Advancements in two-dimensional covalent organic framework nanosheets for electrocatalytic energy conversion: current and future prospects
Abstract
Humanity is confronting significant environmental issues due to rising energy demands and the unchecked use of fossil fuels. Thus, the strategic employment of sustainable and environmentally friendly energy sources is becoming increasingly vital. Additionally, addressing challenges, such as low reactivity, suboptimal energy efficiency, and restricted selectivity, requires the development of innovative catalysts. Two-dimensional (2D) covalent organic frameworks (COFs), known for their limitless structural versatility, are proving to be important materials in energy conversion applications. The exceptional properties of 2D COFs, including an organized arrangement resulting in well-defined active sites and π-π stacking interactions, enable breakthroughs in sustainable energy conversion applications. In this study, we comprehensively investigate universal synthesis methods and specific techniques, such as membrane-based deposition, liquid-phase intercalation, and polymerization. Furthermore, we demonstrate energy-conversion applications of 2D COFs as eco-friendly catalysts for electrochemical processes to promote sustainability and scalability by utilizing them in the hydrogen evolution reaction, oxygen evolution reaction, oxygen reduction reaction, and carbon dioxide reduction reaction. Additionally, we will explore methods for analyzing the physicochemical properties of precisely fabricated 2D COFs. Despite extensive research pertaining to 2D COFs, their practical industrial applications remain limited. Therefore, we propose various perspectives, including enhancing performance, improving synthesis methods, developing binder-free catalysts, expanding catalyst functionality, and advancing full-cell research, to achieve complete industrialization by leveraging their potential.
Keywords
INTRODUCTION
Human civilization has progressed rapidly and significantly, with no indicator of deceleration in the foreseeable future. To sustain and further propel this progress, the amount of energy supply must be increased[1]. However, the predominant reliance on fossil fuels, which currently constitute the largest proportion of energy sources, presents not only the challenge of limited energy resources but also detrimental consequences caused by significant carbon dioxide (CO2) emission during combustion, thus exacerbating global warming[2,3]. Consequently, sustainable and environmentally friendly alternative energy conversion methods are urgently required[4-6]. Considering the constraints posed by the limited conditions and low energy efficiency of renewable energy generation, the integration of catalysts has emerged as a promising solution to address these challenges and foster a sustainable energy future[7]. Significant advancements can be realized to enhance the efficiency and effectiveness of renewable energy conversion processes by leveraging catalytic properties, including those of transition metal dichalcogenides[8,9], transition metal chalcogenides[10], metal-organic frameworks (MOFs)[11], and covalent organic frameworks (COFs)[12,13]. These materials provide a substantial active surface area owing to their high surface-to-volume ratio, which facilitates efficient interactions and enhances the catalytic performance. Moreover, their tunable electronic properties enable catalyst optimization, thus allowing precise control over the catalytic activity, selectivity, and stability through composition and layer stacking strategies[14,15]. In particular, COFs have been investigated continuously since their initial introduction in 2005[16]. As porous materials composed of interconnected organic building blocks formed by covalent bonds, they possess a unique advantage: their exceptional ability for precise structural manipulation enables the creation of tailor-made designs customized to suit specific applications, a distinction that sets them apart from conjugated microporous polymers (CMPs)[17] and hierarchically porous carbons (HCPs)[18], primarily characterized by carbon and nitrogen-based porous structures. Numerous types of COFs with distinctive characteristics have been reported, including the bipyridine-based COF[19], which incorporates heteroatomic nitrogen; the triphenylene-based COF[20], which features a well-ordered and regular structural arrangement; the pyrazine-based COF[21], which is well known for its electrochemical stability; and the polydopamine-based COF[22], which is distinguished by its biocompatibility and surface adhesion properties. In addition, COFs offer precise control over composition, pore size, and functionality, along with design flexibility, to accommodate various applications[23].
Within the realm of COFs, the meticulously structured two-dimensional (2D) COFs stand out due to their well-defined active sites and π-π stacking interactions, which contribute to their heightened catalytic activity and enhanced selectivity[24,25]. Compared to their three-dimensional (3D) counterparts, 2D COFs offer superior capabilities in modulating molecular dimensions and crystallinity. This adaptability aids in fine-tuning intermolecular interactions, thus promoting specific reactions[26]. Additionally, the interactions between layers in 2D COFs enhance their electrochemical properties. This enhancement is achieved through activation processes, added functionality, and improved selective adsorption[27]. In this review, we aim to comprehensively elucidate the chemical methodologies employed in the synthesis of 2D COFs through both top-down and bottom-up approaches, along with the utilization of advanced characterization techniques [Scheme 1]. In the characterization section, we focus on two essential analytical techniques:
SYNTHESIS APPROACHES
The synthesis of 2D COFs can be classified into top-down and bottom-up methods[31]. Top-down methods entail the precision removal or manipulation of a target material from larger structures, enabling the attainment of the desired nano-scale configuration. In contrast, bottom-up methods encompass the assembly of molecules or atoms through chemical reactions and self-assembly processes to construct large-scale structures[32]. In this study, we aim to provide a comprehensive classification that encompasses both universal and specific synthesis approaches, such as deposition techniques, liquid-phase intercalation, and polymerization, along with their respective procedures.
A wide range of porous organic polymer networks have been classified [Scheme 2], such as crystalline COFs (CCOFs) and various amorphous networks, such as hypercrosslinked polymers (HCPs), covalent triazine frameworks (CTFs), porous aromatic frameworks (PAFs), and CMPs.
Scheme 2. Types of porous organic polymer frameworks and their coupling chemistries. Porous polymers from left to right: COFs. Reproduced with permission. Copyright 2021, Elsevier[33]. HCPs. Reproduced with permission. Copyright 2021, Elsevier[34]. CTFs. Reproduced with permission. Copyright 2013, American Chemical Society[35]. PAFs. Reproduced with permission. Copyright 2020, American Chemical Society[36]. Conjugated CMPs. Reproduced with permission. Copyright 2022, Elsevier[37].
Vapor-assisted deposition technique
Chemical vapor-assisted deposition (CVD) techniques are regarded as exceptional synthesis methodologies for controlling the structures and properties of 2D COFs. These techniques exhibit remarkable advantages, including the formation of precise and uniform layers, thus enabling large-scale fabrication and facilitating the creation of diverse material compositions contingent upon deposition or growth conditions. Additionally, they afford meticulous control over the chemical properties (such as the crystallinity, thickness, and other structural characteristics)[38,39]. Moreover, their inherent scalability renders them to be advantageous for industrialization and commercialization, thus enhancing their appeal for developing advanced materials[40]. As shown in Figure 1A, 4,4′,4″,4‴- (PyTTA-TPA), 4,4′,4″,4‴-(1,3,6,8-Tetrakis(4-aminophenyl)pyrene-[2,2′-Bipyridine]-5,5′-dicarboxaldehyde (PyTTA-BPyDCA), and 4,4′,4″,4‴-(1,3,6,8-Tetrakis(4-aminophenyl)pyrene-4,4′-Biphenyldicarboxaldehyde (PyTTA-BPDA) COF films were synthesized using ambient-pressure CVD. The PyTTA monomer was uniformly loaded onto a clean growth substrate through thermal evaporation, thus allowing precise control of the growth thickness[41]. The substrate with PyTTA was placed in a quartz tube inside a tube furnace, whereas TPA powders were placed in a ceramic boat heated to 80 °C. The tube furnace was connected to a bubbler containing an acetic acid solvent, and hydrogen and argon were used as carrier gases to transport H2O, acetic acid, and TPA vapor for COF synthesis on the PyTTA surface. Increasing the temperature from 80 to 150 °C resulted in faster reactions. Based on meticulous screening, a growth temperature of 140 °C was selected to generate large-area COF films on substrates, which is cost-effective for large-scale COF film production. The 2D COF film fabricated using the vapor-induced conversion method exhibited remarkable efficacy as an electrochemical catalyst for the HER. Additionally, Chaki Roy et al. successfully fabricated 2D COFs via CVD, where vapor-induced conversion with PyTTA was used as a pristine precursor[42]. The suggested incorporation of COF366-QD thin films onto a silicon substrate through in-situ low-temperature CVD is promising for promoting progress in the transfer-free and economically viable manufacturing of extensive organic electronics and direct device applications. The structure of the fabricated COF366-QD thin film is shown in Figure 1B. In conclusion, CVD is widely acknowledged as a prominent technique in the production of diverse 2D COFs, primarily because of its inherent benefits, such as high deposition velocity, precise layer control, and the ability to enable large-scale manufacturing.
Figure 1. (A) Schematic representation of growth of imine-linked 2D COF films on SiO2/Si substrates. Reproduced with permission. Copyright 2022, Springer Nature[41]. (B) Schematic illustration of in-situ low-temperature CVD equipment setup and final structural model of COF366-QD thin films. Reproduced with permission. Copyright 2023, Royal Society of Chemistry[42].
Liquid-assisted synthesis method
In this section, we discuss solvent absorption[43], insertion[44], infiltration[45], encapsulation[46], and solvothermal processes[47], with specific focus on their application in the liquid-phase synthesis of 2D COFs. Solvent absorption involves the permeation of solvent molecules into the internal structure of a material, which effectively fills the internal voids[48]. This approach enables the alteration of material properties or imparts new characteristics by leveraging solid-liquid interactions. Kang et al. investigated four distinct 2D COFs: 1,3,5-tris(4-aminophenyl)benzene-terephthalaldehyde (TAPB-TA); 1,3,5-tris(4-aminophenyl)benzene- 2,5-dimethoxyterephthalaldehyde (TAPB-OMeTA); 4,4′,4″,4‴-(1,9-dihydropyrene-1,3,6,8-tetrayl)tetraaniline-terephthalaldehyde (Py-1P), and 2,4,6-tris(4-aminophenyl)-1,3,5-triazine-benzene-1,3,5-tricarbaldehyde (TAPT-BTCA), using the solvation method[49]. Their investigation revealed a significant interlayer-shifting phenomenon in these COFs when exposed to solvents, which resulted in significant changes to their stacking structures. This remarkable flexibility demonstrated by the TAPB-TA, TAPB-OMeTA, Py-1P, and TAPT-BTCA COFs facilitates the accurate determination of the structure of solvated COFs, which requires advanced analysis techniques such as powder XRD (PXRD), simulation modeling, and Pawley refinement. The synthesis of the 2D COFs is illustrated in Figure 2A. Solvothermal synthesis is the predominant method utilized for the fabrication of 2D COFs, and the solvent employed in this process assumes several pivotal roles[50]. (1) The solvent enhances the solubility of the reactants, thereby facilitating improved mixing and interactions between them; (2) The solvent can control the temperature and pressure, thereby facilitating the establishment of targeted reaction conditions and the precise regulation of reaction kinetics; and (3) The solvent contributes significantly to the growth and crystallization of the resulting material, thereby affecting the desired physical and chemical properties. Hence, the solvent used in solvothermal synthesis determines the morphology and characteristics of the desired material. Shevate et al. proposed a modified Langmuir method and incorporated solvothermal synthesis to fabricate large-area 2D COF membranes with adjustable nanopore sizes [Figure 2B][51]. In addition, Ji et al. reported that weakly CCOFs undergo temperature-dependent depolymerization during synthesis, thus further highlighting the potential for improving material quality through repeated depolymerization and repolymerization cycles[52]. Additionally, researchers have investigated solvothermal depolymerization and recrystallization methods to control COF formation and enhance material quality. These processes are similar to molecular recrystallization and provide valuable insights into the formation of imine-linked 2D COFs, thus presenting a novel approach for improving the crystallinity of COFs.
Figure 2. (A) Schematic illustration of 2D COF synthesis processes based on solvation method. Reproduced with permission. Copyright 2020, American Chemical Society[49]. (B) Synthesis process of large-scale nanoporous 2D COF membranes. Reproduced with permission. Copyright 2022, American Chemical Society[51].
Polymerization
Polymerization is a process that involves covalently linking fundamental building blocks to generate extensive molecular chains[54]. As the reaction progresses, bonding occurs and is affected by the surrounding environment, thus resulting in intricate 2D COF architectures. Precise optimization can be achieved by judiciously regulating factors such as the reaction rate, bonding robustness, and structure formation. Polymerization occurs intrinsically during the previously mentioned synthesis methods, where chemical bond formation is catalyzed, thus expediting the overall reaction[55]. This process is particularly important in the COF synthesis pathway and warrants further investigation. Yang et al. successfully fabricated 2D COF membranes with outstanding solvent resistance via interfacial polymerization. They overcame the challenges encountered in previous methodologies to enhance the chemical resistance of polymeric substrates by adopting an innovative approach[56]. As depicted in Figure 3A, the detailed synthesis method involves the carbonization of commercial polyacrylonitrile (PAN) ultrafiltration membranes under an inert atmosphere while incorporating calcium nitrate as a pore-forming agent. This process yielded carbonized PAN substrates featuring crosslinked structures while maintaining finger-like pores with sizes ranging from 100 to 500 nm. Furthermore, these substrates demonstrated exceptional scalability and foldability without adverse effects. Subsequently, in-situ interfacial polymerization was adopted to synthesize interconnected 2D COF membranes on carbonized PAN substrates, where an aldehyde monomer of 1,3,5-triformylphloroglucinol (Tp) and the following four distinct amine monomers were used: hydrazine hydrate (HZ), 1,3,5-tris(4-aminophenyl)benzene (TAPB), p-phenylenediamine (PDA), and 3,3-dihydroxybenzidine (DHBD). Consequently, they successfully created distinct COF membranes designated as Tp-HZ, Tp-TAPB, Tp-PDA, and Tp-DHBD. Zhan et al. conducted a comprehensive examination of polymerization and crystallization to fabricate well-defined crystalline 2D polymers at the solid-liquid interface under ambient conditions[57]. By utilizing state-of-the-art in-situ scanning tunneling microscopy, they obtained valuable insights into the intricate processes. The solid-liquid interface provides a significant advantage by effectively decoupling 2D polymerization from the stacking/destacking processes, which is crucial in the formation of 2D COFs. The self-condensation of pyrene-2,7-diboronic acid (PDBA) was investigated to synthesize various 2D COF structures through dynamic covalent chemistry. Interfacial polymerization for 2D COF synthesis encompasses a range of synthesis methods beyond the solid-liquid approach previously discussed. The choice of method varies, depending on specific applications and requirements. These methods include: (1) Solid-vapor interface: This approach offers the advantage of simply increasing the temperature to accelerate the reaction rate, resulting in higher-quality 2D COF membranes and decreased membrane thickness[58]; (2) Liquid-air interface: This approach enables the fabrication of large-area COF membranes and demonstrates excellent performance in electrochemical reactions due to its high availability[59]; and (3) Liquid-liquid interface: This approach demonstrates remarkable versatility, enabling a diverse spectrum of polymerization reactions by amalgamating two distinct liquid-phase reactants[60].
Figure 3. (A) Schematic diagram illustrating carbonization processes of PAN substrates and the subsequent interfacial polymerization with various monomers to fabricate 2D COF membranes. Reproduced with permission. Copyright 2023, Springer Nature[56]. (B) Schematic diagram illustrating two-step polymerization processes for fabrication of high-quality 2D COFs. Reproduced with permission. Copyright 2018, The American Association for the Advancement of Science[61].
Other synthesis methods
In the realm of 2D COF synthesis, “exfoliation” typically refers to delicately separating pre-formed thick 2D COF layers into thinner, individual layers[62]. This is essential for reducing the thickness of 2D COFs and obtaining layered structures that exhibit exceptional properties tailored for specific applications[63]. Furthermore, exfoliation can extend its scope beyond simply thinning thick layers and encompass the process of forming more complex material structures[64]. To apply exfoliation to 2D COFs, one must disrupt the interlayer interactions. However, exfoliation is not universally applicable to all 2D COFs because its success depends on the structure and characteristics of the synthesized material. The presynthesized microcrystalline COF-43 powder, which was prepared via solvothermal synthesis, was purified thoroughly to ensure the complete removal of residual substances [Figure 4A][65]. Meanwhile, the results of atomic force microscopy (AFM) analysis demonstrated that COF-43 subjected to sonication with dioxane exhibited significantly reduced average heights (1.32 nm) as compared with COF-43 treated with THF [Figure 4B]. This finding strongly implies the formation of a single-layer structure in the dioxane-sonicated COF-43. In addition, the incorporation of an in-situ linker exchange by Zhang et al. is a novel strategy for customizing the properties of 2D COFs[66]. Through the deliberate substitution of molecular linkers within existing frameworks, 2D COFs with precise attributes suitable for diverse applications can be customized and synthesized. As shown in Figure 4C, heterostructured 2D COF membranes were successfully engineered via an in-situ linker exchange approach, wherein a fraction of ACOF-1 was substituted with COF-LZU1. Figure 4D shows a schematic illustration of the stepwise structural transformation of the 2D COF membrane.
Figure 4. (A) Fabrication process of 2D COF membranes using microcrystalline powder of COF-43 via exfoliation methods. (B) AFM images of 2D COF-43. Left: COF-43 treated with THF; right: COF-43 treated with dioxane. Reproduced with permission. Copyright 2013, American Chemical Society[65]. (C) Schematic diagram illustrating fabrication of heterostructure 2D COFs via in-situ growth and linker exchange methods. (D) Structural transformation of 2D COF membrane as a function of synthesis time. Reproduced with permission. Copyright 2022, Royal Society of Chemistry[66].
CHARACTERIZATIONS
XRD analysis
XRD is a highly valuable analytical technique that is widely employed for elucidating material structures and properties; furthermore, it is used extensively to analyze the crystallinity of 2D COFs[28,29,67]. Because of the ordered crystalline structures of 2D COFs, XRD allows one to investigate their molecular alignments and interactions and precisely determine their intermolecular spacing. Moreover, the evaluation of the crystal quality, which directly affects the functional properties of 2D COFs, enables the optimization of manufacturing processes[68]. By monitoring the changes in 2D COF crystal structures under diverse conditions, valuable insights into the stability and physical characteristics, along with the analysis of defects, can be gained. Therefore, XRD provides essential information regarding the structure, crystal quality, lattice constants, structural transformations, and functional properties of 2D COFs, thereby contributing significantly to the development and utilization of these materials[69]. Mahmood et al. fabricated a 2D COF with a fully conjugated fused aromatic structure featuring aromatic amine (-NH2) and hydroxyl (-OH) functionalities within the pores. In particular, a potent polycondensation process (aromatization) involving pentaaminophenol and hexaketocyclohexane (HKH) in trifluoromethanesulfonic acid was performed[70]. Figure 5A illustrates the confirmed crystalline structure of the synthesized F-COF, which was obtained both experimentally and numerically. The results show that F-COF exhibits a hexagonal arrangement with an AA-stacking model and a layer-to-layer distance of 3.30 Å, which does not align with the ab-stacking model [Figure 5B and C]. Li et al. introduced a novel method to prepare imine-linked 2D COF solid solutions by adjusting the ratio of two linear monomers, i.e., PDA and 4,4′-biphenyldicarbaldehyde (BDA)[71]. This innovative approach allows precise control over the monomer feed ratios, thus resulting in enhanced complexity in the composition and structure of 2D COFs. The XRD analysis presented in Figure 5D-F reveals that the (100) diffraction peak remained stable, with a slight shift depending on the BDA monomer ratio (after different synthesis times: 15, 30 min, and 1 h). This shift provides compelling evidence of the successful integration of BDA into the PDA lattice. Cao et al. investigated the intriguing phenomenon of membrane polarity switches by employing a sophisticated multivariate approach (COF-V) to adjust the solution pH[72]. This innovative method involves integrating vinyl groups into COFs and facilitating subsequent post-synthesis functionalization through thiol-ene click chemistry while preserving the crystalline structure of the COFs. The initial COF-V membranes were skillfully synthesized on a PAN support via an interfacial condensation reaction, with particular emphasis on the COF-V-60% membrane, which presented significant characterization outcomes. Figure 5G and H show comparisons of the XRD patterns and grazing incidence wide-angle X-ray scattering (GIWAXS) to validate the crystal structures of the COF-V and cysteine-functionalized COF (COF-Cys) membranes at different concentrations.
Figure 5. (A) PXRD patterns of F-COF, including Pawley-refined patterns. Atomic model of F-COF with (B) AA stacking structures and (C) AB stacking structures. Reproduced with permission. Copyright 2020, Springer Nature[70]. PXRD patterns of 2D COFs synthesized at various BDA monomer ratios. Synthesis time (D): 15 min (E): 30 min (F): 60 min. Reproduced with permission. Copyright 2021, American Chemical Society[71]. (G) XRD patterns of COF-V-x% (30%, 60%, 80%). Inset: GIWAX patterns (H): XRD patterns of COF-Cys-x% (30%, 60%, 80%). Inset: GIWAX patterns. Reproduced with permission. Copyright 2022, Springer Nature[72].
BET measurement
The large specific surface areas and porosities of 2D COFs are their major advantages, and BET measurements are essential for evaluating these characteristics[73]. To elaborate further, a substantial specific surface area and fine-tuned pore size can lead to an increase in electrocatalytic activity by augmenting the number of active sites, facilitating improved mass transport, and enhancing charge transfer processes[74,75]. Chen et al. successfully synthesized stable porphyrin-based 2D COFs containing H2, Co, Ni, and Zn by preparing porphyrinic aldehyde p-MPor-CHO[76]. Subsequently, they performed a condensation reaction using 2,5-diethoxyterephthalohydrazide (DETH) to obtain MPor-DETH-COFs, as depicted in Figure 6A-D. Figure 6E-H shows that the MPor-DETH-COFs incorporating H2, Co, Ni, and Zn feature large surface areas, with values of 820, 940, 770, and 1,020 m²g-1, respectively (with a pore size of approximately 2.4 nm, as shown in the insets of Figure 6E-H). Evans et al. prepared boronate-ester-linked 2D COF colloidal inks and performed spray coating to fabricate high-quality 2D COF thin films on substrates, including polymers, metals, and oxides[77]. As shown in Figure 6I and J, the synthesized 2D COFs exhibited large surface areas (reaching 1,900 m²g-1) and a pore size of 50 nm. This outcome can be attributed to the capability of spray coating to achieve a uniform dispersion of COFs and enable deposition into a thinner layer, thereby facilitating the creation of an enhanced surface structure and micro-porosity. In conclusion, 2D COFs, characterized by their extensive specific surface area as measured by BET, enhance interactions among catalytic particles and sustain stability during prolonged reactions. Moreover, these COFs are notably beneficial for electrochemical transformations due to their ability to enhance interactions among various chemical species.
Figure 6. Atomic structure modeling of MPor-DETH-CO (M: H2, Co, Ni, Zn) (A): H2 (B): Co (C): Ni (D): Zn BET surface area measurement of MPor-DETH-CO (E): H2 (F): Co (G): Ni (H): Zn Reproduced with permission. Copyright 2021, Springer Nature[76]. (I) BET measurement and (J) pore size distribution of COF-5 films. Reproduced with permission. Copyright 2020, John Wiley and Sons[77].
APPLICATIONS
Catalysts can be classified into several categories: electrocatalysts, photocatalysts[24,25,78,79], thermal catalysts, and photochemical catalysts[80,81]. Among them, electrocatalysts have received significant attention because of their ability to function in various environments. Electrocatalysis involves the use of a catalyst to accelerate electrochemical reactions and is critical to numerous energy conversion and storage applications, including water splitting[82-84], CO2 reduction[85,86], fuel cells, and batteries[87,88]. Conventional electrocatalysts typically involve the use of expensive and rare metals, such as platinum and palladium, which limits their widespread adoption. By contrast, 2D COFs offer immense potential for advanced energy conversion and storage applications[89]. 2D COFs possess unique properties and are cost-effective, thus rendering them ideal candidates for overcoming the limitations of conventional catalysts and promoting the development of more sustainable and efficient alternatives[90]. One of the appealing aspects of 2D COFs for electrocatalysis is their modular and customizable features, which allow for the precise control of their structure, pore size, and surface chemistry[91,92]. This enables the customized design and optimization of the catalyst properties. Moreover, the well-defined and ordered pores within 2D COFs enhance mass transport, facilitating the diffusion of reactants and, hence, improving catalytic performance.
In electrocatalysis, 2D COFs can serve as efficient catalysts or support materials for other catalytic species[93]. For example, 2D COFs can be functionalized with specific catalytic sites, such as metal or metal oxide nanoparticles, to enhance their electrocatalytic activity and selectivity[94]. The unique porous structure of 2D COFs provides a stable and confined environment for these catalytic sites, which prevents aggregation and, thus, improves the overall performance[95]. Additionally, 2D COFs can serve as electrochemically active materials that directly participate in electrochemical reactions. Certain COFs can serve as excellent catalysts for important ORR and OER in fuel cells or metal-air batteries. Unlike conventional catalysts, these COFs exhibit high activity, durability, and improved resistance to contaminants[96,97]. Several researchers are actively investigating different synthesis strategies for the design and fabrication of 2D COFs with enhanced electrocatalytic properties[98,99]. These efforts include the development of new 2D COF structures, investigations into different catalytic sites, and the optimization of the electronic and chemical properties of the frameworks to achieve different products and specific reaction requirements. Among them, the HER[100], OER, ORR[101], and CO2RR[102], which are the most investigated aspects in the field of electrocatalysis, are actively being investigated. Additionally, research cases and strategies for each type of 2D COF electrocatalyst have been introduced. Scheme 3 illustrates a representative ligand building block that forms a 2D COF.
Scheme 3. Typical building block types comprising 2D COFs. Reproduced with permission. Copyright 2018, John Wiley and Sons[103].
HER
Recently, hydrogen has received considerable attention as an alternative energy source to conventional fossil fuels[104]. Research pertaining to hydrogen storage and production using various methods is currently being conducted. In particular, a method for generating hydrogen by decomposing water via an electrochemical method is receiving attention because it requires a precursor other than water; this is referred to as the HER[105]. Rare or transition metals (Pt, Pd, Ru, Co, etc.) or ceramics (NiO, Co2O3, transition metal dichalcogenide, etc.) are the most typically investigated electrocatalytic materials for water splitting[106]. However, these materials are not chemically stable under basic or acidic conditions and are expensive. By contrast, 2D COFs via irreversible reactions are suitable as HER catalysts because they are inexpensive and chemically stable. Metal-free catalysts can be manufactured using only 2D COFs. However, the simultaneous use of 2D COFs and metals to improve electrical conductivity has been reported as well.
Metal-free 2D COF for HER
First, a metal-free 2D COF was introduced [Figure 7A]. Bhunia et al. prepared a metal-free 2D COF using two monomers[107]. In particular, 1,3,6,8-tetrakis(4-formylphenyl) pyrene (0.1 mmol, 61 mg) and 5,10,15,20-Tetrakis(4-aminophenyl) porphyrin (0.1 mmol, 67 mg) were added to dimethylacetamide/o-dichlorobenzene(o-DCB) (3.0/1.0 mL) and sonicated. Subsequently, SB-PORPy-COF was fabricated via heat treatment at 120 °C for 7 to 8 h in a vacuum atmosphere. In other words, SB-PORPy-COF was composed of imine-based pyrene and porphyrin. This was verified through PXRD, which confirmed that the stability in acid and base conditions is very high. Also, it is confirmed that the pore size was uniform owing to N2 adsorption. The π electronic conjugation was maximized while pyrene and porphyrin formed AA stacking (eclipsed). This conducting channel participates in the HER and enables the synthesis of metal-free catalysts. The Tafel slope of SB-PORPy-COF was 116 mV dec-1, and the onset potential at 5 mA cm-2 was 380 mV. Zhao et al. fabricated 2D-CTFs using 1,4-phenylenonitrile (DCB 2 mmol, 0.256 g) and CF3SO3H (1 mmol, 0.088 mL)[108]. After cooling in liquid nitrogen, heat treatment in vacuum, and finally immersion in liquid nitrogen, an AB-stacked (staggered) CTF (CTF-1-AB) was obtained. If DCTFB is removed and processed, then F-CTF-1-AB, to which fluorine has been added, can be generated. If additional heat treatment is performed, then the AA stacking (eclipsed) of CTF-1-AA and F-CT-1-AA can be achieved. AA and AB stacking was confirmed through XRD analysis; furthermore, the analysis confirmed that the structure was maintained even after treatment with fluorine. Comparing the HER performances of the four CTFs, the overpotential of F-CT-1-AA was 200 mV [Figure 7B], which reflected the best catalytic efficiency (Tefal slope: 97 mV dec-1). This is because a unique-conjugated one-dimensional channel was formed during AA stacking, and the hydrogen evolution efficiency increased because the band gap was reduced by fluorine, and the number of carriers increased. Ruidas et al. synthesized an imine-based 2D C6-TRZ-TFP-COF using a C3-symmetric triamine and trialdehyde monomer[109]. The 2D C6-TRZ-TFP-COF exhibited an outstanding HER in the absence of metal atoms. Because various hydrogen adsorption sites were considered in various aspects based on DFT calculations and the results of previous studies, a unique result was obtained. Notably, a high density of electrons among π bonds is advantageous to the HER. However, when comparing the sites of carbon bonded to oxygen and carbon bonded to nitrogen, the sites bonded to nitrogen (electron-deficient) indicated a lower adsorption barrier [Figure 7C and D]. This is because the adsorption of hydrogen onto an already electron-deficient surface can compensate for stronger bonding. Therefore, based on this mechanism, we hypothesize that hydrogen production is realizable using only inexpensive 2D COFs without metal atoms. Halder et al. synthesized a triazine-containing polyimide-based COF (TP-COF) using pyromellitic dianhydride (PMDA), melamine, and dimethylformamide (DMF) as precursors[110]. The synthesis involved a relatively simple one-pot condensation method. The surface area and pore size of TP-COF synthesized in this manner were 312.6 m2 g-1 and 1.8 nm, respectively. The surface area of TP-COF-C700, which was heat-treated in N2 atmosphere for HER applications, increased to
Figure 7. (A) Schematic illustration of 2D COF HER catalyst. Copyright 2017, American Chemical Society[107]. (B) Electrochemical measurement of LSV of CTFs in 0.5 mol L-1 H2SO4. Copyright 2023, Elsevier[108]. (C) Free energy plots of HER on unique sites. (D) Model structure showing selected sites for H-atom adsorption. Gray, blue, red, and white spheres represent carbon, nitrogen, oxygen, and hydrogen, respectively. Copyright 2023, John Wiley and Sons[109].
Metal-decorated 2D COF for HER
Next, a catalyst prepared by combining a metal with a 2D COF is introduced. Zhao et al. manufactured a hybrid of Ru particles, reduced graphene oxide (rGO), and a 2D COF to increase the electrical conductivity and improve the HER [Figure 8A][111]. A 2D COF was synthesized by mixing piperazine, cyanuric chloride, and potassium carbonate in 1,4-dioxane, followed by performing reflux at 110 °C for 2D. Ru and rGO were synthesized using the 2D COF and refluxing. During the complexation of Ru, Ru ions were formed in the COF nanosheet in particle form, and uniformly distributed Ru particles were achieved without separate dispersion. The formed Ru particles measured 2-2.4 nm, and the results of TEM analysis confirmed that they were uniformly distributed in the nanosheet. The COF/rGO-Ru indicated an overpotential of 42 mV and a low Tefal slope of 46 mV dec-1, which were significantly better than those of commercially available Pt/C [Figure 8B]. However, they were lower compared with those of other Ru-based catalysts. Maiti et al. improved the HER by encapsulating Ru in a 2D COF, which was synthesized using 3,4-diaminobenzohydrazide and benzene-1,3,5,-tricarboxaldehyde[112]. The Ru encapsulation was dried after stirring the synthesized 2D COF in a RuCl3 solution. Even after Ru encapsulation, the result of XRD analysis confirmed that no structural change occurred in the 2D COF. In addition, the result of energy dispersive
Figure 8. (A) Preparation procedure of COF/rGO-Ru, (B) LSV curves of COF, COF/rGO, COF-Ru, COF/rGO-Ru, and commercial Pt/C. Copyright 2019, American Chemical Society[111]. (C) LSV plot of Ru@COF, bare RuCl3, and COF. (D) Tafel plot of Ru@COF, COF, RuCl3, and bare GC. (E) Nyquist plot of impedance spectra of bare GC, COF, and Ru@COF at onset overpotential of 159 mV. Inset: impedance spectra in a lower frequency range. Copyright 2020, John Wiley and Sons[112]. TEM images of (F) Cryst-2D-PMPI. Copyright 2023 ELSEVIER. Copyright 2021, ELSEVIER[113]. (G) Longtime stability of Mo2C-MoNi4@NC-2 at current densities of 10, 20, 50, 100, 50, 20, and 10 mA cm-2 (each step was set as 20,000 s). Copyright 2023, Elsevier[114].
OER/ORR
Oxygen-related catalysts are a class of materials that contribute significantly to various chemical reactions involving oxygen, such as the ORR[115,116], OER[117-120], and oxygen-based fuel production[121]. These catalysts are designed to facilitate the conversion of oxygen molecules into different chemical species, thus contributing to applications such as fuel cells, metal-air batteries, water splitting, and CO2 reduction[122]. Oxygen-based catalysts can be based on transition metals, metal oxides, perovskites, or organic materials, and their development aims to improve the reaction kinetics, enhance catalytic activity, and achieve high selectivity and durability, thus ultimately advancing the field of energy conversion and storage[123,124]. Additionally, 2D COFs exhibit unique properties, including large surface areas, ultrahigh porosity, tunable pore sizes, relatively high chemical/thermal stability, and a tailorable architecture. These properties make 2D COFs an attractive material for electrocatalytic reactions such as ORR in fuel cells and OER in electrolytic water splitting and metal-air batteries.
OER
Among the methods for synthesizing free-standing COFs, polymerization occurs at the interface of two solutions[125]. However, implementing this method over a large area is challenging because the diffusion of monomers at the interface is relatively slow. Hence, Tang et al. synthesized a 32-nm-thick free-standing Co-porphyrin-based film (CoP-TOB) via polymerization at the interface between air and DMSO
Figure 9. (A) Synthesis of COF films at liquid–liquid and liquid–air interfaces. (B) Photograph of CoP-TOB film floating on water. (C) OER LSV and Tafel slope of CoP-TOB. Copyright 2023, John Wiley and Sons[59]. (D) Thickness and lateral size distribution of exfoliated Cage-COF-1-Ns (horizontal dotted black line indicates the theoretical thickness of a single-layered Cage-COF-1 nanosheet). (E) Tafel slopes of Cage-COF-1-Ns samples in OER. (F) OER polarization curves of Cage-COF-1-Ns/Co before and after 2000 potential cycles. Copyright 2022, John Wiley and Sons[126]. (G) Polarization curves of CoCu-ZIF, GDY, and CoCu-ZIF@GDY in two-electrode electrolyzers based on scan rate of 10 mV s-1; (H) Comparison with reported catalysts under overpotential of 10 mA cm-2 in two-electrode electrolyzers; (I) Long-term durability test of CoCu-ZIF@GDY electrolyzers at 10 mA cm-2 and photograph showing water splitting (the inset). Copyright 2020, Elsevier[128].
ORR
Fabricating a metal-free catalyst via pyrolysis is inexpensive and useful; however, this technique does not allow the atomic position or structure of COFs to be controlled easily. Martínez-Fernández et al. fabricated an ORR catalyst by preparing a metal-free, non-pyrolyzed material[130]. Using this fabrication method, the structure of the link center of XDI0.19-CPF (X = N, Bz, and P), which is a diimide-based material, was formed by classifying it into three types, and the differences were confirmed through rigorous analysis [Figure 10A]. This fabrication method is referred to as the click post-synthesis methodology. The bulk material prepared via this method was used to evaluate the performance of the electrochemical catalyst. Among the three types of yielded COFs, BzDI0.17-COF was the most suitable material because of the formation of a four-electron pathway under an applied voltage of 0.77 V. The Tafel slope of BzDI0.17-COF was -79.9 mV dec-1 [Figure 10B and C]. The click post-synthesis methodology presented herein provides effective guidelines for catalyst design. Chang et al. fabricated a 2D COF that exhibited two unique properties. Cyclotriphosphazene-based COFs contain abundant C-atom active sites because of their highly electrophilic structure[131]. In addition, the active sites of C atoms are well exposed by the bilayer stacking of the [6+3] imine-linked backbone, and mass diffusion occurs between catalytic reactions. In addition to these two properties, thin COF nanosheets can be fabricated relatively easily via weak interlayer π-π interactions. The π-π interaction energy can be determined by the interlayer spacing, and 14.0 Å was determined to be the appropriate spacing. JUC-610-CON fabricated via this process featured a thickness of 4 nm and a length of 800 nm and can be peeled off [Figure 10D-F]. The Tafel slope of the OER was 61.5 mV dec-1. In addition, the JUC-610-CON as a Zn-air battery demonstrated a delivered power density of 156 mW cm-2 at
Figure 10. (A) Active ORR sites detected within NDI, BzDI, and PDI moieties via DFT calculations and denoted as C1 (blue circles) and C2 (red circles). (B) Hydrodynamic linear sweep voltammetry and (C) number of electrons exchanged in ORR at different potentials using RRDE of GC/Pt (disc/ring) modified with BzDI0.17-COF/Carbon SuperP (black), NDI0.17-COF/Carbon SuperP (red), PDI0.17-COF/Carbon SuperP (blue), TAPB-DMTA-COF/Carbon SuperP (cyan), and Carbon Super P (green) in O2 saturated 0.1 M NaOH solution at 10 mV/s and 1,000 rpm. Copyright 2023, American Chemical Society[130]. (D) AFM image of JUC-610-CON, and (E) the corresponding height curves for selected areas in (D). (F) LSV curves of JUC-610-CON, JUC-610, JUC-611, and JUC-612 at 1,600 rpm in O2- saturated 0.1 M KOH electrolyte. (G) Discharge curves of JUC-610-CON-based ZAB at different current densities (25, 100, 200, and 300 mA cm-2). (H) Stability of JUC-610-CON-based ZAB at 5 mA cm-2 in ambient air conditions, and photographs of red “COF” LED panel powered by two ZABs in series (inset). Copyright 2023, Springer Nature[131].
CO2RR
Because CO2 is one of the most significant contributors to global warming, technology for reducing CO2 using various methods has been continuously developed[102,134-138]. Recently, the utilization of CO2RR beyond storage has received significant attention[139-141]. Unlike previous types of electrocatalysts for the CO2RR, metallic materials are particularly advantageous. Among them, Cu is well known for its ability to generate various products via the CO2RR[142]. However, increasing and controlling product selectivity is difficult. In addition, various metal atoms, such as Ni, Co, and Pd, are alloyed and used in a few cases; however, identifying the CO2RR site is difficult, which renders it challenging to secure stability. Single-atom catalysis has been extensively investigated as an alternative to metallic materials; however, achieving single-atom control is difficult. Therefore, 2D COF CO2RR catalysts should be developed to be less dependent on metal elements compared to the catalysts composed of alloys or single metal atoms. In general, because the electrical conductivity of 2D COFs is low, it can be improved using various methods. CO2RR catalysts can be synthesized using 2D COFs containing donor-acceptor heterojunctions (D-A heterojunctions). Wu et al. developed a 2D cobalt porphyrin-based COF (TT-Por(Co)-COF) via the Schiff base reaction of thieno[3,2-b]thiophene-2,5-dicarbaldehyde (TT) containing an electron donor and Co-TAPP containing an acceptor[143]. The electrical properties of the prepared catalysts were improved by D-A heterojunctions. The electrical conductivity was 1.38 × 10-8 S m-1, and the carrier mobility was 0.18 cm2 V-1 s-1. In terms of the CO2RR performance of TT-Por(Co)-COF, the Faraday efficiency of CO (FECO) was 91.4% at -0.6 V, and an extremely high current density of 7.28 mA cm2 was indicated at -0.7 V [Figure 11A, B and C]. Using a similar strategy, Zhu et al. fabricated highly crystalline metalloporphyrin-TTF-based COFs (M-TTCOFs) by assembling metallized 5,10,15,20-tetrakis (4-aminophenyl) porphinato (MTAPP, M = Co or Ni) and 2,3,6,7-tetra (4-formylphenyl)tetrathiafulvalene (4-formyl-TTF)[144]. In this regard, TTF served as an electron donor, and an electron path was constructed using metalloporphyrin. DFT calculations confirmed that these electron donors and paths provided more favorable The unit of mobility is in this format.conditions for *COOH and *CO production by providing abundant electrons to the Co atom sites [Figure 11D and E]. The FECO at -0.7 V of the Co-TTCOF nanosheet prepared as such was 91.3%, which remained stable even after a reaction time exceeding 40 h. The maximum FECO was 99.7% at -0.8 V [Figure 11F and G].
Figure 11. (A) LSV curves in CO2-saturated and Ar-saturated 0.5 m KHCO3 under scan rate of 10 mV s-1. (B) FECO and (C) Jco from -0.6 to -0.9 V vs. RHE of TT-Por(Co)-COF and COF-366-Co. Copyright 2020, John Wiley and Sons[143]. (D) Relative energy diagrams of CO2 reduction to CO for M-TTCOFs (M = Co or Ni). (E) Proposed schematic mechanism for electrocatalytic CO2RR on Co-TTCOF. (F) FECO based on a calculated overpotential range from -0.5 to -0.9 V. (G) Cycling stability test of Co-TTCOF at potential of -0.7 V vs. RHE. Copyright 2020, Springer Nature[144].
The second strategy involves the addition of metal atoms and conductive materials. Lu et al. improved the CO2RR by adding Co-porphyrin and amino-functionalized carbon nanotubes (CNTs) to a synthesized COF[145]. The 2D COF was synthesized using TAPP. To add Co atoms, TAPP and Co(CH3COO)2·4H2O were added to a DMF solution, and reflux was performed. Subsequently, Co-TAPP, terephthalaldehyde, and the dispersion of CNTs were placed in ethanol, followed by sonication; finally, COF-366-Co@CNT was achieved by degassing in liquid nitrogen. A catalyst with an excellent CO2RR was achieved owing to the strong binding force between the CNT and TAPP. COF-366-Co@CNT indicated a FECO of 93.6% at -0.68 V [Figure 12A]. Subsequently, Zhang et al. synthesized a 2D conductive Ni-phthalocyanine-based COF (NiPc-COF) using 2,3,9,10,16,17,23,24-octaaminophthalocyanato Ni(II) and tert-butylpyrene-tetraone[146]. NiPc-COF confirmed that Ni ions were evenly distributed through TEM analysis, and the area and thickness of the nanosheet were 3 μm and 0.74 nm, respectively, based on AFM measurement. The CO2RR indicated a FECO exceeding 93% over a wide voltage range of -0.6 to -1.1 V. In particular, at -0.9 V, it was approximately 100% [Figure 12B and C]. In addition, the CO generation efficiency was maintained even after 10 h of catalytic reaction. CO2 was adsorbed on the Ni center as COOH*, converted to CO, and desorbed again. This study presents an effective strategy for electrocatalysis using a 2D COF nanosheet. Meanwhile, sacrificial materials were used to control active sites in previous studies. After synthesizing Co-COF on hydrotalcite, Miao et al. increased the number of CoN2O2 sites via heat and acid treatment[147]. Hydrotalcite has a hexagonal base crystal structure, and the Co-COF grown on it exhibits a regular mesoporous structure, depending on the crystallinity of the substrate [Figure 12D]. In the pyrolysis process, the substrate assumes two roles. First, Co-ion aggregation is prevented, and CoN2O2 formation is advantageous because of the sufficient oxygen supply. DFT calculations confirmed that CoN2O2 exhibited the lowest COOH* adsorption energy barrier compared with any other sites. In addition, the binding of COOH* to CoN2O2 was promoted because the 2p orbital of COOH* overlapped with the t2g orbital of CoN2O2
Figure 12. (A) FEs of COF-366-Co@CNT, COF-366-(OMe)2-Co@CNT, COF-366-(OH)2-Co@CNT, and COF-366-(F)4-Co@CNT at a scan rate of 5 mV s-1. Copyright 2020, American Chemical Society[145]. (B) LSV curves of NiPc-COF in CO2-saturated and N2-saturated 0.5 m KHCO3 electrolytes at a scan rate of 10 mV S-1. (C) FECO and FEH2 from -0.6 to -1.1 V of NiPc-COF in CO2-saturated 0.5 m KHCO3 electrolytes. Copyright 2020, John Wiley and Sons[146]. (D) Proposed schematic mechanism for electrocatalytic CO2RR on 2D-Co-COF500. (E) PDOS before COOH adsorbed on 2D-Co-COF500. (F) PDOS before COOH adsorbed on Co-COF500. Copyright, 2022 Elsevier[147].
Various electrocatalysts using 2D COFs
| The type of catalysis | COF | BET surface area (m2 g-1) | Electrolyte | Tafel slope (mV dec-1) | Overpotential (mV) | Faradaic efficiency (%) | Product | Reference |
| HER | SB-PORPy-COF | 869 | 0.5 M H2SO4 | 116 | [107] | |||
| F-CT-1-AA | 741 | 97 | 200 | [108] | ||||
| 2D C6-TRZ-TFP-COF | 928 | 0.5 M H2SO4 | 82 | 200 | [109] | |||
| COF/rGO | 1.0 M KOH | 46 | 42 | [111] | ||||
| Ru@COF | 1.5 M H2SO4 | 79 | 212 | [112] | ||||
| Cryst-2D-PMPI-Ru | 0.5 M H2SO4 1.0 M KOH | 36.5 | 55.3 | [113] | ||||
| Mo2C-MoNi4@NC-2 | 108.7 | 0.5 M H2SO4 | 93 | 104 | [114] | |||
| TP-COF-C700 | 672.2 | 1.0 M KOH | 128 | 94 | [110] | |||
| OER | CoP-TOB | 0.1 M KOH | 89 | 450 | [59] | |||
| 2D COF Cage-COF-1-Ns/Co | 1 M KOH | 56 | 330 | [126] | ||||
| 2D-COF-C4N | 1 M KOH | 64 | 349 | [127] | ||||
| CoCu-ZIF@GDY | 0.1 M KOH | 57 | 250 | [128] | ||||
| Co-COF-C4N | 0.5 M Na2SO4 | 43 | 280 | [129] | ||||
| ORR | NDI0.17-CPF | 158 | 0.1 M NaOH | 79.9 | [130] | |||
| Q3CTP-COFs (JUC-610-CON) | 475 | 0.1 M KOH | 61.95 | [131] | ||||
| DAPT-TFP-COF | 200 | 0.1 M NaOH | [132] | |||||
| 2D FePc-COF | 0.1 M KOH | 80 | [133] | |||||
| CO2RR | TT-Por(Co)-COF | 748 | 0.5 M KHCO3 | 91.4 (-0.6 V) | CO | [143] | ||
| TTF-based COFs (Co-TTCOFs) | 0.5 M KHCO3 | 237 | 99.7 (-0.8 V) | CO | [144] | |||
| COF-366-(OMe)2-Co@CNT | 196 | 0.5 M KHCO3 | 190 | 93.6 (-0.68 V) | CO | [145] | ||
| Ni-phthalocyanine-based COF (NiPc-COF) | 358 | 0.5 M KHCO3 | ~100 (-0.9 V) | CO | [146] | |||
| 2D-Co-COF500 | 123 | 0.5 M KHCO3 | 96.5 (-0.8 V) | CO | [147] | |||
| Cu-NC@CuPc-COF | 1 M KOH | 74.3 (-1.0 V) | CH4 | [148] |
CONCLUSION AND OUTLOOK
These 2D COFs have yielded promising results. However, their development is still in the early stages, and their practical application in industry is not foreseeable in the near future. A comprehensive analysis of the studies introduced earlier reveals that whether they are metal-free or support metal-based catalysts, COF catalysts are highly chemically stable. The advantage allows for applications across a wide range of pH conditions and oxidizing or reducing environments, presenting clear opportunities for various industrial uses. Nevertheless, while 2D COFs show promise, their immediate applications in the industry remain uncertain. In a recent study, the number of electrochemical catalyst sites was compared and analyzed according to the COF dimensions (1D, 2D, and 3D)[148]. The biggest problem with 2D COFs is that they lack catalytically active sites when compared to 1D COFs. Additionally, numerous problems remain, such as performance competitiveness with metal catalysts and sustainability of productivity. To fully exploit the potential of 2D COF catalysts, a wide range of materials should be developed and their placement optimized. Additionally, 2D COF materials offer several advantages, such as low cost, high stability in acidic or basic environments, and specialized mass transport through crystallinity or porosity. Furthermore, their usability is enhanced by their ability to predict catalytic effects and sites through precursor selection and framework design. Whereas the efficiency of small-scale catalysts may be limited, they are expected to contribute significantly to large-scale industrial production lines for reactions such as the HER, OER, ORR, and CO2RR. To exploit these advantages, the following developments must be addressed.
Enhancing Performance: Currently, pure 2D COFs perform worse than catalysts composed of bulky metal or single metal atoms. Increasing the current density during catalytic reactions is essential. This can be achieved by increasing the number of catalytic sites or improving the electrical conductivity. However, matching the performances of metal-based catalysts is challenging. Therefore, extensive research is required to investigate the use of other materials and heterostructures that can serve as cocatalysts or facilitate material transport to maximize the surface area. Strategies aimed at maximizing these roles must be continuously pursued.
Improving Synthesis Methods: The synthesis of 2D COF materials is generally time-consuming and can hinder productivity, even when inexpensive materials are used. These aspects are major disadvantages to price competitiveness. As most 2D COFs use organic precursors, low process temperatures are typically required, rendering mass synthesis and production unfavorable owing to the slow decomposition, diffusion, and rebonding of the precursors. The low speed and yield of the synthesized materials pose significant challenges. Hence, appropriate techniques must be developed to enable the rapid synthesis of large quantities of 2D COFs. One potential solution is to synthesize a 2D COF directly on an electrode to eliminate the additional binding steps. The development of binder-free catalysts can result in higher yields. However, the biggest obstacle to the industrial application of the current catalysts is the manufacturing of large-area electrodes. Given that the process temperature of COF is relatively low, it is expected to be advantageous for manufacturing large-area electrodes. This implies that the development of mass and large-area production is essential to gain industrial merit in the field of 2D COF catalyst usage.
Identifying Active Sites: Determining the active sites in catalyst materials is crucial; however, it involves predicting atomic-level interactions that are complex and require extreme caution. Although DFT calculations are typically performed, they should be applied early in the design stage of catalytic materials instead of solely to confirm active sites. Designing a new 2D COF involves predicting the site and optimizing the performance through continued cross-validation, adjustment steps, performance evaluation, and DFT calculations. This approach maximizes the advantages of 2D COFs by enabling the synthesis of various structures and components.
Expanding CO2RR Catalyst Functionality: Research pertaining to CO2RR catalysts using COFs is currently limited to products primarily involving CO-. Compared with metal-based catalysts, COF catalysts exhibit inferior functionalities. To achieve various products such as CH4, C2H4, and formic acid, the adsorption of CO is crucial. Designing sites that can adsorb CO is challenging as the ability of 2D COF materials to adsorb CO is inherently inferior to that of metal atoms because of the favorability of the d-orbital in most adsorption sites. Consequently, the inclusion of transition metal atoms must be considered in studies pertaining to the CO2RR. The development of optimized catalyst materials that combine metal atoms and 2D COFs is crucial for industrial applications.
Advancing Full-Cell Research: Whereas recent studies pertaining to catalyst materials focus primarily on half-cells, comprehensive investigations into full cells are essential. The current understanding of electrocatalysts is primarily derived from a material science perspective. However, for wider applicability, the electrolyte, CO2, product transport, and counter electrodes must be optimized.
Stability or recyclability/reusability: 2D COFs hold promises for catalytic applications due to their stability under various chemical and thermal conditions and their potential for recyclability and reusability in heterogeneous catalysis. However, the success of COFs as catalysts depends on their design to withstand specific reaction conditions, addressing catalyst deactivation issues, and optimizing the practical aspects of recycling and regeneration processes to make them cost-effective and environmentally sustainable. Researchers should explore and develop COF-based catalysts with improved stability and recyclability for a wide range of applications.
Structural design can be classified into three types: completely metal-free, using metal atoms, and supporting a metal catalyst. As mentioned previously, COFs are generally chemically stable. For each COF, its active catalytic site, mechanical properties, and performance are diverse. Therefore, we propose research aimed at improving system efficiency by using cocatalysts that contribute actively rather than merely serving as structural supports for other catalysts. To develop such systems, it is crucial to investigate the effects that arise when these materials are combined with others. For instance, in the earlier mentioned studies, where Cu clusters and 2D COFs were combined, the COF effectively functioned as a catalyst in the reduction reaction. Therefore, it is essential to establish a distinct and active role in catalytic reactions, as opposed to merely serving as a passive support material.
In conclusion, the development of catalysts using 2D COF materials is highly promising. However, several challenges must be addressed to enable practical industrial applications. Enhancing the performance, improving synthesis methods, identifying active sites, expanding electrocatalyst functionality, and advancing full-cell research are key areas that require focused attention to unravel the full potential of 2D COF catalysts in various industrial processes.
DECLARATIONS
Authors’ contributions
Proposed the topic of this review: Yu HK, Kim SY
Prepared the manuscript: Cho JH, Kim Y
Availability of data and materials
Not applicable.
Financial support and sponsorship
This research was supported by the National Research Foundation of Korea (NRF), funded by the Korean government (2021R1A4A3027878, 2022M3H4A1A01012712).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2024.
REFERENCES
1. Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future. Nature 2012;488:294-303.
2. Meys R, Kätelhön A, Bachmann M, et al. Achieving net-zero greenhouse gas emission plastics by a circular carbon economy. Science 2021;374:71-6.
3. Duan X, Xu J, Wei Z, et al. Metal-free carbon materials for CO2 electrochemical reduction. Adv Mater 2017;29:1701784.
4. Wang J, Dou S, Wang X. Structural tuning of heterogeneous molecular catalysts for electrochemical energy conversion. Sci Adv 2021;7:eabf3989.
5. Habtamu A, Ujihara M. The mechanism of water pollutant photodegradation by mixed and core-shell WO3/TiO2 nanocomposites. RSC Adv 2023;13:12926-40.
6. Motora KG, Wu C, Chala TF, Chou M, Kuo CJ, Koinkar P. Highly efficient photocatalytic activity of Ag3VO4/WO2.72 nanocomposites for the degradation of organic dyes from the ultraviolet to near-infrared regions. Appl Surf Sci 2020;512:145618.
7. Motora KG, Wu C. Magnetically separable highly efficient full-spectrum light-driven WO2.72/Fe3O4 nanocomposites for photocatalytic reduction of carcinogenic chromium (VI) and organic dye degradation. J Taiwan Inst Chem Eng 2020;117:123-32.
8. Zhou Y, Sung J, Brutschea E, et al. Bilayer Wigner crystals in a transition metal dichalcogenide heterostructure. Nature 2021;595:48-52.
9. Nguyen TV, Nguyen TP, Le QV, Dao DV, Ahn SH, Kim SY. Synthesis of very small molybdenum disulfide nanoflowers for hydrogen evolution reaction. Appl Surf Sci 2023;607:154979.
10. Shin H, Eom W, Lee KH, Jeong W, Kang DJ, Han TH. Highly electroconductive and mechanically strong Ti3C2Tx MXene fibers using a deformable MXene gel. ACS Nano 2021;15:3320-9.
11. Do HH, Tekalgne MA, Le QV, Cho JH, Ahn SH, Kim SY. Hollow Ni/NiO/C composite derived from metal-organic frameworks as a high-efficiency electrocatalyst for the hydrogen evolution reaction. Nano Converg 2023;10:6.
12. Lee MK, Shokouhimehr M, Kim SY, Jang HW. Two-dimensional metal-organic frameworks and covalent-organic frameworks for electrocatalysis: distinct merits by the reduced dimension. Adv Energy Mater 2022;12:2003990.
13. Xue Y, Zhang Q, Wang W, Cao H, Yang Q, Fu L. Opening two-dimensional materials for energy conversion and storage: a concept. Adv Energy Mate 2017;7:1602684.
14. Mu Q, Zhu W, Li X, et al. Electrostatic charge transfer for boosting the photocatalytic CO2 reduction on metal centers of 2D MOF/rGO heterostructure. Appl Catal B 2020;262:118144.
15. Voiry D, Yang J, Chhowalla M. Recent strategies for improving the catalytic activity of 2D TMD nanosheets toward the hydrogen evolution reaction. Adv Mater 2016;28:6197-206.
16. Côté AP, Benin AI, Ockwig NW, O'Keeffe M, Matzger AJ, Yaghi OM. Porous, crystalline, covalent organic frameworks. Science 2005;310:1166-70.
17. Mohamed Samy M, Mekhemer IM, Mohamed MG, et al. Conjugated microporous polymers incorporating Thiazolo[5,4-d]thiazole moieties for sunlight-driven hydrogen production from water. Chem Eng J 2022;446:137158.
18. Dutta S, Bhaumik A, Wu KC. Hierarchically porous carbon derived from polymers and biomass: effect of interconnected pores on energy applications. Energy Environ Sci 2014;7:3574-92.
19. Chen J, Tao X, Li C, et al. Synthesis of bipyridine-based covalent organic frameworks for visible-light-driven photocatalytic water oxidation. Appl Catal B 2020;262:118271.
20. Contreras-pereda N, Pané S, Puigmartí-luis J, Ruiz-molina D. Conductive properties of triphenylene MOFs and COFs. Coord Chem Rev 2022;460:214459.
21. Bian G, Yin J, Zhu J. Recent Advances on conductive 2D covalent organic frameworks. Small 2021;17:e2006043.
22. Shen J, Zhang R, Su Y, et al. Polydopamine-modulated covalent organic framework membranes for molecular separation. J Mater Chem A 2019;7:18063-71.
23. Chen L, Wang W, Tian J, et al. Imparting multi-functionality to covalent organic framework nanoparticles by the dual-ligand assistant encapsulation strategy. Nat Commun 2021;12:4556.
24. Spitler EL, Koo BT, Novotney JL, et al. A 2D covalent organic framework with 4.7-nm pores and insight into its interlayer stacking. J Am Chem Soc 2011;133:19416-21.
25. Deng JH, Luo J, Mao YL, et al. π-π stacking interactions: non-negligible forces for stabilizing porous supramolecular frameworks. Sci Adv 2020;6:eaax9976.
26. Jati A, Dey K, Nurhuda M, Addicoat MA, Banerjee R, Maji B. Dual metalation in a two-dimensional covalent organic framework for photocatalytic C-N cross-coupling reactions. J Am Chem Soc 2022;144:7822-33.
27. Zhang M, Tong Y, Sun Z, et al. Two-dimensional covalent organic framework with synergistic active centers for efficient electrochemical sodium storage. Chem Mater 2023;35:4873-81.
28. Khalid NR, Ilyas S, Ali F, et al. Novel Sn-doped WO3 photocatalyst to degrade the organic pollutants prepared by green synthesis approach. Electron Mater Lett 2024;20:85-94.
29. Dalapati S, Addicoat M, Jin S, et al. Rational design of crystalline supermicroporous covalent organic frameworks with triangular topologies. Nat Commun 2015;6:7786.
30. Farha OK, Eryazici I, Jeong NC, et al. Metal-organic framework materials with ultrahigh surface areas: is the sky the limit? J Am Chem Soc 2012;134:15016-21.
31. Rodríguez-San-Miguel D, Montoro C, Zamora F. Covalent organic framework nanosheets: preparation, properties and applications. Chem Soc Rev 2020;49:2291-302.
32. Jin F, Wang T, Zheng H, et al. Bottom-up synthesis of covalent organic frameworks with quasi-three-dimensional integrated architecture via interlayer cross-linking. J Am Chem Soc 2023;145:6507-15.
33. Abuzeid HR, El-mahdy AF, Kuo S. Covalent organic frameworks: design principles, synthetic strategies, and diverse applications. Giant 2021;6:100054.
34. Cai Y, Wen X, Wang Y, et al. Preparation of hyper-crosslinked polymers with hierarchical porous structure from hyperbranched polymers for adsorption of naphthalene and 1-naphthylamine. Sep Purif Technol 2021;266:118542.
35. Katekomol P, Roeser J, Bojdys M, Weber J, Thomas A. Covalent triazine frameworks prepared from 1,3,5-tricyanobenzene. Chem Mater 2013;25:1542-8.
37. Saber AF, Sharma SU, Lee J, El-mahdy AF, Kuo S. Carbazole-conjugated microporous polymers from Suzuki-Miyaura coupling for supercapacitors. Polymer 2022;254:125070.
38. Park CG, Yang JW, Hwang NM. TEM Observations of metastable nanocarbon allotropes in the initial stage of diamond growth at 300 °C during diamond hot filament CVD. Electron Mater Lett 2023;19:316-24.
39. Hao S, Zhang T, Fan S, Jia Z, Yang Y. Preparation of COF-TpPa1 membranes by chemical vapor deposition method for separation of dyes. Chem Eng J 2021;421:129750.
40. Zheng W, Tsang C, Lee LYS, Wong K. Two-dimensional metal-organic framework and covalent-organic framework: synthesis and their energy-related applications. Mater Today Chem 2019;12:34-60.
41. Liu M, Liu Y, Dong J, et al. Two-dimensional covalent organic framework films prepared on various substrates through vapor induced conversion. Nat Commun 2022;13:1411.
42. Roy N, Kundu T. Photoresponse of CVD grown crystalline quantum dot-embedded covalent organic framework thin film. RSC Adv 2023;13:3669-76.
43. Li Y, Zhang M, Guo X, et al. Growth of high-quality covalent organic framework nanosheets at the interface of two miscible organic solvents. Nanoscale Horiz 2018;3:205-12.
44. Shao M, Zhang Q, Wei X, et al. Twisted node modulation of 2D-COFs for programmable long-afterglow luminescence. Cell Rep Phys Sci 2023;4:101273.
45. Sick T, Rotter JM, Reuter S, et al. Switching on and off interlayer correlations and porosity in 2D covalent organic frameworks. J Am Chem Soc 2019;141:12570-81.
46. Belov AS, Voloshin YZ, Pavlov AA, et al. Solvent-induced encapsulation of cobalt(II) ion by a boron-capped tris-pyrazoloximate. Inorg Chem 2020;59:5845-53.
47. Wei H, Chai S, Hu N, Yang Z, Wei L, Wang L. The microwave-assisted solvothermal synthesis of a crystalline two-dimensional covalent organic framework with high CO2 capacity. Chem Commun 2015;51:12178-81.
48. Zhu D, Zhu Y, Yan Q, et al. Pure crystalline covalent organic framework aerogels. Chem Mater 2021;33:4216-24.
49. Kang C, Zhang Z, Wee V, et al. Interlayer shifting in two-dimensional covalent organic frameworks. J Am Chem Soc 2020;142:12995-3002.
50. Huang W, Jiang Y, Li X, et al. Solvothermal synthesis of microporous, crystalline covalent organic framework nanofibers and their colorimetric nanohybrid structures. ACS Appl Mater Interfaces 2013;5:8845-9.
51. Shevate R, Shaffer DL. Large-area 2D covalent organic framework membranes with tunable single-digit nanopores for predictable mass transport. ACS Nano 2022;16:2407-18.
52. Ji W, Hamachi LS, Natraj A, et al. Solvothermal depolymerization and recrystallization of imine-linked two-dimensional covalent organic frameworks. Chem Sci 2021;12:16014-22.
53. Xu L, Ding SY, Liu J, Sun J, Wang W, Zheng QY. Highly crystalline covalent organic frameworks from flexible building blocks. Chem Commun 2016;52:4706-9.
54. Evans AM, Strauss MJ, Corcos AR, et al. Two-dimensional polymers and polymerizations. Chem Rev 2022;122:442-564.
55. Peng L, Guo Q, Song C, et al. Ultra-fast single-crystal polymerization of large-sized covalent organic frameworks. Nat Commun 2021;12:5077.
56. Yang H, Xu J, Cao H, Wu J, Zhao D. Recovery of homogeneous photocatalysts by covalent organic framework membranes. Nat Commun 2023;14:2726.
57. Zhan G, Cai ZF, Strutyński K, et al. Observing polymerization in 2D dynamic covalent polymers. Nature 2022;603:835-40.
58. Khan NA, Zhang R, Wu H, et al. Solid-vapor interface engineered covalent organic framework membranes for molecular separation. J Am Chem Soc 2020;142:13450-8.
59. Tang J, Liang Z, Qin H, et al. Large-area free-standing metalloporphyrin-based covalent organic framework films by liquid-air interfacial polymerization for oxygen electrocatalysis. Angew Chem Int Ed 2023;62:e202214449.
60. Zhou D, Tan X, Wu H, Tian L, Li M. Synthesis of C-C bonded two-dimensional conjugated covalent organic framework films by suzuki polymerization on a liquid-liquid interface. Angew Chem Int Ed 2019;58:1376-81.
61. Evans AM, Parent LR, Flanders NC, et al. Seeded growth of single-crystal two-dimensional covalent organic frameworks. Science 2018;361:52-7.
62. Jin E, Li J, Geng K, et al. Designed synthesis of stable light-emitting two-dimensional sp2 carbon-conjugated covalent organic frameworks. Nat Commun 2018;9:4143.
63. Wang S, Wang Q, Shao P, et al. Exfoliation of covalent organic frameworks into few-layer redox-active nanosheets as cathode materials for lithium-ion batteries. J Am Chem Soc 2017;139:4258-61.
64. Liu J, Lyu P, Zhang Y, Nachtigall P, Xu Y. New layered triazine framework/exfoliated 2D polymer with superior sodium-storage properties. Adv Mater 2018:30.
65. Bunck DN, Dichtel WR. Bulk synthesis of exfoliated two-dimensional polymers using hydrazone-linked covalent organic frameworks. J Am Chem Soc 2013;135:14952-5.
66. Zhang Z, Xiao A, Yin C, Wang X, Shi X, Wang Y. Heterostructured two-dimensional covalent organic framework membranes for enhanced ion separation. Chem Commun 2022;58:7136-9.
67. Feng X, Ding X, Chen L, et al. Two-dimensional artificial light-harvesting antennae with predesigned high-order structure and robust photosensitising activity. Sci Rep 2016;6:32944.
68. Lukose B, Kuc A, Heine T. The structure of layered covalent-organic frameworks. Chemistry 2011;17:2388-92.
69. Yu F, Liu W, Ke SW, Kurmoo M, Zuo JL, Zhang Q. Electrochromic two-dimensional covalent organic framework with a reversible dark-to-transparent switch. Nat Commun 2020;11:5534.
70. Mahmood J, Ahmad I, Jung M, et al. Two-dimensional amine and hydroxy functionalized fused aromatic covalent organic framework. Commun Chem 2020;3:31.
71. Li RL, Yang A, Flanders NC, Yeung MT, Sheppard DT, Dichtel WR. Two-dimensional covalent organic framework solid solutions. J Am Chem Soc 2021;143:7081-7.
72. Cao L, Chen IC, Li Z, et al. Switchable Na+ and K+ selectivity in an amino acid functionalized 2D covalent organic framework membrane. Nat Commun 2022;13:7894.
73. Wu X, Han X, Liu Y, Liu Y, Cui Y. Control interlayer stacking and chemical stability of two-dimensional covalent organic frameworks via steric tuning. J Am Chem Soc 2018;140:16124-33.
74. Zhuang S, Lei L, Nunna B, Lee ES. New nitrogen-doped graphene/MOF-modified catalyst for fuel cell systems. ECS Trans 2016;72:149-54.
75. Duresa LW, Kuo D, Bekena FT, Kebede WL. Simple room temperature synthesis of oxygen vacancy-rich and In-doped BiOBr nanosheet and its highly enhanced photocatalytic activity under visible-light irradiation. J Phys Chem Solids 2021;156:110132.
76. Chen R, Wang Y, Ma Y, et al. Rational design of isostructural 2D porphyrin-based covalent organic frameworks for tunable photocatalytic hydrogen evolution. Nat Commun 2021;12:1354.
77. Evans AM, Bradshaw NP, Litchfield B, et al. High-sensitivity acoustic molecular sensors based on large-area, spray-coated 2D covalent organic frameworks. Adv Mater 2020;32:e2004205.
78. Yu H, Wang J, Xie F, et al. A stack-guiding unit constructed 2D COF with improved charge carrier transport and versatile photocatalytic functions. Chem Eng J 2022;445:136713.
79. Kim KH, Choi C, Choung S, et al. Continuous oxygen vacancy gradient in TiO2 photoelectrodes by a photoelectrochemical-driven “self-purification” process. Adv Energy Mater 2022;12:2103495.
80. Biswas S, Dey A, Rahimi FA, Barman S, Maji TK. Metal-free highly stable and crystalline covalent organic nanosheet for visible-light-driven selective solar fuel production in aqueous medium. ACS Catal 2023;13:5926-37.
81. Lee SA, Yang JW, Lee TH, et al. Multifunctional nano-heterogeneous Ni(OH)2/NiFe catalysts on silicon photoanode toward efficient water and urea oxidation. Appl Catal B 2022;317:121765.
82. Bae S, Lee S, Ryu H, Lee W. Improvement of photoelectrochemical properties of CuO photoelectrode by Li doping. Korean J Met Mater 2022;60:577-86.
83. Nguyen VH, Nguyen BS, Hu C, et al. Novel architecture titanium carbide (Ti3C2Tx) MXene cocatalysts toward photocatalytic hydrogen production: a mini-review. Nanomaterials 2020;10:602.
84. Do HH, Nguyen DLT, Nguyen XC, et al. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: a review. Arab J Chem 2020;13:3653-71.
85. Nguyen V, Nguyen B, Jin Z, et al. Towards artificial photosynthesis: sustainable hydrogen utilization for photocatalytic reduction of CO2 to high-value renewable fuels. Chem Eng J 2020;402:126184.
86. Nguyen TP, Nguyen DLT, Nguyen VH, et al. Recent advances in TiO2-based photocatalysts for reduction of CO2 to fuels. Nanomaterials 2020;10:337.
87. Cho JH, Ma J, Kim SY. Toward high-efficiency photovoltaics-assisted electrochemical and photoelectrochemical CO2 reduction: strategy and challenge. Exploration 2023;3:20230001.
88. Yang Y, Shen Y, Wang L, Song Y, Wang L. Three-dimensional porous carbon/covalent-organic framework films integrated electrode for electrochemical sensors. J Electroanal Chem 2019;855:113590.
89. Zhu Y, Shao P, Hu L, et al. Construction of interlayer conjugated links in 2D covalent organic frameworks via topological polymerization. J Am Chem Soc 2021;143:7897-902.
90. Zhao X, Pachfule P, Thomas A. Covalent organic frameworks (COFs) for electrochemical applications. Chem Soc Rev 2021;50:6871-913.
91. Yang C, Mao C, Deng Q, Yang Y, Zhou Y, Zhang Y. One-Pot synthesis of flavones catalyzed by an Au-mediated covalent organic framework. J Colloid Interface Sci 2023;642:283-91.
92. Li C, Yu G. Controllable synthesis and performance modulation of 2D covalent-organic frameworks. Small 2021;17:e2100918.
93. Tang J, Su C, Shao Z. Covalent organic framework (COF)-based hybrids for electrocatalysis: recent advances and perspectives. Small Methods 2021;5:e2100945.
94. Sun K, Xiao F, Yu B, He W. Photo-/electrocatalytic functionalization of quinoxalin-2(1H)-ones. Chinese J Catal 2021;42:1921-43.
95. Liu C, Li H, Liu F, et al. Intrinsic activity of metal centers in metal-nitrogen-carbon single-atom catalysts for hydrogen peroxide synthesis. J Am Chem Soc 2020;142:21861-71.
96. Liu Q, Li J, Wang J. Research of covalent organic frame materials based on porphyrin units. J Incl Phenom Macrocycl Chem 2019;95:1-15.
97. Haase F, Lotsch BV. Solving the COF trilemma: towards crystalline, stable and functional covalent organic frameworks. Chem Soc Rev 2020;49:8469-500.
98. An S, Li X, Shang S, et al. One-dimensional covalent organic frameworks for the 2e- oxygen reduction reaction. Angew Chem Int Ed 2023;62:e202218742.
99. Gao Z, Yu Z, Huang Y, et al. Flexible and robust bimetallic covalent organic frameworks for the reversible switching of electrocatalytic oxygen evolution activity. J Mater Chem A 2020;8:5907-12.
100. Chang C, Wei Y, Kuo W. Free-standing CuS-ZnS decorated carbon nanotube films as immobilized photocatalysts for hydrogen production. Int J Hydrog Energy 2019;44:30553-62.
101. Wang X, Sun L, Zhou W, Wu H, Deng W. Iron single-atom catalysts confined in covalent organic frameworks for efficient oxygen evolution reaction. Cell Rep Phys Sci 2022;3:100804.
102. Qiu XF, Huang JR, Yu C, et al. A Stable and conductive covalent organic framework with isolated active sites for highly selective electroreduction of carbon dioxide to acetate. Angew Chem Int Ed 2022;61:e202206470.
103. Yusran Y, Fang Q, Qiu S. Postsynthetic covalent modification in covalent organic frameworks. Israel J Chem 2018;58:971-84.
104. Jin B, Cho Y, Park C, et al. A two-photon tandem black phosphorus quantum dot-sensitized BiVO4 photoanode for solar water splitting. Energy Environ Sci 2022;15:672-9.
105. Lee MG, Yang JW, Park H, et al. Crystal facet engineering of TiO2 nanostructures for enhancing photoelectrochemical water splitting with BiVO4 nanodots. Nanomicro Lett 2022;14:48.
106. Tian C, Liu R, Zhang Y, Yang W, Wang B. Ru-doped functional porous materials for electrocatalytic water splitting. Nano Res 2023.
107. Bhunia S, Das SK, Jana R, et al. Electrochemical stimuli-driven facile metal-free hydrogen evolution from pyrene-porphyrin-based crystalline covalent organic framework. ACS Appl Mater Interfaces 2017;9:23843-51.
108. Zhao Y, Li T, Gu J, et al. Covalent triazine frameworks based on different stacking model as electrocatalyst for hydrogen evolution. Appl Surface Sci 2023;618:156697.
109. Ruidas S, Mohanty B, Bhanja P, et al. Metal-free triazine-based 2D covalent organic framework for efficient H2 evolution by electrochemical water splitting. ChemSusChem 2021;14:5057-64.
110. Halder S, Pradhan AK, Khan S, Chakraborty C. Generation of covalent organic framework-derived porous N-doped carbon nanosheets for highly efficient electrocatalytic hydrogen evolution. Energy Adv 2023;2:1713-23.
111. Zhao Q, Chen S, Ren H, Chen C, Yang W. Ruthenium nanoparticles confined in covalent organic framework/reduced graphene oxide as electrocatalyst toward hydrogen evolution reaction in alkaline media. Ind Eng Chem Res 2021;60:11070-8.
112. Maiti S, Chowdhury AR, Das AK. Electrochemically facile hydrogen evolution using ruthenium encapsulated two dimensional covalent organic framework (2D COF). ChemNanoMat 2020;6:99-106.
113. Pan R, Wu J, Wang W, Cheng C, Liu X. Robust crystalline aromatic imide-linked two-dimensional covalent organic frameworks confining ruthenium nanoparticles as efficient hydrogen evolution electrocatalyst. Colloids Surf A Physicochem Eng Asp 2021;621:126511.
114. Wang W, Zhang L, Gao C, et al. Covalent organic framework derived Mo2C-MoNi4 chainmail catalysts for hydrogen evolution. Appl Surf Sci 2023;627:157322.
115. Xu Q, Tang Y, Zhang X, Oshima Y, Chen Q, Jiang D. Template conversion of covalent organic frameworks into 2D conducting nanocarbons for catalyzing oxygen reduction reaction. Adv Mater 2018;30:e1706330.
116. Das SK, Kumar G, Das M, Dey RS. A 2D covalent organic framework as a metal-free electrode towards electrochemical oxygen reduction reaction. Mater Today Proc 2022;57:228-33.
117. Zhang J, Zhao Z, Xia Z, Dai L. A metal-free bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Nat Nanotechnol 2015;10:444-52.
118. Ji J, Zhang C, Qin S, Jin P. First-principles investigation of two-dimensional covalent-organic framework electrocatalysts for oxygen evolution/reduction and hydrogen evolution reactions. Sustain Energy Fuels 2021;5:5615-26.
119. Tavakoli E, Kakekhani A, Kaviani S, et al. In situ bottom-up synthesis of porphyrin-based covalent organic frameworks. J Am Chem Soc 2019;141:19560-4.
120. Li S, Gao Y, Li N, Ge L, Bu X, Feng P. Transition metal-based bimetallic MOFs and MOF-derived catalysts for electrochemical oxygen evolution reaction. Energy Environ Sci 2021;14:1897-927.
121. Cai M, Ding S, Gibbons B, Yang X, Kessinger MC, Morris AJ. Nickel(II)-modified covalent-organic framework film for electrocatalytic oxidation of 5-hydroxymethylfurfural (HMF). Chem Commun 2020;56:14361-4.
122. Lee SJ, Park YS. Effect of synthesis temperature on oxygen evolution reaction of cobalt-iron layered double hydroxide. Korean J Met Mater 2022;46:326-9.
123. Xu J, Tang W, Yang C, et al. A highly conductive COF@CNT electrocatalyst boosting polysulfide conversion for Li-S chemistry. ACS Energy Lett 2021;6:3053-62.
124. Zhang Z, Wang W, Wang X, Zhang L, Cheng C, Liu X. Ladder-type π-conjugated metallophthalocyanine covalent organic frameworks with boosted oxygen reduction reaction activity and durability for zinc-air batteries. Chem Eng J 2022;435:133872.
125. Hao Q, Zhao C, Sun B, et al. Confined synthesis of two-dimensional covalent organic framework thin films within superspreading water layer. J Am Chem Soc 2018;140:12152-8.
126. Yan Y, Qin H, Ding D, et al. Ultrathin cage-based covalent organic framework nanosheets as precursor for pyrolysis-free oxygen evolution reaction electrocatalyst. ChemNanoMat 2022;8:e202200305.
127. Yang C, Yang Z, Dong H, et al. Theory-driven design and targeting synthesis of a highly-conjugated basal-plane 2D covalent organic framework for metal-free electrocatalytic OER. ACS Energy Lett 2019;4:2251-8.
128. Cui J, Liu J, Wang C, et al. Efficient electrocatalytic water oxidation by using the hierarchical 1D/2D structural nanohybrid of CoCu-based zeolitic imidazolate framework nanosheets and graphdiyne nanowires. Electrochimica Acta 2020;334:135577.
129. Zhang R, Liu W, Zhang F, Yang Z, Zhang G, Zeng XC. COF-C4N nanosheets with uniformly anchored single metal sites for electrocatalytic OER: from theoretical screening to target synthesis. Appl Catal B 2023;325:122366.
130. Martínez-Fernández M, Martínez-periñán E, Martínez JI, et al. Evaluation of the oxygen reduction reaction electrocatalytic activity of postsynthetically modified covalent organic frameworks. ACS Sustain Chem Eng 2023;11:1763-73.
131. Chang J, Li C, Wang X, et al. Quasi-three-dimensional cyclotriphosphazene-based covalent organic framework nanosheet for efficient oxygen reduction. Nanomicro Lett 2023;15:159.
132. García-Arroyo P, Martínez-periñán E, Cabrera-trujillo JJ, et al. Pyrenetetraone-based covalent organic framework as an effective electrocatalyst for oxygen reduction reaction. Nano Res 2022;15:3907-12.
133. Kumar A, Ubaidullah M, Pandit B, Yasin G, Gupta RK, Zhang G. Fe-phthalocyanine derived highly conjugated 2D covalent organic framework as superior electrocatalyst for oxygen reduction reaction. Discov Nano 2023;18:109.
134. Chi S, Chen Q, Zhao S, et al. Three-dimensional porphyrinic covalent organic frameworks for highly efficient electroreduction of carbon dioxide. J Mater Chem A 2022;10:4653-9.
135. Lei K, Yu Xia B. Electrocatalytic CO2 reduction: from discrete molecular catalysts to their integrated catalytic materials. Chemistry 2022;28:e202200141.
136. Nie W, Tarnopol DE, Mccrory CC. The effect of extended conjugation on electrocatalytic CO2 reduction by molecular catalysts and macromolecular structures. Curr Opin Electrochem 2021;28:100716.
137. Zhang M, Lu M, Yang M, et al. Ultrafine Cu nanoclusters confined within covalent organic frameworks for efficient electroreduction of CO2 to CH4 by synergistic strategy. eScience 2023;3:100116.
138. Xu K, Dai Y, Ye B, Wang H. Two dimensional covalent organic framework materials for chemical fixation of carbon dioxide: excellent repeatability and high selectivity. Dalton Trans 2017;46:10780-5.
139. Yang YL, Wang YR, Dong LZ, et al. A honeycomb-like porous crystalline hetero-electrocatalyst for efficient electrocatalytic CO2 reduction. Adv Mater 2022;34:e2206706.
140. Zhao Q, Wang Y, Li M, et al. Organic frameworks confined Cu single atoms and nanoclusters for tandem electrocatalytic CO2 reduction to methane. SmartMat 2022;3:183-93.
141. Cho JH, Lee C, Hong SH, et al. Transition metal ion doping on ZIF-8 enhances the electrochemical CO2 reduction reaction. Adv Mater 2023;35:e2208224.
142. Kim S, Kim KH, Oh C, Zhang K, Park JH. Artificial photosynthesis for high-value-added chemicals: old material, new opportunity. Carbon Energy 2022;4:21-44.
143. Wu Q, Mao MJ, Wu QJ, Liang J, Huang YB, Cao R. Construction of donor-acceptor heterojunctions in covalent organic framework for enhanced CO2 electroreduction. Small 2021;17:e2004933.
144. Zhu HJ, Lu M, Wang YR, et al. Efficient electron transmission in covalent organic framework nanosheets for highly active electrocatalytic carbon dioxide reduction. Nat Commun 2020;11:497.
145. Lu Y, Zhang J, Wei W, Ma DD, Wu XT, Zhu QL. Efficient carbon dioxide electroreduction over ultrathin covalent organic framework nanolayers with isolated cobalt porphyrin units. ACS Appl Mater Interfaces 2020;12:37986-92.
146. Zhang MD, Si DH, Yi JD, Zhao SS, Huang YB, Cao R. Conductive phthalocyanine-based covalent organic framework for highly efficient electroreduction of carbon dioxide. Small 2020;16:2005254.
147. Miao Q, Lu C, Xu Q, et al. CoN2O2 sites in carbon nanosheets by template-pyrolysis of COFs for CO2RR. Chem Eng J 2022;450:138427.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Cho JH, Kim Y, Yu HK, Kim SY. Advancements in two-dimensional covalent organic framework nanosheets for electrocatalytic energy conversion: current and future prospects. Energy Mater 2024;4:400013. http://dx.doi.org/10.20517/energymater.2023.72
AMA Style
Cho JH, Kim Y, Yu HK, Kim SY. Advancements in two-dimensional covalent organic framework nanosheets for electrocatalytic energy conversion: current and future prospects. Energy Materials. 2024; 4(2): 400013. http://dx.doi.org/10.20517/energymater.2023.72
Chicago/Turabian Style
Cho, Jin Hyuk, Youngho Kim, Hak Ki Yu, Soo Young Kim. 2024. "Advancements in two-dimensional covalent organic framework nanosheets for electrocatalytic energy conversion: current and future prospects" Energy Materials. 4, no.2: 400013. http://dx.doi.org/10.20517/energymater.2023.72
ACS Style
Cho, JH.; Kim Y.; Yu HK.; Kim SY. Advancements in two-dimensional covalent organic framework nanosheets for electrocatalytic energy conversion: current and future prospects. Energy Mater. 2024, 4, 400013. http://dx.doi.org/10.20517/energymater.2023.72
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 5 clicks
Cite This Article 5 clicks



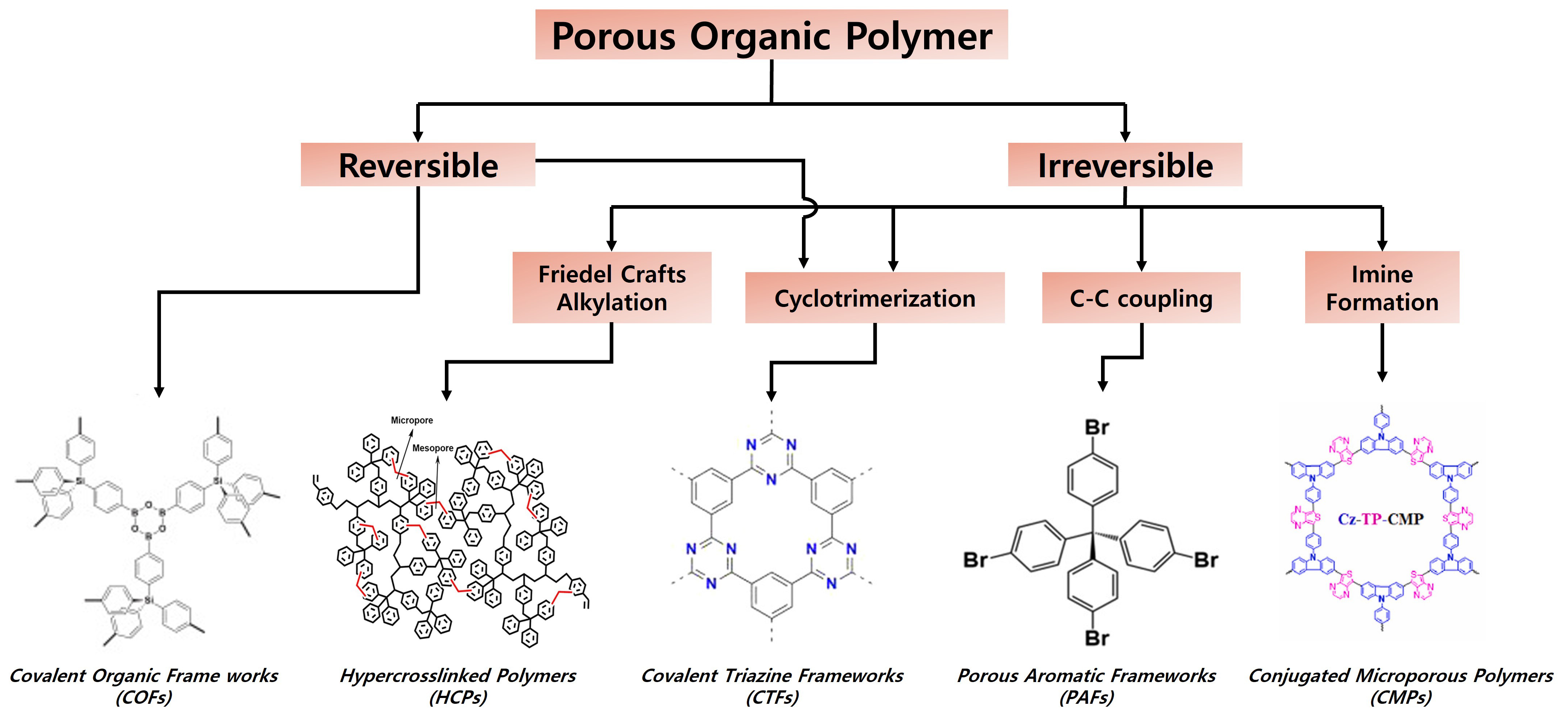
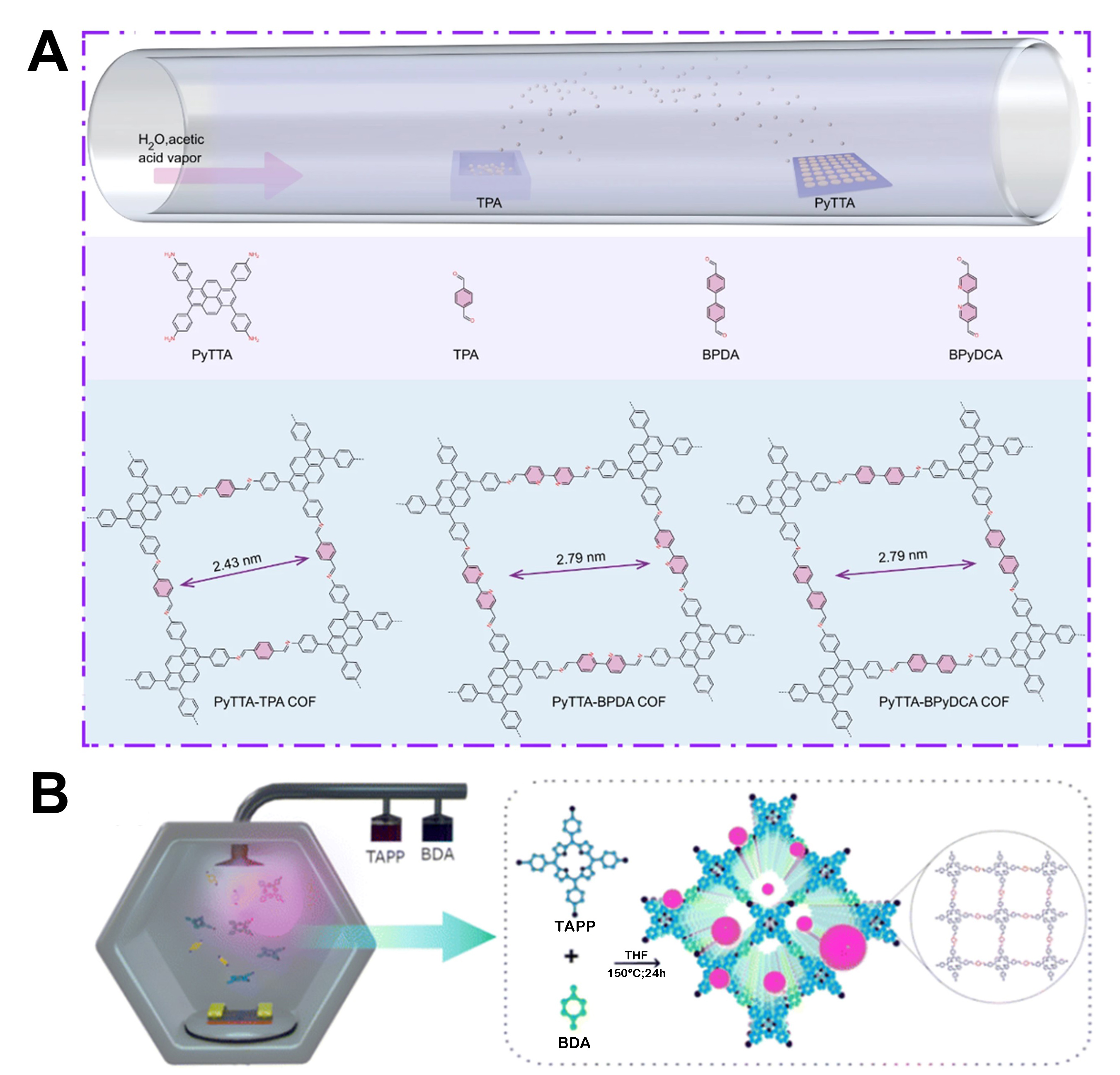


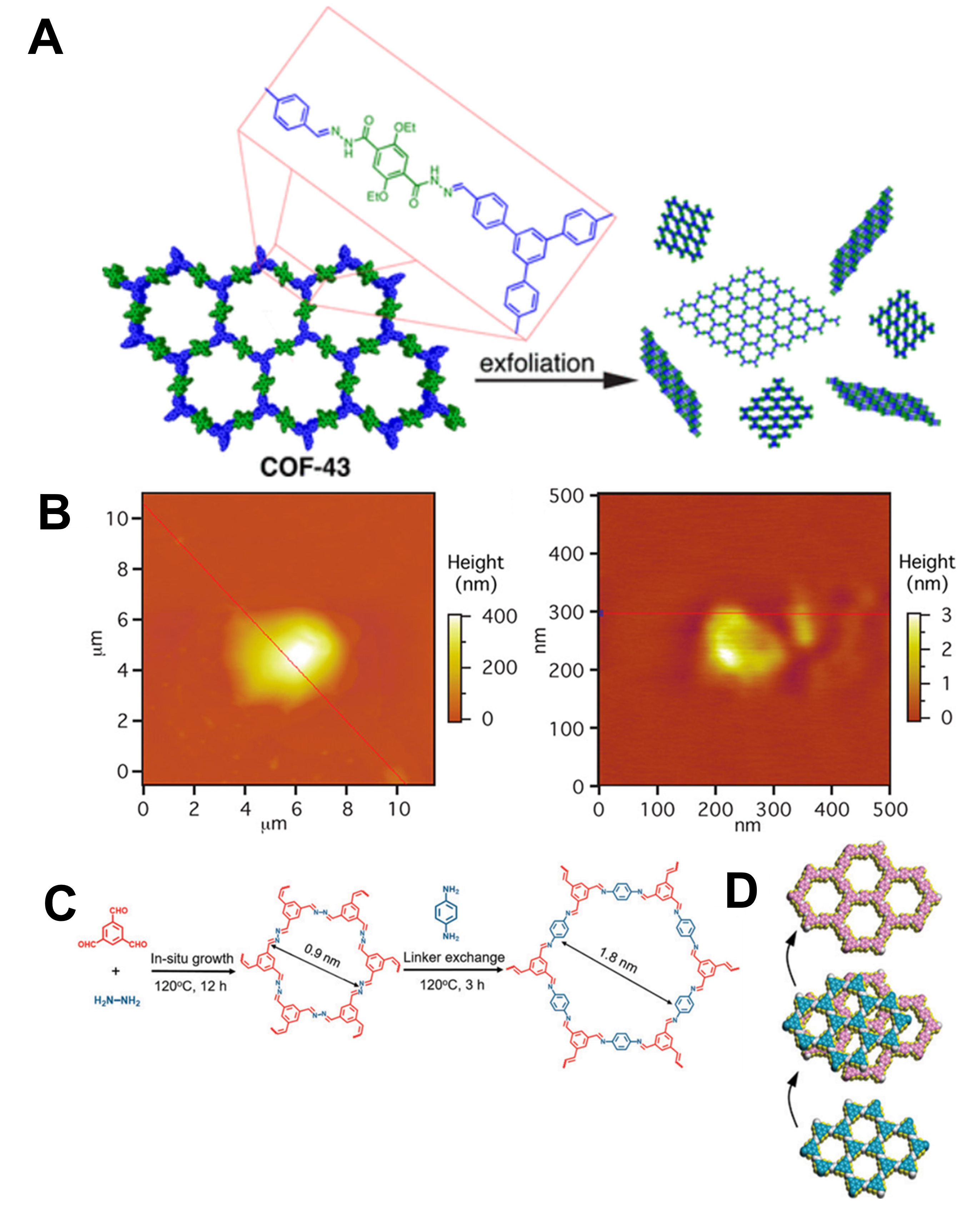
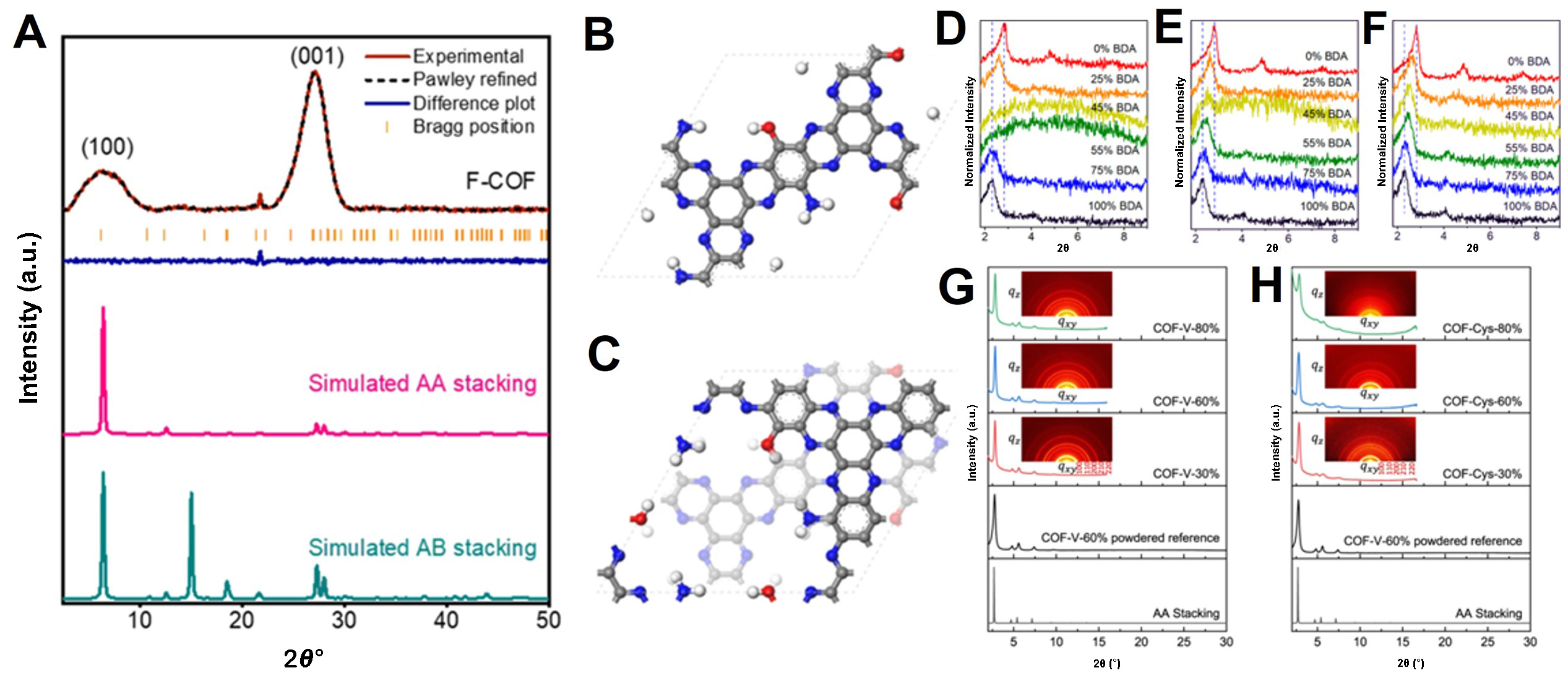


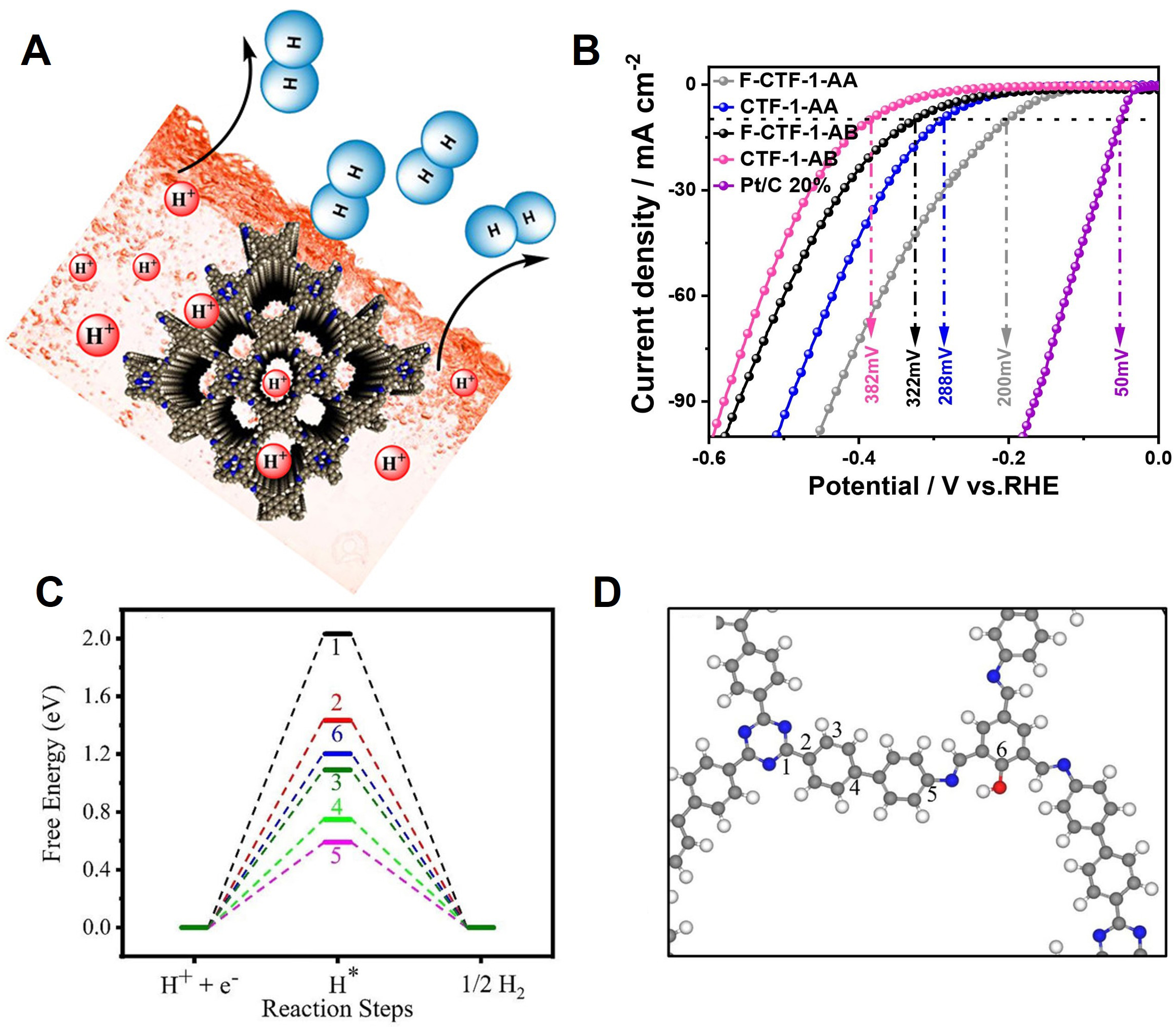

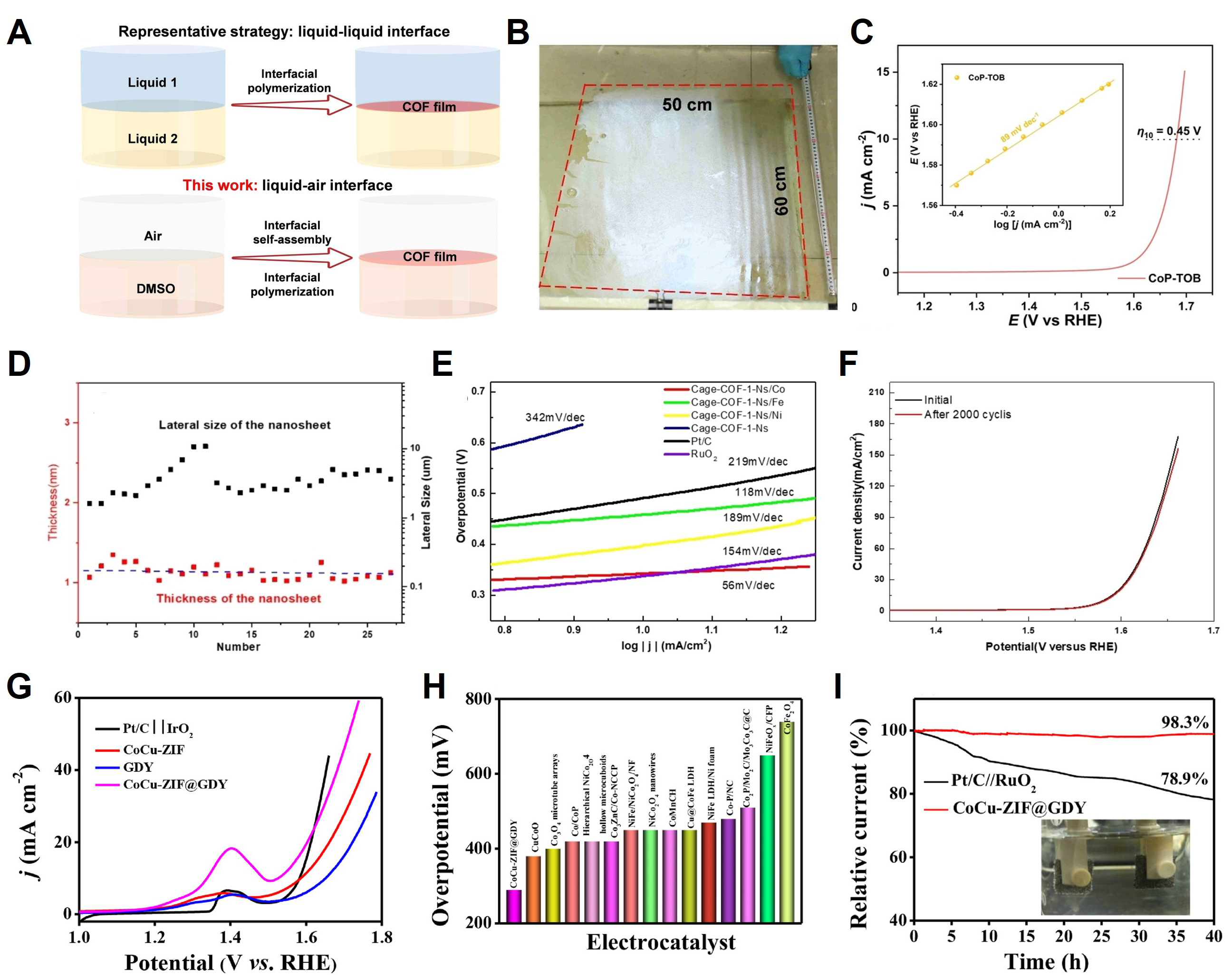
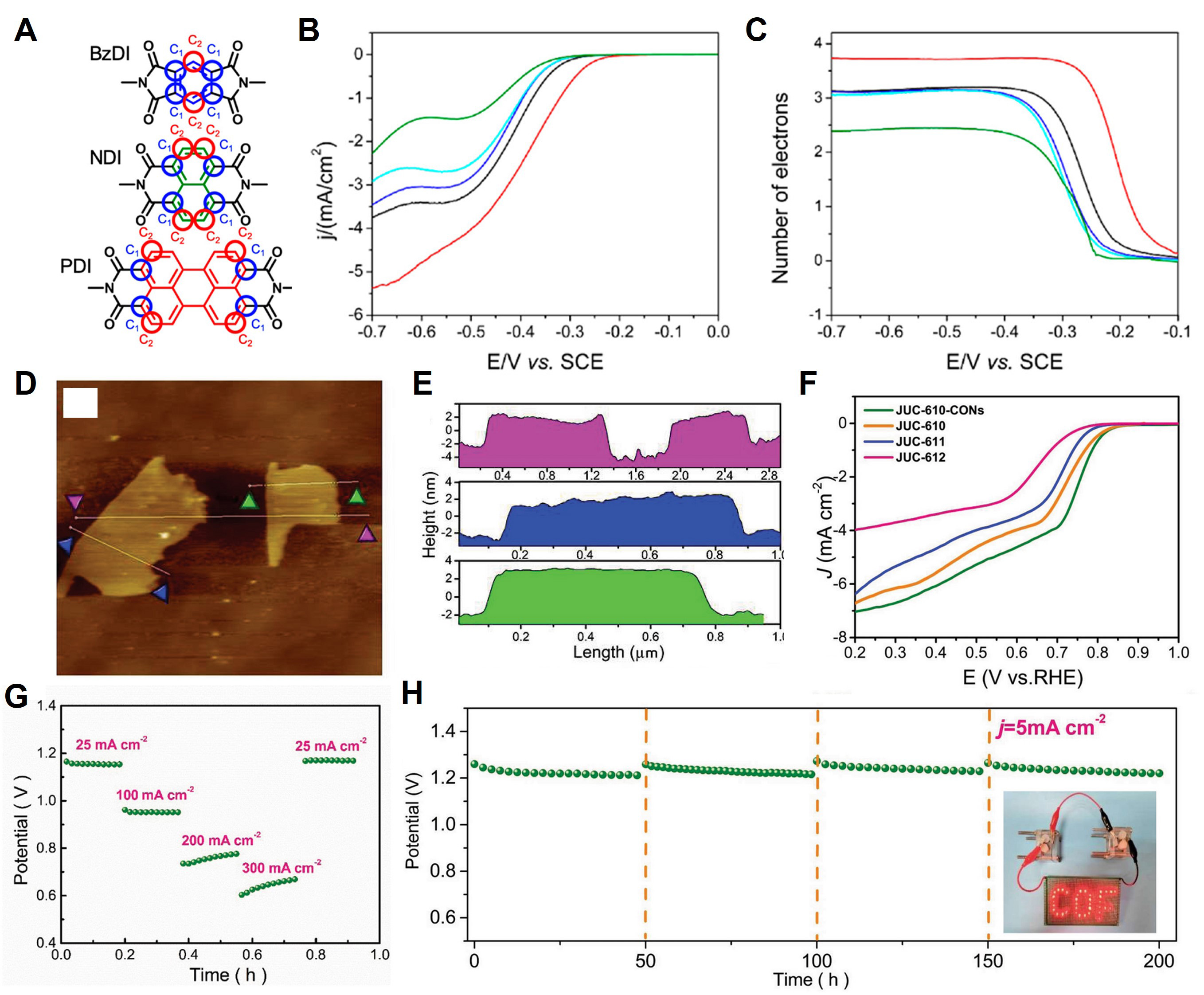

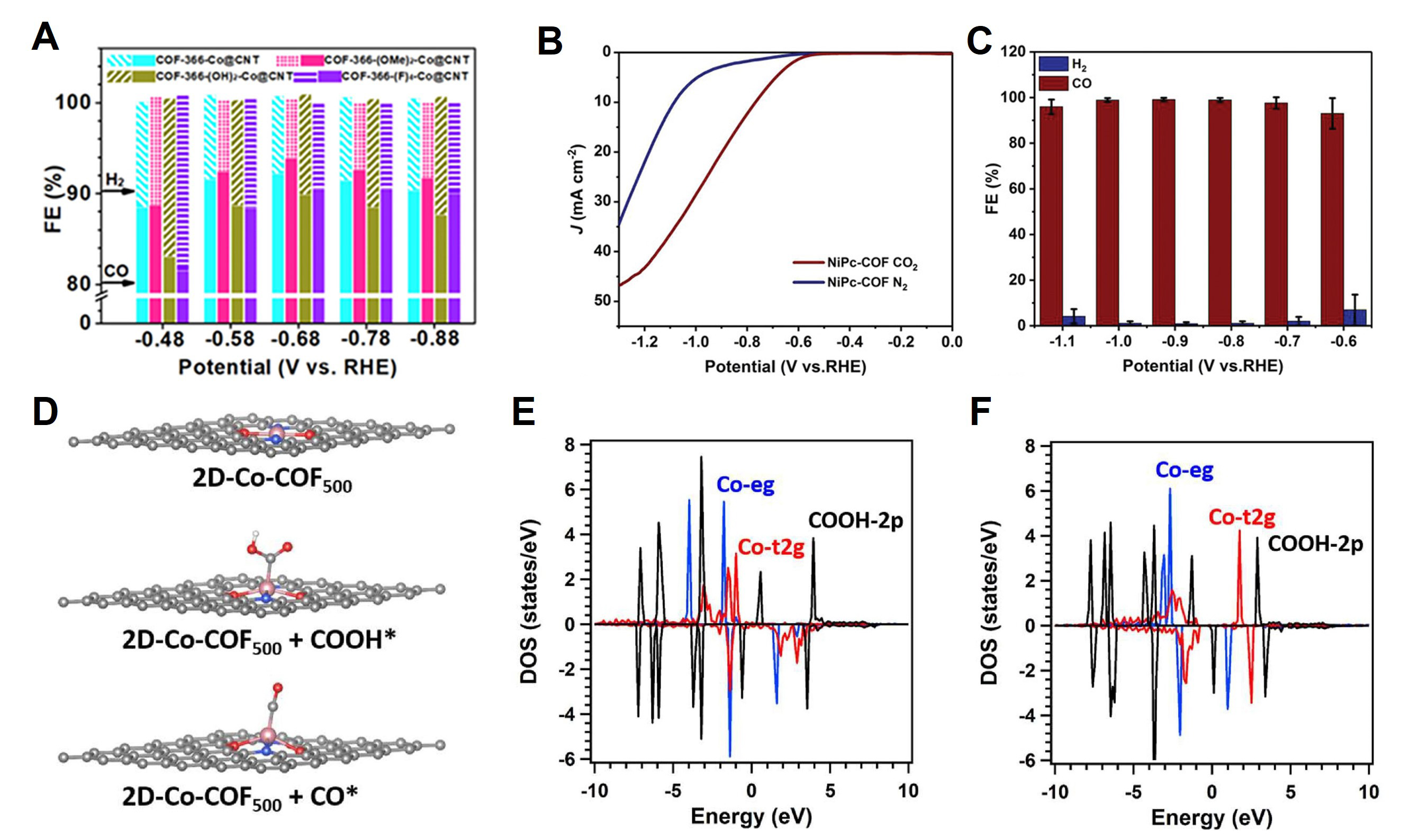











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.