C, Ganapathy S, Wagemaker M. Improving Li-ion interfacial transport in hybrid solid electrolytes. Nat Nanotechnol 2022;17:959-67.
140. Arai N, Watanabe H, Yamaguchi T, et al. Dynamic chelate effect on the Li+-ion conduction in solvate ionic liquids. J Phys Chem C 2019;123:30228-33.
141. Arai N, Watanabe H, Nozaki E, et al. Speciation analysis and thermodynamic criteria of solvated ionic liquids: ionic liquids or superconcentrated solutions? J Phys Chem Lett 2020;11:4517-23.
142. Shigenobu K, Shibata M, Dokko K, Watanabe M, Fujii K, Ueno K. Anion effects on Li ion transference number and dynamic ion correlations in glyme-Li salt equimolar mixtures. Phys Chem Chem Phys 2021;23:2622-9.
143. Takahashi K, Ishino Y, Murata W, et al. Physicochemical compatibility of highly-concentrated solvate ionic liquids and a low-viscosity solvent. RSC Adv 2019;9:24922-7.
144. Eyckens DJ, Henderson LC. A review of solvate ionic liquids: physical parameters and synthetic applications. Front Chem 2019;7:263.
145. Ueno K, Tatara R, Tsuzuki S, et al. Li+ solvation in glyme-Li salt solvate ionic liquids. Phys Chem Chem Phys 2015;17:8248-57.
146. Yoshida K, Nakamura M, Kazue Y, et al. Oxidative-stability enhancement and charge transport mechanism in glyme-lithium salt equimolar complexes. J Am Chem Soc 2011;133:13121-9.
147. Ueno K, Yoshida K, Tsuchiya M, Tachikawa N, Dokko K, Watanabe M. Glyme-lithium salt equimolar molten mixtures: concentrated solutions or solvate ionic liquids? J Phys Chem B 2012;116:11323-31.
148. Phiri I, Bon CY, Kim S, et al. Effects of novel benzotriazole based zwitterionic salt as electrolyte additive for lithium ion batteries. Curr Appl Phys 2020;20:122-31.
149. Guan Z, Zhang Z, Du B, Peng Z. A non-flammable zwitterionic ionic liquid/ethylene carbonate mixed electrolyte for lithium-ion battery with enhanced safety. Materials 2021;14:4225.
150. Nguyen DQ, Loi Nguyen T, Loan Phung Le M, Phong Mai T, Sik Kim H. A zwitterionic salt with one sulfonate and two ether functional groups as an additive for lithium-ion battery electrolyte. Electrochem Commun 2022;137:107269.
151. Byrne N, Howlett PC, Macfarlane DR, Forsyth M. The zwitterion effect in ionic liquids: towards practical rechargeable lithium-metal batteries. Adv Mater 2005;17:2497-501.
152. Yoshizawa M, Narita A, Ohno H. Design of ionic liquids for electrochemical applications. Aust J Chem 2004;57:139-44.
153. Phiri I, Ko S, Kim S, et al. Zwitterionic osmolyte-inspired additives as scavengers and low temperature performance enhancers for lithium ion batteries. Mater Lett 2021;288:129366.
154. Byrne N, Howlett PC, Macfarlane DR, et al. Effect of zwitterion on the lithium solid electrolyte interphase in ionic liquid electrolytes. J Power Sources 2008;184:288-96.
155. Gómez E, Cojocaru P, Magagnin L, Valles E. Electrodeposition of Co, Sm and SmCo from a deep eutectic solvent. J Electroanal Chem 2011;658:18-24.
156. Malaquias JC, Steichen M, Thomassey M, Dale PJ. Electrodeposition of Cu-In alloys from a choline chloride based deep eutectic solvent for photovoltaic applications. Electrochim Acta 2013;103:15-22.
157. Abbott AP, Capper G, Mckenzie KJ, Ryder KS. Electrodeposition of zinc-tin alloys from deep eutectic solvents based on choline chloride. J Electroanal Chem 2007;599:288-94.
158. Xie Y, Dong H, Zhang S, Lu X, Ji X. Solubilities of CO2, CH4, H2, CO and N2 in choline chloride/urea. Green Energy Environ 2016;1:195-200.
159. Xu Q, Qin LY, Ji YN, et al. A deep eutectic solvent (DES) electrolyte-based vanadium-iron redox flow battery enabling higher specific capacity and improved thermal stability. Electrochim Acta 2019;293:426-31.
160. Lindberg D, de la Fuente Revenga M, Widersten M. Deep eutectic solvents (DESs) are viable cosolvents for enzyme-catalyzed epoxide hydrolysis. J Biotechnol 2010;147:169-71.
161. Loow YL, Wu TY, Yang GH, et al. Deep eutectic solvent and inorganic salt pretreatment of lignocellulosic biomass for improving xylose recovery. Bioresour Technol 2018;249:818-25.
162. Liao HG, Jiang YX, Zhou ZY, Chen SP, Sun SG. Shape-controlled synthesis of gold nanoparticles in deep eutectic solvents for studies of structure-functionality relationships in electrocatalysis. Angew Chem Int Ed 2008;47:9100-3.
163. Chirea M, Freitas A, Vasile BS, Ghitulica C, Pereira CM, Silva F. Gold nanowire networks: synthesis, characterization, and catalytic activity. Langmuir 2011;27:3906-13.
164. Dong JY, Lin CH, Hsu YJ, Lu SY, Wong DS. Single-crystalline mesoporous ZnO nanosheets prepared with a green antisolvent method exhibiting excellent photocatalytic efficiencies. CrystEngComm 2012;14:4732-7.
165. Huang Y, Shen F, La J, et al. Synthesis and characterization of CuCl nanoparticles in deep eutectic solvents. Part Sci Technol 2013;31:81-4.
166. Raghuwanshi VS, Ochmann M, Hoell A, Polzer F, Rademann K. Deep eutectic solvents for the self-assembly of gold nanoparticles: a SAXS, UV-Vis, and TEM investigation. Langmuir 2014;30:6038-46.
167. Mjalli FS, Abdel Jabbar NM. Acoustic investigation of choline chloride based ionic liquids analogs. Fluid Phase Equilibria 2014;381:71-6.
168. Mezzomo L, Pianta N, Ostroman I, et al. Deep eutectic solvent electrolytes based on trifluoroacetamide and LiPF6 for Li-metal batteries. J Power Sources 2023;561:232746.
169. Li W, Liu W, Huang B, et al. Suppressing growth of lithium dendrites by introducing deep eutectic solvents for stable lithium metal batteries. J Mater Chem A 2022;10:15449-59.
170. Hu Y, Li H, Huang X, Chen L. Novel room temperature molten salt electrolyte based on LiTFSI and acetamide for lithium batteries. Electrochem Commun 2004;6:28-32.
171. Liang Y, Wu W, Li D, et al. Highly stable lithium metal batteries by regulating the lithium nitrate chemistry with a modified eutectic electrolyte. Adv Energy Mater 2022;12:2202493.
172. Joos B, Volders J, da Cruz RR, et al. Polymeric backbone eutectogels as a new generation of hybrid solid-state electrolytes. Chem Mater 2020;32:3783-93.
173. Jaumaux P, Liu Q, Zhou D, et al. Deep-eutectic-solvent-based self-healing polymer electrolyte for safe and long-life lithium-metal batteries. Angew Chem Int Ed 2020;59:9134-42.
174. Li Z, Zhang S, Jiang Z, Cai D, Gu C, Tu J. Deep eutectic solvent-immobilized PVDF-HFP eutectogel as solid electrolyte for safe lithium metal battery. Mater Chem Phys 2021;267:124701.
175. Wang S, Chen Y, Fang Q, et al. Facilitating uniform lithium deposition via nanoconfinement of free amide molecules in solid electrolyte complexion for lithium metal batteries. Energy Stor Mater 2023;54:596-604.
176. Li Q, Zhang Z, Li Y, et al. Rapid self-healing gel electrolyte based on deep eutectic solvents for solid-state lithium batteries. ACS Appl Mater Inter 2022;14:49700-8.
177. Wu W, Li Q, Cao M, et al. Non-flammable dual-salt deep eutectic electrolyte for high-voltage lithium metal battery. Crystals 2022;12:1290.
178. Du Z, Wood DL, Belharouak I. Enabling fast charging of high energy density Li-ion cells with high lithium ion transport electrolytes. Electrochem Commun 2019;103:109-13.
179. Hu Z, Xian F, Guo Z, et al. Nonflammable nitrile deep eutectic electrolyte enables high-voltage lithium metal batteries. Chem Mater 2020;32:3405-13.
180. Hammond OS, Bowron DT, Edler KJ. The effect of water upon deep eutectic solvent nanostructure: an unusual transition from ionic mixture to aqueous solution. Angew Chem Int Ed 2017;56:9782-5.
181. Wang H, Song J, Zhang K, et al. A strongly complexed solid polymer electrolyte enables a stable solid state high-voltage lithium metal battery. Energy Environ Sci 2022;15:5149-58.
182. Zhang H, Zhou L, Du X, et al. Cyanoethyl cellulose-based eutectogel electrolyte enabling high-voltage-tolerant and ion-conductive solid-state lithium metal batteries. Carbon Energy 2022;4:1093-106.
183. Mazzapioda L, Tsurumaki A, Di Donato G, Adenusi H, Navarra MA, Passerini S. Quasi-solid-state electrolytes - strategy towards stabilising Li|inorganic solid electrolyte interfaces in solid-state Li metal batteries. Energy Mater 2023;3:300019.
184. Pervez SA, Kim G, Vinayan BP, et al. Overcoming the interfacial limitations imposed by the solid-solid interface in solid-state batteries using ionic liquid-based interlayers. Small 2020;16:e2000279.
185. Xiong S, Liu Y, Jankowski P, et al. Design of a multifunctional interlayer for NASCION-based solid-state Li metal batteries. Adv Funct Mater 2020;30:2001444.
186. Cao Y, Lou S, Sun Z, et al. Solvate ionic liquid boosting favorable interfaces kinetics to achieve the excellent performance of
187. Kim HW, Manikandan P, Lim YJ, Kim JH, Nam S, Kim Y. Hybrid solid electrolyte with the combination of Li7La3Zr2O12 ceramic and ionic liquid for high voltage pseudo-solid-state Li-ion batteries. J Mater Chem A 2016;4:17025-32.
188. Tsurumaki A, Rettaroli R, Mazzapioda L, Navarra MA. Inorganic-organic hybrid electrolytes based on Al-doped Li7La3Zr2O12 and ionic liquids. Appl Sci 2022;12:7318.
189. Abdelmaoula AE, Shu J, Cheng Y, et al. Core-shell MOF-in-MOF nanopore bifunctional host of electrolyte for high-performance solid-state lithium batteries. Small Methods 2021;5:e2100508.
190. Wu JF, Guo X. Nanostructured metal-organic framework (MOF)-derived solid electrolytes realizing fast lithium ion transportation kinetics in solid-state batteries. Small 2019;15:e1804413.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Palluzzi M, Tsurumaki A, Adenusi H, Navarra MA, Passerini S. Ionic liquids and their derivatives for lithium batteries: role, design strategy, and perspectives. Energy Mater 2023;3:300049. http://dx.doi.org/10.20517/energymater.2023.48
AMA Style
Palluzzi M, Tsurumaki A, Adenusi H, Navarra MA, Passerini S. Ionic liquids and their derivatives for lithium batteries: role, design strategy, and perspectives. Energy Materials. 2023; 3(6): 300049. http://dx.doi.org/10.20517/energymater.2023.48
Chicago/Turabian Style
Palluzzi, Matteo, Akiko Tsurumaki, Henry Adenusi, Maria Assunta Navarra, Stefano Passerini. 2023. "Ionic liquids and their derivatives for lithium batteries: role, design strategy, and perspectives" Energy Materials. 3, no.6: 300049. http://dx.doi.org/10.20517/energymater.2023.48
ACS Style
Palluzzi, M.; Tsurumaki A.; Adenusi H.; Navarra MA.; Passerini S. Ionic liquids and their derivatives for lithium batteries: role, design strategy, and perspectives. Energy Mater. 2023, 3, 300049. http://dx.doi.org/10.20517/energymater.2023.48
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 8 clicks
Cite This Article 8 clicks



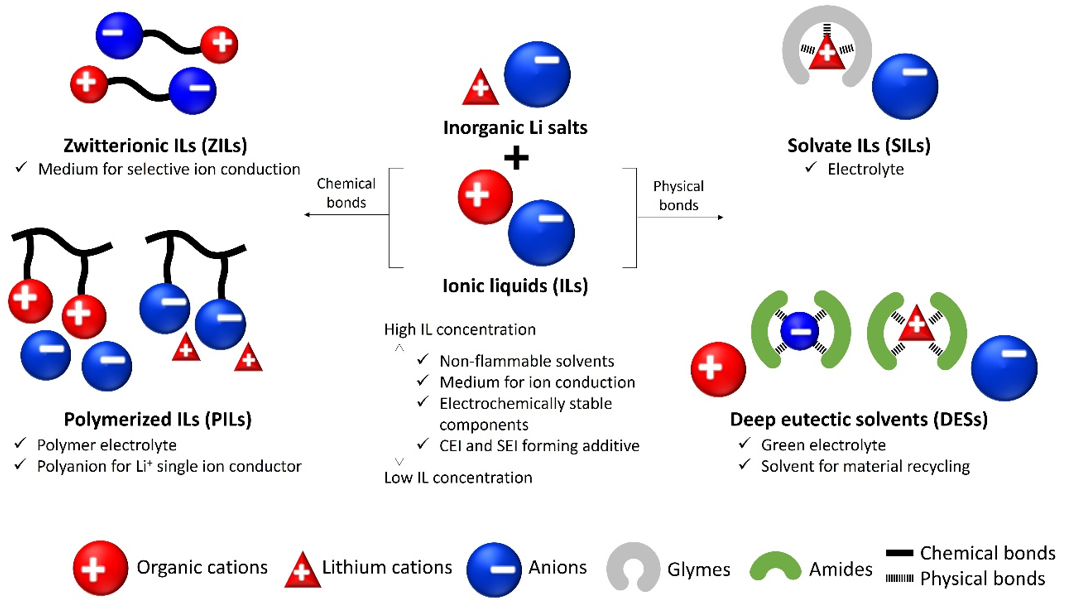


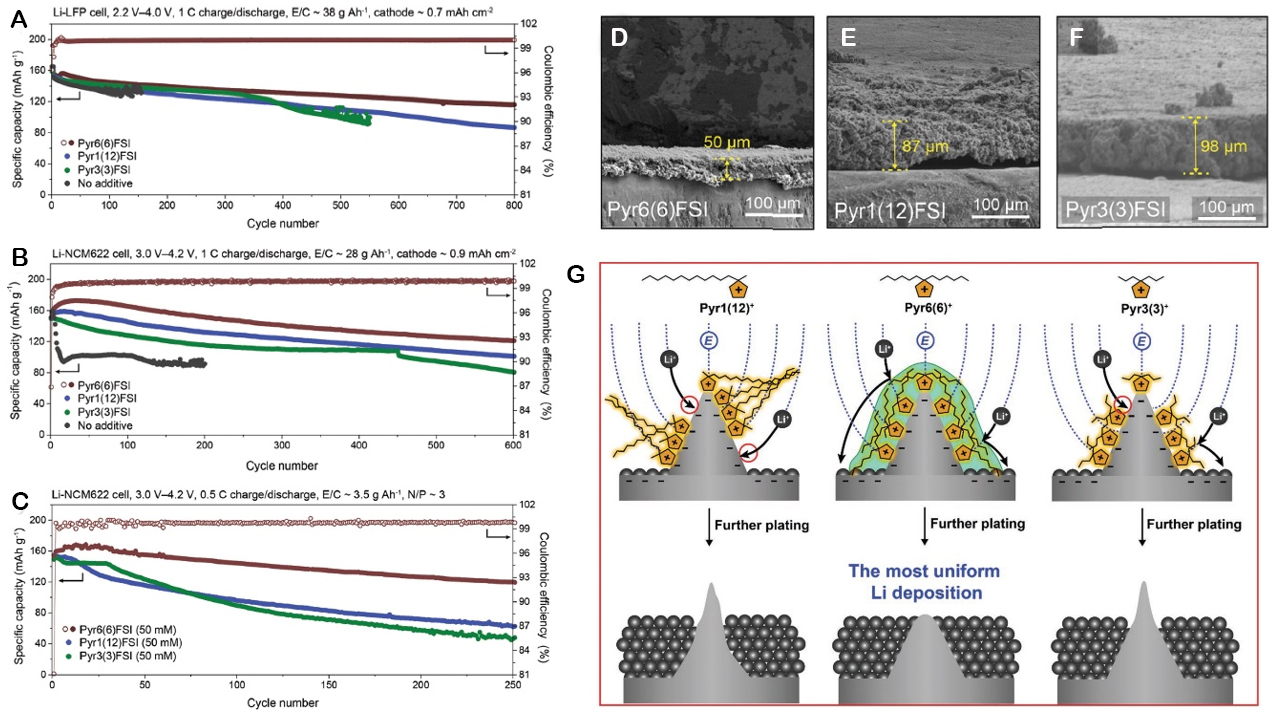
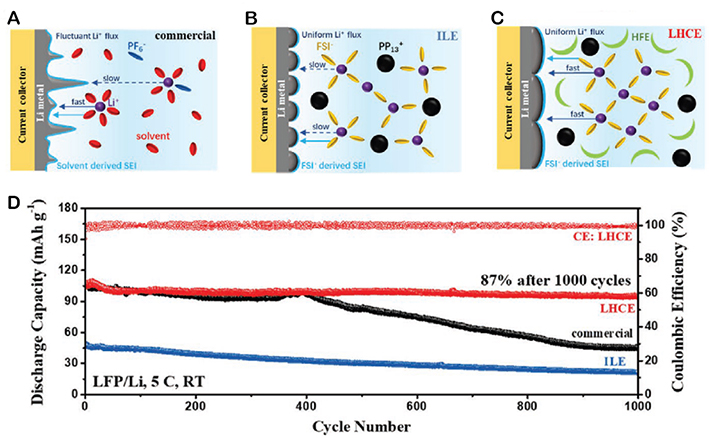

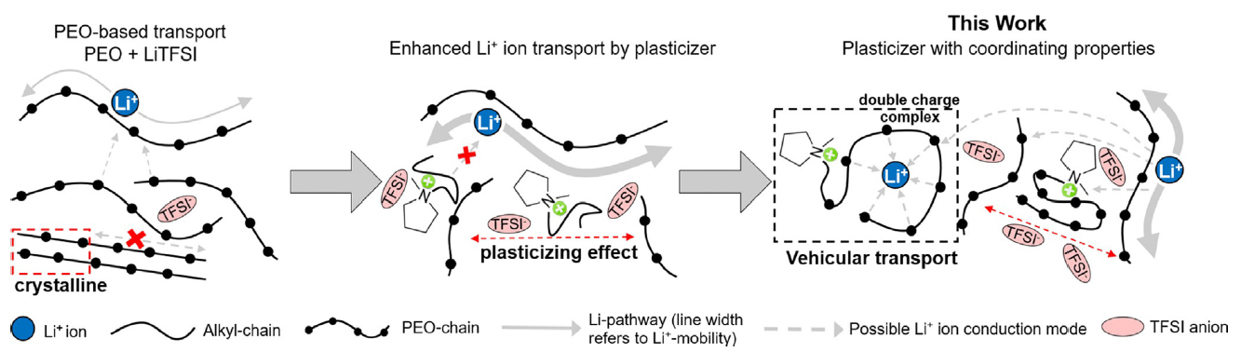
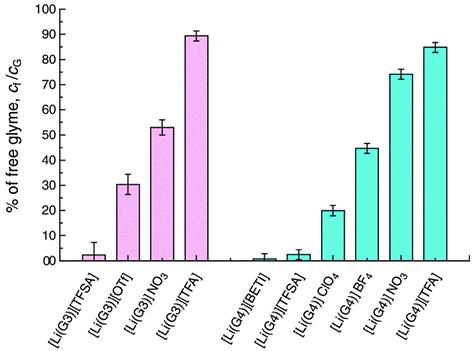
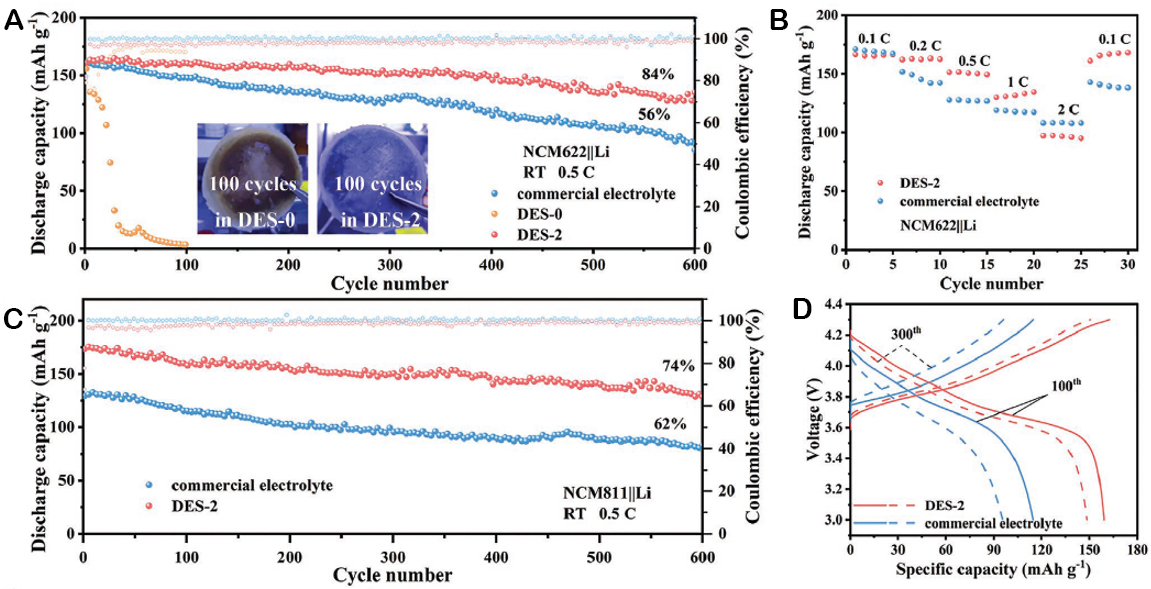
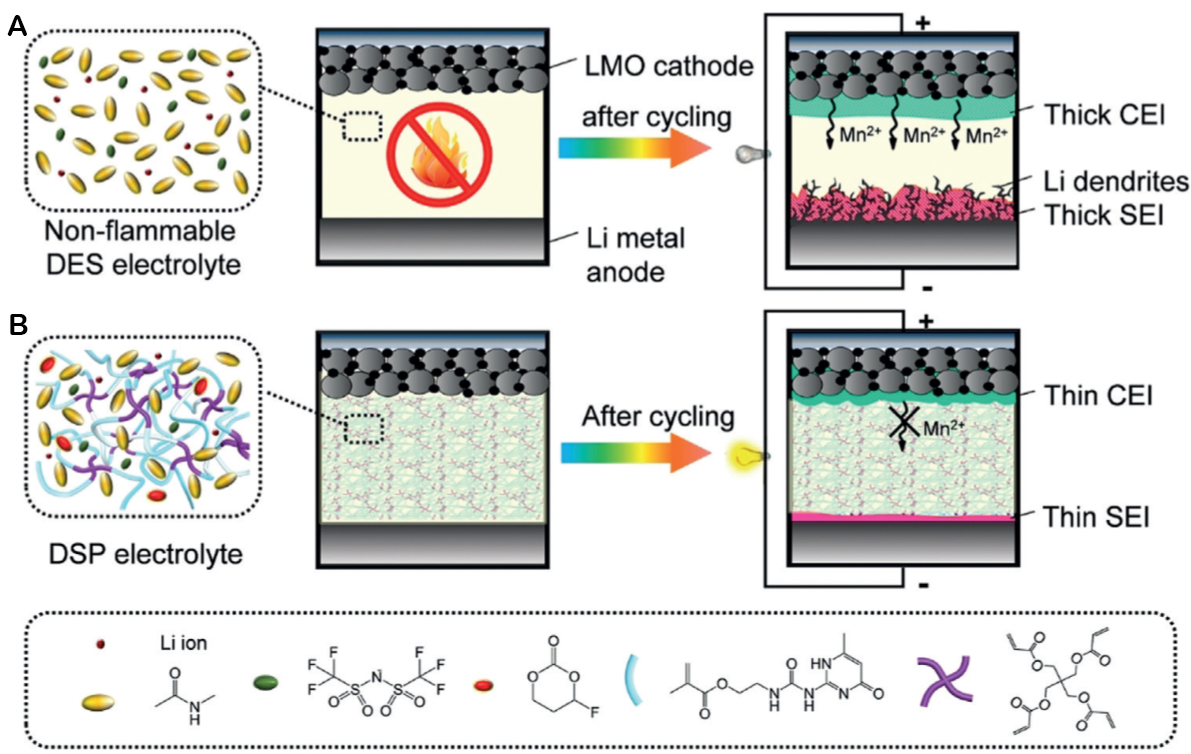
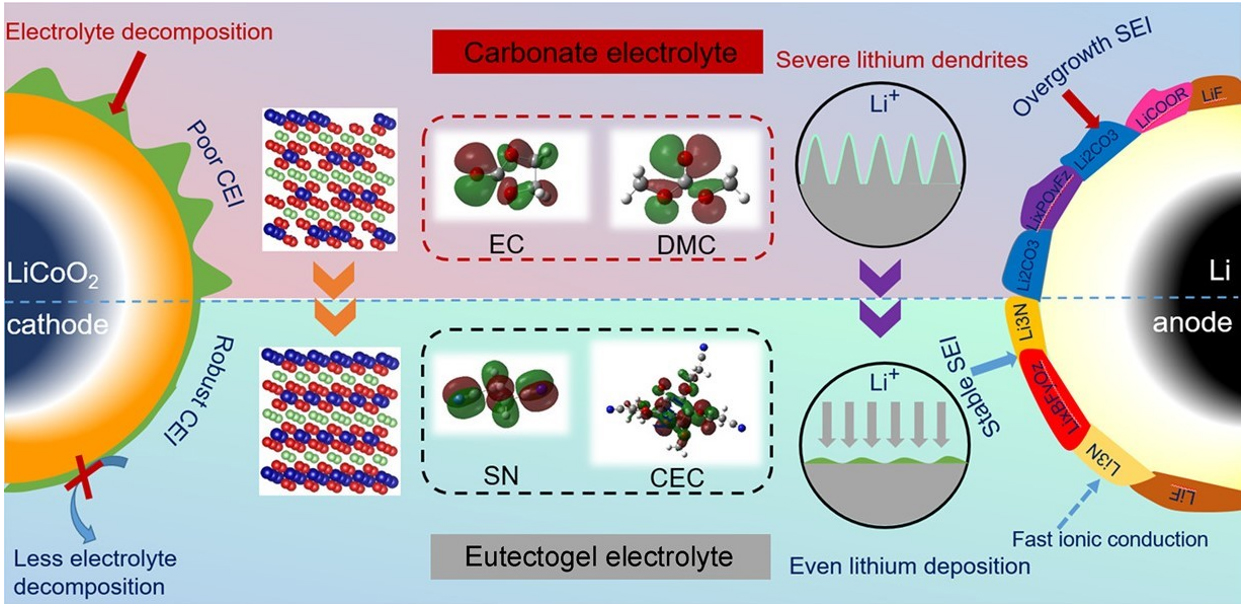











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.