Insights into the design of mildly acidic aqueous electrolytes for improved stability of Zn anode performance in zinc-ion batteries
Abstract
Mildly acidic aqueous zinc (Zn) batteries are promising for large-energy storage but suffer from the irreversibility of Zn metal anodes due to parasitic H2 evolution, Zn corrosion, and dendrite growth. In recent years, increasing efforts have been devoted to overcoming these obstacles by regulating electrolyte structures. In this review, we investigate progress towards mildly acidic aqueous electrolytes for Zn batteries, with special emphasis on how the microstructures (in the bulk phase and on the surface of Zn anodes) affect the performance of Zn anodes. Moreover, effective computational simulations and characterization measurements for the structures of bulk electrolytes and Zn/electrolyte interfaces are discussed, along with perspectives for the direction of further investigations.
Keywords
INTRODUCTION
Zinc (Zn) batteries have been regarded as one of the advanced large-energy storage systems owing to their huge natural abundance, low cost, and high theoretical capacity (820 mAh g-1 and 5,854 mAh cm-3) of metallic Zn[1-3]. In addition, compared with strongly acidic and alkaline electrolytes, using mildly acidic aqueous electrolytes (pH ranging from 3 to 7) contributes to Zn batteries with excellent environmental compatibility and inherent safety[4,5]. However, the major obstacle to the implementation of Zn batteries is the intrinsic thermodynamic instability of Zn metal anodes in mildly acidic aqueous electrolytes, which involves H2 evolution, Zn corrosion, and dendritic growth[6-9]. These undesirable results will degrade battery performance or even result in battery failure.
In recent years, many strategies have been proposed to develop highly stable Zn anode systems, such as surface coating[10-14], host materials design[15], separator alteration[16,17], and electrolyte optimization[18,19]. Among them, the electrolyte optimization strategy is recognized as one of the most viable and effective approaches to improve the electrochemical performance of the Zn anode due to the features of facile preparation and cost effectiveness[20]. In parallel to developing novel electrolytes, research into the influence mechanisms of the structural compositions of electrolytes on the performance of Zn anodes is in full swing. Researchers have realized that the fundamental structures in electrolytes directly determine the bulk and interfacial electrolyte properties and hence the Zn battery performance. Understanding the complicated structures and their correlations with electrolyte properties is of great significance for achieving the rational design of Zn battery electrolytes beyond conventional trial-and-error approaches[21]. Recently, some reviews have introduced electrolytes and their development stages, which help to solve complicated chemical/electrochemical issues of Zn anodes in aqueous media. However, a comprehensive and detailed review of how electrolyte structures (in the bulk phase and at the Zn surface) affect the stability and reversibility of Zn anodes has not been published so far[22].
In this review, we concentrate on investigating the microstructures of mildly acidic aqueous electrolytes and their functional mechanisms in stabilizing Zn anodes. As shown in Figure 1, the principle models and characterization techniques for the structures of bulk electrolytes and Zn/electrolyte interfaces are examined in detail. We also present recent advances in mildly acidic aqueous electrolytes for Zn batteries. The effects of electrolyte compositions, including the salt (with salt concentration), solvent, and additive, are mentioned and critically evaluated. Perspectives on future research directions towards such electrolytes are also outlined. We hope this review can provide a guideline for the rational design of advanced electrolytes and boost the development of this field.
UNDERSTANDING THE MOLECULAR ORIGIN OF THE ENHANCED STABILITY OF WATER IN BULK ELECTROLYTES
Two different water environments in controlling HER
Metallic Zn is a desirable anode material for aqueous batteries because of its high specific capacity, low redox potential [-0.76 V vs. the standard hydrogen electrode (SHE)], low cost, good water compatibility, and intrinsic safety[2,3]. However, in the charging process, except for Zn ion (Zn2+) deposition
Figure 2. Overview of two water environments to interpret the mechanism of HER and the associated corrosion, passivation, and dendrite growth of the Zn anode in mildly acidic aqueous electrolytes. (A) One view suggests that HER originates from solvated water in the Zn2+ solvation sheath. (B) Another view claims that free water (without interaction with Zn2+) is the origin of HER.
Solvated water
HER can be inhibited by regulating the compositions and structures of electrolytes. For example,
Free water
As is known, water consumption occurs not only during the Zn deposition process
Obviously, both of the two explanations only emphasize the influence of the electrolyte local structure on HER, and it is still unclear which mechanism is in control of water activity. Very recently, Yang et al. delved into the origin of HER and suggested that HER primarily originates from solvated water rather than free water[4]. They found that in ZnCl2 electrolytes, an increase in the ZnCl2 concentration will promote H2 evolution. The results of Fourier transform infrared (FTIR) and Raman spectroscopy reveal that the amount of solvated water also increases together with the concentration of ZnCl2. Using the density functional theory (DFT) calculation, the deprotonation energies of free and solvated water were computed at -0.49 and -4.94 eV, respectively, which further underscores that deprotonation of solvated water is more energetically favorable than for the free states. In addition, Chen et al. presented that cation-solvent complexes exhibit much lower lowest unoccupied molecular orbital (LUMO) energy levels compared with pure solvents[30]. In theory, the solvent with a lower LUMO energy level is more easily reduced. Therefore, solvents decompose more easily on metal anodes once they are complexed with metal cations in electrolytes. Zn2+-H2O has a more negative LUMO energy level than H2O (-2.54 vs. 2.46 eV), so solvated water is easier to be reduced than free water[31]. But even then, it still cannot be ruled out the role of free water. In contrast to alkaline and neutral electrolytes, mildly acidic aqueous electrolytes contain a large amount of H+ (the reactant for HER) produced by hydrolysis. A majority of H+ predominantly reside in the H-bond network of free water, and the free water structure has an important impact on their activity. In addition, electrolyte modification usually alters the Zn2+ solvation structure and the H-bond network of free water simultaneously, so a more trustworthy conclusion may require a combination of the two aforementioned mechanisms.
The correlation effects in bulk electrolytes
Understanding the underlying molecular interactions between electrolyte compositions provides a useful framework for guiding the rational design of electrolytes with satisfactory performance. Here, we provided a comprehensive survey of the interactions between each composition (including Zn salt (Zn2+ and anion), water, and additives or cosolvents) in mildly acidic aqueous electrolytes. As shown in Figure 3A, these interactions can be roughly categorized into three types at different levels of detail, including the cation-anion interaction, the interaction between ion and water (cation-water and anion-water interaction), and the interaction of additive/cosolvent with water or Zn salt (additive/cosolvent-water interaction or additive/cosolvent-ion interaction).
Figure 3. The correlation effects in bulk electrolytes. (A) Compositions of mildly acidic aqueous electrolytes and their interactions.
The cation-anion interaction
For ions in solution, there are three aggregation states of ions, including solvent-separated ion pair (SSIP), contact ion pair (CIP), and aggregate (AGG). As shown in Figure 3B, SSIP refers to the ion pairs which are separated by more than one solvent. Each ion in the SSIP state holds its individual solvation shell and is only slightly affected by the counterion. CIP is usually the closest-distance contact ion pair, which is formed through the electrostatic forces between the cation and the anion. The AGG state corresponds to the presence of ions in the form of clusters. Ionic aggregation states are influenced by the ion species, properties of the solvent molecule, and the most influential ion concentrations[32]. Most ion complexes in aqueous solutions are dominated by SSIP when the salt concentration is less than 1 mol L-1 (M). As the salt concentration increases, ions engage in each other’s solvation shells, resulting in CIP. AGG states are mostly found in high-concentration water-in-salt electrolytes, where the solvation shell of cation/anion is entirely surrounded by opposite-charged ions and contains almost no water. The occurrence of AGG has also been observed for specific Zn salt with bulky anions, such as TFSI-[23] and trifluoromethanesulfonate (OTf-)[33].
The ion-water interaction
Salt dissolution can be considered as the process in which cations and anions in an ionic crystal overcome the attraction force between each other, dissociate from the crystal lattice to become gas ions, and then enter the water solution and combine with polar water molecules to form hydrated ions. As shown in Figure 3C, the Zn2+ solvation structure is usually composed of Zn2+ and six water molecules. There are many different kinds of anions (including TFSI-, OTf-, SO42-, Cl-, ClO42-, etc.) being used in Zn batteries. Compared with Zn2+, anions have different solvation structures due to their differences in sizes, shapes, and even charges. As one of the few empirical endeavors toward the anion effects in water, Collins et al. introduced the concepts of kosmotropic and chaotropic solutes: Smaller anions like Cl- are kosmotropes, meaning that they are water structure makers[34]; larger ions like OTf- and TFSI- are chaotropes, which emphasizes that these species have to be considered as water structure breakers[34,35]. This theory well explains the promoted stability of Zn anodes using bulky anionic salts such as Zn(OTf)2[36] and Zn(TFSI)2[23]. These bulky anions, as water structure breakers, strongly disrupt the H-bond network of water, inhibiting HER and stabilizing Zn electrodes.
The interaction of additive/cosolvent with water or Zn salt
Except for Zn salt and water, a common aqueous electrolyte in Zn batteries contains additional compositions in terms of additives or cosolvents. As shown in Figure 3D, the additive/cosolvent can interact with ions, water, and itself in electrolytes. At present, three concepts - chelation, electron acceptor/donor number (AN/DN), and hydrophilic and hydrophobic effect - are offered to describe the impacts of an additive/cosolvent on the remaining species in electrolytes. Chelation is a chemical reaction in which two or more coordination atoms of a multidentate ligand form a chelating ring with a cation. Chelating agents are chemicals containing multidentate ligands, which can bind with cations to product chelates. Chelates usually have high thermodynamic and thermal stability. Inspired by this, the di-2-picolylamine (DPA) suggested by Geng et al. exhibits a strong affinity to Zn2+ and successfully controls random Zn2+ diffusion, realizing highly reversible Zn plating/stripping with satisfactory coulombic efficiency (CE) at high current settings[37]. To categorize solvents with respect to their cation solvation properties, Gutmann et al. developed the so-called DN, which accounts for the electron-donating property of a solvent molecule[38]. Generally, the additives/cosolvents with high DN value are willing to participate in the Zn2+ solvation structure. For example, Cao et al. have proposed that dimethyl sulfoxide (DMSO) is able to replace water in the Zn2+ solvation sheath as it has a greater DN (29.8) than water (18)[39]. AN, which contrasts with DN, explains the electron-withdrawing property of a solvent molecule. It directly reflects a solvent’s propensity to donate protons to create H bonds or salt bridges with anions. As mentioned above, however, there is little research about the anion solvation structure. The function of solvents with high AN values is uncertain. Most additives/cosolvents used in Zn batteries are organic. In light of their interactions with water, they can be roughly divided into hydrophilic and hydrophobic organics. According to the empirical “like-dissolves-like” rule, the hydrophobicity of a molecule is inversely proportional to its polarity, suggesting that a low-polarity molecule exhibits a high hydrophobicity. On the contrary, the molecules containing polar groups have a great affinity to water and exhibit hydrophilicity. Some polar organics, like alcohols[27,40-45], sulfones[39,46], and nitriles[47-49], are widely utilized as additives/cosolvents in Zn batteries. Their introduction can not only maintain the homogeneity of electrolytes but also restrict water activity by breaking the H-bond network of water. Theoretically, hydrophobic solvents such as most esters[31,50] have a strong mutual repulsion with water, which makes them potentially feasible to significantly disrupt the H-bond network of water when hydrophobic-aqueous systems are realized. Besides, once the hydrophobic additive/cosolvent coordinates into the Zn2+ solvation sheath, it would favorably diminish the solvation interaction between solvated water and Zn2+. However, due to the immiscibility of the hydrophobic organic with water, it is intuitive that hydrophobic organics may not meet the demands of the homogeneity of electrolytes and are therefore rarely used as cosolvents in aqueous electrolytes. Fortunately, Miao et al. successfully prepared a series of hydrophobic carbonate-based hybrid aqueous electrolytes by coupling Zn(OTf)2[31]. Their results show that the amphiprotic OTf- anion with hydrophilic -SO3 and hydrophobic -CF3 groups acts as a linker interplaying with both water and carbonate to make them miscible. This work emphasizes how important it is to consider the interactions between different compositions when designing electrolytes.
Although mildly acidic aqueous electrolytes only contain Zn salt, water, and additives/cosolvents, electrolyte microstructures are still intricate. This is primarily caused by the complicated interactions between ions and molecules. These interactions are roughly divided into three types, including the ion-ion, ion-water, and additive/cosolvent-ion (or water) interaction. Different ion-pair states, which can be roughly categorized as SSIP, CIP, and AGG, are present in ion complexes. The occurrence of these states mainly depends on ion concentrations. The proposed concepts of kosmotropic and chaotropic anions well elaborate on and distinguish the effects of different anions on water. The molecular properties of additives/cosolvents, including hydrophilicity and hydrophobicity, chelation, and AN/DN value, have significant influences on electrolyte structural evolution. Although the universality of the effects above is still far from being achieved, using these theories enables us to identify key principles and research guidelines for an enhancement of electrolyte solutions for future Zn battery technologies.
Characterization techniques of bulk electrolyte structures
Computational methodologies
Computational simulations have been widely utilized to understand the principles behind electrolyte modification strategies at the molecular level. According to previous reports, commonly used computational techniques include DFT calculation, molecular dynamics (MD) simulation, high-throughput virtual screening (HTVS), and machine learning (ML) [top of Figure 4]. DFT calculation is primarily used to explore electronic structure properties and interactions between the compositions in electrolytes. For example, the reducibility of chemicals can be discerned by computing the energy of molecular orbitals (MOs) via DFT. According to MO theory[51,52], the lower the LUMO energy level of a molecule, the easier it is to capture electrons and demonstrate reducing activity. In addition, it was reported that Zn2+ can induce a decrease in the LUMO energy of water[53]. Thus, if the LUMO energy of Zn2+-X (X: the modifier in electrolyte regulation) is higher than that of Zn2+-H2O, the modified electrolyte will have enhanced reduction stability. DFT calculation is also able to understand the Zn2+ solvation process. As is known, Zn2+ forms a solvation structure with a hydration layer composed of six water molecules, namely Zn(H2O)62+. As mentioned above, these water molecules are solvated by Zn2+ and are highly reductive. Therefore, to expel water from the Zn2+ solvation sheath, the chemical used for electrolyte modification should be more zincophilic than water; That is, it should have a higher binding energy to Zn2+, so as to construct an altered Zn2+ solvation structure with the lower solvation energy than Zn(H2O)62+[54]. In addition, DFT calculation provides insight into the electronic structure properties of electrolytes. Methods of wave function analysis, including molecular surface electrostatic potential (MSEP) and deformation charge density (DCD), can be used to visually describe the evolution of electrons before and after the interactions between the compositions in the electrolyte. In addition, DFT calculation can also identify molecule shapes, electrophilic and nucleophilic characteristics, and other parameters related to solution chemistry, providing a wealth of theoretical information for electrolyte modification. However, DFT calculation has its own shortcomings: Even though DFT-based molecular dynamics can be used to model the molecular structure of electrolytes, it is only capable of doing so at the atomic scale of 102-105, which is not enough to accurately describe the electrolyte structure[32].
Figure 4. Computational simulations and experimental characterization techniques to understand the bulk electrolyte structure from a microscale perspective. Theoretical calculating methods include DFT, MD, HTVS, and ML. Electronic structure characteristics and interactions between the compositions in electrolytes are primarily investigated using DFT calculations. Electrolyte structures are usually determined by MD simulations. HTVS is employed to hasten the search for optimal electrolyte materials. Building structure-property relationships in electrolytes can be done quickly and affordably using the technique of ML. Experimental characterization techniques include NMR, Raman, FTIR, XAFS, and SANS.
In contrast, MD simulation can deal with a large-scale system of up to 104-105 atoms, for which they are popularly used to identify the electrolyte structure. The spatial distribution of species around Zn2+ can be determined by computing radial distribution functions [RDFs, g(r)] using MD simulations. The result of RDFs can be further used to calculate the coordination number [N(r)] of other electrolyte compositions or even specific elements to Zn2+, thus confirming the Zn2+ solvation structure. In addition, MD simulation can be used to determine the number of H bonds around each water molecule, which implicitly reflects the H-bond network structure of water. The physical properties of solutions, such as density, viscosity, and diffusion coefficient, can all be computed via MD simulations. Note that in MD simulations, the choice and modification of the force field as well as the simulation time should be carefully considered as they significantly influence the computational accuracy.
Recent developments in computer architecture and parallelization have made theoretical screening of electrolytes possible, which saves time and high costs associated with traditional trial-and-error methods. The process of utilizing a combination of high-performance computer architecture and theoretical models to screen through a large chemical database represents the idea of HTVS[55]. The “computational funnel approach” is the sequential scheme that HTVS commonly uses. It can be interpreted as follows: An individual phase of the screening is assigned to each quantity of interest, and unsuitable electrolytes from the candidate pool are eliminated at each step according to a certain criterion, leaving behind a substantially reduced set of electrolytes as candidates for the following step. A sizable and effective database is required prior to the execution of high-throughput screening. Despite the high accuracy of DFT and MD algorithms in predicting material properties, these techniques are computationally expensive and fail to build material databases with millions of molecules. The screening process can be sped up by using ML. By training a reliable model using the current data, the low-cost computational prediction of specific properties of new materials can be made. Combined with HTVS, it enables quick electrolyte design[56]. To the best of our knowledge, however, HTVS and ML have not been performed in developing aqueous electrolytes in Zn batteries, which provides more room for further research in this field.
Experimental characterizations
In addition to theoretical calculations, spectroscopic techniques like nuclear magnetic resonance (NMR), FTIR, and Raman spectroscopy can reveal the structural property of electrolytes [bottom of Figure 4]. NMR is a significant and useful technique for examining the electrolyte structure, which can reveal details about the geometry, chemical environment, and the number of nuclei. Especially for light elements (such as H, O, and C), the strength of the interaction between atoms can be quantitatively determined. For instance, the disruption of the H-bond network of water can be identified by 1H NMR spectra[40]. When the H-bond interaction in water weakens, it will lower the electron density of H atoms in water, leading to the 1H peak shifting to a lower field with a larger chemical shift value. In addition, the evolution of the water H-bond network can be illustrated by 17O NMR[50]. The 17O chemical shift in water will experience a downshift (high field) if the H-bond network of water is broken. This is because breaking the water H-bond network leads to an increase in the electron density on O atoms in water, resulting in a stronger shielding effect to external electric fields.
The aggregation states of water can be illustrated via FTIR as well. In liquid water, water molecules associate with each other by H bonds. In order to reduce the system energy, water molecules tend to form large clusters through strong H bonds, which is represented in FTIR as a redshift of the O-H stretching peak in water. Otherwise, the FTIR peak gradually blueshifts as the H-bond interaction wanes[57]. By analyzing the evolution of the O-H stretching peak in water, the H-bond network structure of water can be identified. According to Gaussian fitting[50], the broad O-H stretching band (3,200-3,600 cm-1) for water is usually divided into three states: (i) “Network water” (~3,205 cm-1) is the water molecules with about four strong H bonds; (ii) “Intermediate water” (~3,410 cm-1) corresponds to the water molecules with distorted H bonds but unable to form a fully connected network; and (iii) “Multimer water” (~3,560 cm-1) refers to the water molecules that are poorly connected to their surroundings and exist as a free monomer, dimer, or trimer. Raman spectroscopy is a complementary partner to FTIR in studying electrolyte structures. FTIR is responsible for the detection of polar bonds, while Raman spectroscopy detects the vibration of non-polar bonds. Raman spectroscopy can identify the aggregation states of ions by performing a deconvolution analysis. In general, the Raman peak of anions gradually shifts to a higher frequency as ion aggregate states from SSIP, CIP to AGG[58].
X-ray absorption fine structure (XAFS) spectroscopy including X-ray absorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS), as a potent technique for characterizing the coordination environment and the atomic site structures of the absorbing center with high sensitivity, can be used to reveal the solvation and electronic structure of Zn2+ in electrolytes[59]. In general, XANES can provide details on the geometry of the coordinating atom and the valence state of the absorbing atom. For instance, in the Zn K-edge XANES spectroscopy, the larger white-line peak energy corresponds to the higher Zn valence state[39]; The strength of the white-line peak reflects the N(r) of Zn2+, where the N(r) gradually declines as the white-line peak intensity increases from large to tiny[60]. The bond length information of the coordination atom can be determined by examining the R space generated by EXAFS[61]. Wavelet transform is a new technique for identifying and visualizing EXAFS atomic contributions. It has the advantage of simultaneously exhibiting the length of the coordination bond and the type of coordination atom, with the peak’s position to the right indicating the bigger atomic number[61]. Small-angle neutron scattering (SANS) measurements are another effective method to understand the electrolyte structure[23]. It has the ability to evaluate different RDFs in solution and validate the intermediate-range electrolyte structures predicted by MD simulations.
In a nutshell, theoretical computation and experimental characterization complement one another by allowing the former to explain the experimental data and the latter to verify computational accuracy. Each calculational or experimental method has specific and irreplaceable strengths, and meanwhile, it has its own limitations. Therefore, multi-method research is indispensable to achieving a full understanding of the electrolyte properties in Zn batteries. In fact, we have not been able to actually observe the electrolyte structure so far. The dynamic evolution of the electrolyte structure has not been directly examined yet. Thus, there are still a lot of challenges and room for advancement in characterization techniques for viewing and understanding the electrolyte structure.
UNDERSTANDING OF ZN/ELECTROLYTE INTERFACES
Interfacial structures and reactions
The Zn/electrolyte interfacial structure is another critical aspect related to the performance of the Zn anode. The composition and behavior of the electrolyte at the Zn surface are completely different from that of the bulk electrolyte[62]. Through physical electrostatic interactions or chemical adsorptions, the Zn electrode captures molecules or ions from the bulk electrolyte to produce an electric double layer (EDL). The structure of EDL determines the ionic solvation and desolvation process, the compositions and properties of solid electrolyte interphase (SEI), Zn2+ transports crossing SEI, and the depositional mode of Zn2+. Figure 5A illustrates the electrochemical deposition process of Zn2+ in the EDL, approximately divided into five
Figure 5. The influence of electrolyte regulation on the Zn/electrolyte interfacial electrochemistry. (A) A schematic representation of the energy storage mechanisms of Zn deposition. (B-D) Three strategies for facilitating the even Zn deposition: (B) the impact of overpotential on the energy barrier of Zn nucleation as well as the size and density of Zn nuclei; (C) the influence of surface texture on the Zn morphology, in which planar and non-dendritic Zn hexagons can be made by adsorbing functional additives/cosolvents; and (D) the even Zn plating triggered by the mechanisms of zincophilicity and electrostatic shielding. (E) A schematic of SEI acting on Zn anodes, including promoting the homogeneous Zn deposition and preventing HER.
Regulation of nucleation process
The electrochemical deposition of Zn begins with the Zn nucleation process. The free energy diagram for the Zn reduction reaction [left of Figure 5B] shows that the formation of Zn metal needs to overcome a nucleation energy barrier[67]. The nucleation barrier can be changed by adjusting the reduction reaction overpotential. The nucleation overpotential is known as the difference between the tip potential and the subsequent stable potential, which represents the thermodynamic cost of creating the initial Zn atomic cluster. Usually, an increase in the nucleation overpotential reduces the Zn nucleation barrier. The nucleation overpotential is also an important factor influencing the nucleation size and the number of nucleation sites: (i) The Zn nucleation size is inversely proportional to the overpotential; and (ii) The number of nucleation sites is positively correlated with the overpotential [right of Figure 5B][68]. As demonstrated in the literature[41,69-71], these two rules only are appropriate for most electrolytes containing organic molecules. For example, by increasing the Zn2+ desolvation energy, some electrolyte additives/cosolvents [e.g., ethylene glycol (EG)[41], tetraglyme (G4)[69], and hexaoxacyclooctadecane
Adjustion of surface texture
The surface morphology of Zn anodes has been reported to be related to the Zn surface texture[76,77]. The texture reflects the desired orientation of the Zn anode, which controls the corrosion resistance of the Zn electrode and the direction of Zn dendrite growth[78]. The growth of Zn dendrite is favorable when the angle between the Zn anode surface and the growth direction of Zn crystal is large[79]. The crystalline orientation affects the crystal growth direction, which in turn influences the surface morphology and dendrite growth direction[77]. Figure 5C (left) depicts the Zn growth direction in different crystalline orientations. When the Zn orientation index is (100) or (110), the angle between the Zn growth direction and the substrate is
Restriction of Zn2+ deposition site
During the Zn nucleation stage, in order to reduce the nuclear energy barrier, Zn2+ diffusing along the Zn anode surface will aggregate together to form Zn nuclei. In the stage of crystal growth, due to the high electric field density on these nuclei sites, the later Zn2+ is attracted to these nuclei, which leads to the Zn uneven deposition and dendrite formation[82]. Regulating Zn2+ migration and uniformizing the Zn surface electric field prevent the uneven Zn deposition, which can be achieved by adsorbing additives/cosolvents on the Zn electrode surface[83-87]. For example, some polymers such as polyacrylamide (PAM)[83] and polyethylene oxide (PEO)[84] are able to adsorb on the Zn surface. The polar functional groups of these polymers have strong attractions to Zn2+ and show zincophilicity, which leads to the homogeneous dispersion of Zn2+ on the surface and achieves the uniform Zn deposition [left of Figure 5D]. In addition to zincophilicity, the uneven Zn deposition can be suppressed through the electrostatic shielding mechanism. The cations (e.g., Na+[85] and tetrabutylammonium ions (TBA+)[86]) which have a lower redox potential than Zn2+ and highly polar organic molecules (e.g., diethyl ether (Et2O)[87]) can act as “electrostatic shielding” to protect Zn anodes from the growth of dendrites. These materials adhere to the initial Zn nuclei and provide a positive electrostatic shield around it. As two like electric charges repel each other, the later Zn2+ will be deposited elsewhere, thereby preventing the Zn growth on the initial tip and inhibiting Zn dendrite formation [right of Figure 5D].
To control Zn dendrite growth, constructing coating functional layers on Zn metal anodes is also an efficient strategy since it is able to guide homogeneous Zn2+ diffusion and shield water and anion to suppress side reactions. The surface coating prevents the Zn dendrite formation can be categorized as electronic regulators, ion regulators (including organic and inorganic ion regulators), and electronic-ionic mixed conductive coatings. Coating electronic materials on the Zn surface is an effective approach to evenly distribute charge to redirect uniform electrodeposition, such as metal nanoparticles (e.g., Au[10] and
Construction of SEI
The interfacial reactions between the electrolyte and the Zn anode trigger the formation of the SEI layer. The SEI layer is electrically insulating but ionically conductive. It is highly permeable to Zn2+ while limiting the penetration of electrolytes. Most SEI layer is made up of an inorganic layer close to the anode from the decomposition of ions and an organic layer exposed to the electrolyte side from the decomposition of organics. The SEI layer plays an important role in stabilizing the electrode/electrolyte interface and determines the battery performance. However, in initial aqueous electrolytes, the products of the reactions between water and Zn metal are ZnO, Zn(OH)2, and alkaline Zn salt, which have discrete structures and cannot be regarded as the SEI. An effective SEI can be formed by optimizing the electrolyte composition, such as by adding organic additives/cosolvents or inorganic additives that comprise C, F, and P[89-92] into the electrolyte. Unlike the artificial SEI layer, which is not self-repairable and gradually loses its protective function, the SEI layer generated by in-situ electrolyte decomposition can continuously regulate the behaviors of electrons and Zn2+ at the Zn/electrolyte interface, thus promoting the uniform Zn deposition[90]. Moreover, the SEI layer can prevent the electrolyte from directly contacting with Zn metal, thus inhibiting interfacial side reactions [Figure 5E][63].
Briefly, the electrolyte structure on the Zn anode surface has a significant impact on the performance of Zn anodes. The side reactions on the Zn anode surface, like dendritic formation, corrosion, and H2 evolution, can be alleviated by modifying the interfacial electrolyte structure. Controlling the Zn nucleation overpotential, adjusting the Zn surface texture, and introducing “electrostatic shielding” and zincophilic additives/cosolvents, can all promote the uniform Zn2+ flux and the electric field distribution at the Zn surface, leading to an even deposition of Zn. By preventing side reactions from directly contacting with water and Zn metal, the in-situ formed SEI layer at the Zn/electrolyte interface can also help stabilize Zn anodes.
Analytical methodologies of interfacial structures
A full understanding of the interfacial electrolyte structure, the Zn deposition behavior, and their internal linkages is essential to guide the rational design of Zn/electrolyte interfaces. In order to qualitatively or quantitatively assess the effectiveness of interface modification brought on by electrolyte design, various characterization methods are utilized. These approaches make it possible to observe the surface morphology evolution of deposited Zn, interfacial compositions and structures, underlying mechanisms, and electrochemical properties.
Morphology
The electron microscope technology allows for the observation of the surface morphology of Zn anodes [Figure 6A]. A camera, for example, gives the general profile of Zn plates. By using in-situ optical microscopy, the processes of Zn deposition and dendrite growth are observed. Optical microscopy with three-dimensional (3D) imaging can not only view the morphological changes of Zn electrodes in real time but also quantitatively measure the Zn plating rates at different locations of 3D structures. The morphology of Zn deposition is effectively examined using scanning electron microscopy (SEM). The SEM images of the Zn surface can estimate the formation and thickness of an SEI layer. Transmission electron microscopy (TEM) is another common tool to visualize the Zn deposition morphology at better resolution as well as its lattice plane spacing. In addition, atomic force microscopy (AFM) has been used to directly monitor the roughness of Zn surface and the Zn nucleation process.
Figure 6. Characterization techniques of Zn/electrolyte interfaces. (A) Information on the Zn morphology at different spatial scales, which can be gathered using a camera, optical microscopies with/without 3D imaging, SEM, TEM, and AFM. Copyright from Ref.[1,3,25,42,43,60]. (B) Characterization approaches for studying Zn/electrolyte interfacial structures and compositions, including Raman, FTIR, NMR, XPS, and XRD. Copyright from Ref.[13,37,39,46]. (C) Theoretical calculations to explore the mechanisms at the interfaces. DFT and MD simulations are used to explore the interfacial property and structure at the atomic scale. It is possible to analyze the electric field density and the Zn2+ flux on the Zn anode surface using multi-physical simulation technologies. Copyright from Ref.[9,27,31]. (D) Electrochemical characterization methods with regards to Zn anodes, including LSV, CV, CA, EDLC, and EIS.
Structure and composition
As shown in Figure 6B, the composition and structure of Zn/electrolyte interfaces can be characterized by various spectroscopic techniques. For instance, Raman and FTIR spectroscopy can identify specific functional groups that are present on Zn surfaces, allowing for inferring interfacial reaction products. Moreover, the Raman mapping is performed to examine the distribution of these products on the Zn surface. The apparent components of the SEI layer can be identified using solid-state NMR technology and X-ray photoelectron spectroscopy (XPS). Especially in the XPS, the internal composition of SEI can be determined as long as prolonging argon ion (Ar+) sputtering time. The X-ray diffraction (XRD) analysis contributes to understanding the structural modifications and material transformations brought on by the corrosion response of Zn metal in contact with electrolytes. The suggested corrosion mechanism is supported by comparing the obtained XRD profile to the powder diffraction files (PDFs) of the Zn corrosion byproducts (e.g., ZnO, Zn(OH)2, and alkaline Zn salt).
Theoretical mechanism
The Zn/electrolyte interfacial structure and property can be examined at the atomic level using theoretical simulations [Figure 6C]. Some physicochemical properties at Zn surfaces can be explored via DFT calculation. For instance, DFT calculation is performed to simulate the Zn2+ diffusion path at SEI, and the resulting diffusion energy barrier is used to measure ion conductivity[88]. In addition, the wettability of Zn metal in electrolytes can be assessed by DFT calculation. The strong binding energy between the adsorbate (which is an electrolyte composition) and the Zn anode allows for the demonstration of the high Zn affinity that the adsorbate exhibits in electrolytes. By using species density profiles from MD simulation, it is possible to determine the distribution of electrolyte compositions perpendicular to Zn anodes, visualizing the Zn/electrolyte interfacial structure. The local current density and the electric field distribution at the Zn electrode surface can be simulated by multi-physical simulations, which contributes to explaining where Zn is initially deposited and further extended during cycling.
Electrochemical properties
The evolution of working ions and electrons during the charge/discharge process can be demonstrated by electrochemical testing [Figure 6D]. Methods for electrochemical measuring of the Zn/electrode interface include linear sweep voltammetry (LSV), cyclic voltammetry (CV), chronoamperometry (CA), electric double-layer capacitance (EDLC), and electrochemical impedance spectroscopy (EIS). Among them, LSV is conducted to investigate the activity of HER that happens at the Zn/electrode interface. The potential corresponding to the current density at 10 mA cm-2 is usually regarded as the onset potential of HER[93]. The lower the onset potential, the better the electrolyte used inhibits HER. Not only can the reversibility of Zn be assessed by CV testing, but it can also roughly estimate the Zn nucleation overpotential. Testing with CA can reveal the Zn diffusion modes. An abrupt decrease in the current density represents the 2D diffusion of Zn2+. This process can easily lead to the formation of dendrites. When the CA curve becomes flat, the Zn2+ transport switches to the 3D diffusion mode, which promotes uniform Zn deposition[67]. The results of EDLC in Zn||Zn symmetrical cells can determine whether the additives/cosolvents have priority over water to adsorb on the Zn surface[83]. In general, when the EDLC value decreases, more additives/cosolvents are absorbed on the Zn surface. Interfacial conductivity can be observed in the EIS spectrum. Moreover, in-situ EIS testing is capable of assessing the stability of SEI and Zn anodes.
In short, the Zn/electrolyte interfacial structure can be characterized using a variety of ways depending on the requirements and goals. An in-depth understanding of the Zn/electrolyte interfacial structure and reaction requires a combination use of the aforementioned methodologies. The Zn/electrolyte interfacial structure is actually very complicated and only can be deduced or indirectly detected by the existing theories and methods so far. In order to visually examine the Zn/electrolyte interfacial structure and its response, it may call for the employment of in-situ techniques and their combinations, or the development of more exact and new characterization instruments.
Characterization challenges for analyzing the structures of bulk electrolytes and Zn/electrolyte interfaces
Although electrolyte modification is an effective strategy to optimize battery performance, there are still some long-standing challenges that hinder the design of high-performance aqueous electrolytes, starting with inadequate characterization techniques [Figure 7]. The traditional FTIR, Raman, and NMR spectra characterization can obtain bulk electrolyte information. However, Raman and FTIR are not sensitive to weak interactions between electrolyte microstructures, leading to incomplete insights into the electrolyte mechanisms. In addition, Raman and FTIR also struggle to decipher the local electrolyte properties, such as the electrolyte compositions at the Zn surface[57]. While NMR can sensitively detect structural variations at the atomic scale, researches still focus on strong interactions between cation and solvent molecules. Anion-solvent and solvent-solvent interactions are often overlooked.
Figure 7. Characterization challenges for studying aqueous electrolytes as well as Zn anode surfaces.
DFT calculation provides insight into the interactions between molecules/ions in the electrolyte. But limited by the power of computers, it is not capable of accurately describing electrolyte structures. Although MD simulation can characterize electrolyte microstructures, the simulation time and the choice of the force field have an impact on the computational accuracy; that is, the simulated electrolyte structure is significantly affected by the anthropogenic factor. Analogously, multi-physical simulation technologies are heavily perturbed by the parameter settings, which should be carefully considered. In addition, the study of HTVS and ML in aqueous electrolytes for Zn batteries is a void at present, which presents opportunities and challenges for researchers.
The morphological information of Zn anodes at different spatial scales can be obtained by cameras, optical microscopes, SEM, and TEM. However, these techniques can only observe the morphology of the Zn surface and the structure under the surface cannot be detected. In addition, some methods for characterizing the surface composition of Zn anodes have their limitations. For example, using tools such as XRD to study interface identification corrosion also has limitations, as peaks of different phases can overlap and accurate identification becomes challenging[86]. In addition, various electrochemical testing techniques are affected by factors such as the experimental environment, the test equipment, battery row materials, and parameter settings, leading to the challenge of ensuring the repeatability of experiments.
THE ADVANCES IN MILDLY ACIDIC AQUEOUS ELECTROLYTES FOR ZN ANODES
In this section, we will systematically discuss recent advances in electrolyte optimization based on each composition of mildly acidic aqueous electrolytes. The advantages and disadvantages of optimization strategies for each electrolyte composition are examined, along with their future prospects. Then, we analyze the functional principles of each electrolyte component modification strategy to improve the performance of Zn anodes. Finally, the research status of mildly acidic aqueous electrolytes on extending the cycling life and improving the utilization of the Zn anode are reviewed.
Salt
Recent advances in mildly acidic aqueous electrolyte modification during the past few years are summarized in Figure 8. Based on the electrolyte compositions, they are classified into three categories: salt (with salt concentration), cosolvents, and additives. As an important component of electrolytes, Zn salt serves as a current carrier in batteries. In addition, since different Zn salts have different solvation structures and compatibility with Zn metal, the type of Zn salt has an important effect on the performance of Zn electrodes. Issues of Zn anodes can be solved by changing the kinds of Zn salt. Zn salt, including ZnSO4[94], Zn(NO3)2[95], ZnCl2[54], Zn(OTf)2[36], Zn(ClO4)2[96], Zn(CH3COO)2 (Zn(OAc)2)[96], Zn(TFSI)2[23], and
Figure 8. Summary of electrolyte modification examples based on the compositions of mildly acidic aqueous electrolytes in Zn batteries[3,17,18,23,25,27,31,33,36,37,39-50,59-61,69-75,83-91,94-97,98-109,112-126,130-167]. Every example is marked with a point formatted in bold, and the number within the square bracket to the upper right of the example corresponds to the reference label. Note that the abbreviation used in the literature is adopted for each substance here. Due to space limitation, the full name of each substance is not provided.
Salt concentration
Previous studies have suggested that altering salt concentrations can change the Zn2+ solvation structure and the SEI composition[23]. Electrolytes with high-concentration salt are known as water-in-salt electrolytes. Anions coordinate with Zn2+ instead of water molecules in such electrolytes[113,114]. With little solvated water, water activity in such electrolytes is extremely low, which inhibits water-induced side reactions and results in an excellent CE for Zn anodes[115]. Besides, such electrolytes induce the formation of SEI composed of the anion decomposition products at the Zn surface during the initial cycling[116,117]. Sheltered by the SEI layer, the Zn anode is protected from corrosion by electrolytes. Water-in-salt electrolytes provide novel routes to highly reversible Zn anodes. However, excessive voltage polarization is a common issue with such electrolytes. The increase in salt concentrations results in decreased ionic conductivity and increased viscosity of the electrolyte, which negatively affects the rate performance of batteries. In addition, such electrolytes are more expensive than dilute electrolytes, which may make them difficult to be applied on a large scale. To address the issues of water-in-salt electrolytes, researchers started experimenting with adding diluents into the electrolyte. The function of diluents is to make the solution less viscous while promoting the formation of a localized high-concentration salt structure in the electrolyte. Thus, the diluents must be water-miscible but not dissolve Zn salt. Because of this property of diluents, it is challenging to explore available diluents. To our best knowledge, solely trimethyl phosphate (TMP)[118] and 1,4-dioxane (1,4-DX)[119] have been used as diluents in Zn batteries so far.
Solvent
In addition to the solute, the solvent in the electrolyte not only provides a residence for current carriers, but also plays a vital role in stabilizing Zn electrodes[25,39,41,50]. Aqueous electrolytes have garnered tremendous attention in Zn batteries due to their inherent benefits, such as affordability, safety, and environmental friendliness. However, due to the high reactivity of water in electrolytes, water-induced side reactions inevitably occur during the Zn plating/striping process, which results in low CEs and poor cycling performance of Zn anodes[2,6]. Due to the relatively high cost and flammability of organic solvents, organic electrolytes have drawn less attention. However, organic electrolytes enable a wide electrochemical stable window and alleviate side reactions faced by aqueous electrolytes. In light of this, combining the merits of aqueous electrolytes and organic electrolytes, researchers have developed organic/aqueous hybrid electrolytes, where the organic solvents are regarded as cosolvents in aqueous electrolytes. Alcohols[27,40-45], ethers[25,69,120,121], esters[50,31,122-124], sulfones[39,46], nitriles[47-49], and amides[125,126] are the currently known organic cosolvents. Abundant carboxyl groups in alcohols can be seen as H-bond acceptors; these carboxyl groups interact with water and break water H-bond networks, thereby reducing the activity of water. Different from the mechanism of inhibiting HER by alcohols, carbonyl groups in ethers have strong affinities to Zn2+, which allows ether molecules to participate in the Zn2+ solvation sheath and inhibit HER by reducing the amount of solvated water. In addition, these ethers have the ability to adsorb on the Zn metal surface, which helps to control random Zn2+ diffusion and prevent the growth of Zn dendrites. Owing to the presence of hydrophobic methyl groups, most ester solvents are not water-soluble. Therefore, it is often necessary to add amphoteric anions, such as OTf- and TFSI-, to make them soluble in water. These hydrophobic ester-based aqueous electrolytes exhibited high inhibition of HER due to their superior ability to disrupt the H-bond network of water. Sulfone solvents (with sulfonyl group), nitrile solvents (with nitrile group), and amide solvents (with acyl group) all contain functional groups that can bind to Zn2+, so they solvate with Zn2+ and exclude water from the Zn2+ solvation sheath, preventing the occurrence of water-induced side reactions. The hydrated eutectic electrolyte, which is another organic/aqueous hybrid electrolyte, has recently been reported. This kind of electrolyte is a derivative of the deep eutectic electrolyte (DEE)[127-129]. The DEE is a eutectic mixture characterized by a solidification temperature lower than that of its individual component. Owing to the numerous appealing properties, e.g., high electrochemical stability, ease of synthesis, and low cost, such an electrolyte has gained significant study interest in Zn batteries. But because of being nonaqueous, they have much lower ionic conductivity and are hence less attractive. Unlike this kind of electrolyte, a hydrated eutectic electrolyte not only maintains most eutectic properties but also has a high level of ionic conductivity as a result of the addition of water[18,130-132]. Water molecules in the electrolyte are mainly confined to the internal interaction network of eutectic solvents by H bonds, so this kind of electrolyte has low water freezing points, enabling it to be used in low-temperature batteries[131]. Overall, although organic/aqueous hybrid electrolytes significantly improve the performance of Zn anodes, there are still safety issues caused by the addition of flammable organic solvents. In addition, most organic solvents are toxic and not environmental-friendly, thus limiting their further development.
Additive
In contrast, adding a small number of additives (with total mass ratios lower than 5 wt%) into aqueous electrolytes has been experimentally developed to enhance battery safety and achieve high CEs of Zn plating/stripping. The additives used in Zn batteries can be divided into inorganic additives[72-75] and organic additives[37,70,71,83,84,87]. Organic additives have better water solubility than inorganic additives and are thus widely studied. Organic additives can be further classified into organic small molecule additives[37,70,87] and organic polymer additives[71,83,84,157,158]. Compared with organic polymer additives, organic small molecule additives have been more intensively studied due to their diversity, simple structure, ease of synthesis, and pro-environment. At present, adding organic small molecule additives into aqueous electrolytes is a promising commercially viable electrolyte modification strategy for Zn batteries.
The mechanisms of different electrolyte compositions
Interest in Zn batteries has been increasing recently, and some inventive electrolyte design strategies have appeared. In order to clearly understand the rationale behind each one, different electrolyte modification strategies are compared using a radar graph [Figure 9] which considers the Zn2+ solvation structure, H-bond network of water, Zn nucleation process, Zn anode surface texture, Zn deposition behavior, and construction of SEI. As depicted in Figure 9A, three mechanisms - constructing an SEI, modifying the Zn2+ solvation structure, and modulating the Zn2+ flux at the Zn/electrolyte interface - are primarily used by the salt modification to impact Zn anodes. There is no instance where the idea of controlling the Zn nucleation process has been used for the salt regulation strategy. The only instance of an effect on the Zn anode through the surface texture is the report about OTf- mentioned above[81]. The impact of the salt concentration on the Zn anode stability is depicted in Figure 9B. It is not difficult to find that the main driver behind modifying the salt concentration strategy to support the Zn stability is a change in the Zn2+ solvation structure. Similarly, the primary mechanism by which organic cosolvents improve the stability of Zn anodes is to reduce water activity [Figure 9C]. Additionally, some organic cosolvents have the propensity to decompose and form SEI layers on the Zn surface, upgrading the stability of Zn metal anodes. The stabilizing mechanism of the additives to Zn anodes, as shown in Figure 9D, involves altering the Zn2+ solvation structure to reduce HER and adsorbing on the Zn anode surface to uniformly distribute Zn2+ flux and the electric field. Some additives also have the ability to produce SEI, which can assist in extending the cycle life of Zn anodes. Due to its modest concentration, it is challenging for additives to impact the H-bond network of water.
Zn cycle life and CE
We sum up the current states of mildly acidic aqueous electrolytes on stabilizing Zn anodes in terms of the Zn cycle life and the CE, as shown in Figure 10. The cycle life of Zn anodes employing Zn||Zn symmetric cells in various mildly acidic aqueous electrolytes is summarized in Figure 10A. We compared the Zn lifespans of these data based on three testing parameters: the plating/stripping current density, a crucial parameter reflecting the battery charge-discharge rate; the areal capacity of Zn plated during each cycle, reflecting the Zn utilization (or depth of discharge, DOD); and the cycling life of Zn. Four takeaways can be seen in Figure 10A. First, two-thirds of Zn||Zn symmetric cells are able to achieve the cycle life of over
Figure 10. (A) Summary of the cycle performance of Zn||Zn symmetric cells in various electrolytes. Three parameters - cycle time
In addition to the cycle life of Zn anodes, another important metric for assessing the Zn anode performance is the Zn plating/stripping CE. In order to attain a high energy density, the Zn mass should be kept to a minimum, which calls for a high CE. The reported CE values for Zn plating/stripping obtained in the above-mentioned electrolyte modifications from various test protocols and cell setups are summarized in Figure 10B. We compare three test parameters for CE, including the cumulative capacity of the Zn plating during short-circuit or excessive resistance formation, which is an important indicator of the battery life, areal capacity, and CE value. Similar to Figure 10A for the cycle life of Zn anodes, the parameters chosen for these CE measurements are highly dispersed and arbitrary. Two-thirds of newly published electrolytes claim CEs > 99% in the literature. However, in terms of the cumulative plated capacity, less than a tenth of the electrolytes can exceed 1 Ah cm-2. To our knowledge, the salt-NH4OAc (point 33)[108] is the record holder for contributing to a cumulative plated capacity of 6.88 Ah cm-2. The additive-gamma butyrolactone (GBL, point 95)[143] helps achieve the highest CE of 99.93% with a cumulative plated capacity of 3.1 Ah cm-2. When contrasting various electrolyte alteration strategies for Zn reversibility, the strategy of modifying salt concentrations also has no advantages, and the results of the other three strategies are difficult to differentiate.
CONCLUSIONS AND OUTLOOK
Large-scale energy storage with Zn batteries has bright futures. However, the side reactions and dendritic growth on the Zn anode surface seriously obstruct the commercialization of Zn batteries. Recently, electrolyte modification has drawn a lot of interest as a potential and efficient remedy for the issues of Zn anodes. In this review, we have a systematic summary of mildly acidic aqueous electrolytes used in Zn batteries. We emphatically discussed the relationship between the electrolyte microstructures and the performance of Zn anodes. The relevant principles and characterization techniques are mentioned and evaluated. Thereafter, recent progress on the manipulation strategy of each composition of mildly acidic aqueous electrolytes for Zn batteries is systematically summarized. Although the Zn anode performance has been improved thanks to the electrolyte modification, further effort is still needed to make progress [Figure 11].
Figure 11. Schematic illustration of the future research on electrolyte design for high-performance Zn anodes.
The structure-function relationship between electrolyte structures and Zn anode performance
So far, most theories about the impact of the electrolyte structure on the Zn anode performance are still in the infancy stage. This is mostly due to the fact that although the electrolyte compositions in mildly acidic aqueous electrolytes are only Zn salt, solvent, and additives/cosolvents, their interactions and microstructures are complicated. It has been confirmed that altering the Zn2+ solvation structure and the water H-bond network affects the probability of HER, but the effects of the Zn2+-outer, anion-solvation, and additive/cosolvent-anion structures are unknown. This results in difficulty in establishing a correlation between the electrolyte structure and the Zn anode performance. Meanwhile, different compositions interact with each other, and a comprehensive consideration of the interactions between different compositions contributes to the design of better electrolytes. In addition, it is well known that due to the influence of the Zn metal anode, the electrolyte structure on the Zn surface is obviously different from that in the bulk phase. However, there are few studies involving the electrolyte structure on the Zn surface. In fact, the interfacial electrolyte structure is worth studying, because it has an important influence on the Zn2+ desolvation process, which in turn affects the Zn nucleation process.
Combinations and developments of characterization techniques
The key to providing guidelines for designing high-performance electrolytes is to understand the electrolyte structure and properties. They can be analyzed using a variety of advanced characterization techniques. The combined computational and experimental study can provide a more in-depth understanding of the influence mechanism of the electrolyte structure on the performance of Zn anodes. However, most characterization approaches can only perform static observation, making it impossible to understand chemical reaction processes in real time. Hence the need for in-situ technologies. Additionally, it is unable to directly observe the electrolyte structure either in the bulk phase or on the Zn anode surface at present. In most cases, the electrolyte structure is only inferred from the signal generated by characterization procedures. Therefore, in order to investigate the relationship between the electrolyte structure and the Zn anode performance, it is important to develop and employ more advanced characterization techniques.
Synergistic impacts for enhancing Zn anode performance
Each electrolyte modification approach has merits and drawbacks. For instance, the Zn anode lifespan can be prolonged without damaging the rate performance of the battery by altering the type of Zn salt in electrolytes. However, this electrolyte regulation strategy is pricey. Particularly for the strategy to increase salt concentrations, the cost increases significantly. Although adding organic cosolvents to electrolytes saves cost, doing so will decrease ionic conductivity and increase voltage polarization. Moreover, the majority of organic solvents are flammable, which presents security gaps for the battery. Currently, using additives to stabilize Zn anodes appears to be the most promising approach. However, there is no clear advice on the selection and design of additives. We speculate that the strategy of integrating the modification mechanism of each electrolyte composition is a possible way to design optimal additives. In addition, the present approaches for testing the Zn cycle life and CE have yet to be standardized, which poses an obstacle to comparing the effectiveness of different electrolyte modification strategies. Thus, it is urgent to develop universal testing standards.
DECLARATIONS
Authors’ contributionsConceptualization, literature search, writing - original draft: Miao L
Discussion, writing - review and editing: Guo Z
Conceptualization, writing - review and editing, funding acquisition, supervision: Jiao L
Availability of data and materialsNot applicable.
Financial support and sponsorshipThis work was financially supported by the National Natural Science Foundation of China (52025013, 52071184, 52171228), the 111 Project (B12015), and the Fundamental Research Funds for the Central Universities.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
REFERENCES
1. Feng G, Guo J, Tian H, et al. Probe the localized electrochemical environment effects and electrode reaction dynamics for metal batteries using in situ 3D microscopy. Adv Energy Mater 2022;12:2103484.
2. Han D, Cui C, Zhang K, et al. A non-flammable hydrous organic electrolyte for sustainable zinc batteries. Nat Sustain 2022;5:205-13.
3. Cao L, Li D, Pollard T, et al. Fluorinated interphase enables reversible aqueous zinc battery chemistries. Nat Nanotechnol 2021;16:902-10.
4. Yang F, Yuwono JA, Hao J, et al. Understanding H2 evolution electrochemistry to minimize solvated water impact on zinc-anode performance. Adv Mater 2022;34:e2206754.
5. Fan W, Sun Z, Yuan Y, et al. High cycle stability of Zn anodes boosted by an artificial electronic-ionic mixed conductor coating layer. J Mater Chem A 2022;10:7645-52.
6. Yang H, Chang Z, Qiao Y, et al. Constructing a super-saturated electrolyte front surface for stable rechargeable aqueous zinc batteries. Angew Chem Int Ed 2020;59:9377-81.
7. Kang L, Cui M, Jiang F, et al. Nanoporous CaCO3 coatings enabled uniform Zn stripping/plating for long-life zinc rechargeable aqueous batteries. Adv Energy Mater 2018;8:1801090.
8. Zhao K, Wang C, Yu Y, et al. Ultrathin surface coating enables stabilized zinc metal anode. Adv Mater Interfaces 2018;5:1800848.
9. Sun P, Ma L, Zhou W, et al. Simultaneous regulation on solvation shell and electrode interface for dendrite-free Zn ion batteries achieved by a low-cost glucose additive. Angew Chem Int Ed 2021;60:18247-55.
10. Cui M, Xiao Y, Kang L, et al. Quasi-isolated Au particles as heterogeneous seeds to guide uniform Zn deposition for aqueous zinc-ion batteries. ACS Appl Energy Mater 2019;2:6490-6.
11. Cui J, Li Z, Xu A, Li J, Shao M. Confinement of zinc salt in ultrathin heterogeneous film to stabilize zinc metal anode. Small 2021;17:e2100722.
12. Shen C, Li X, Li N, et al. Graphene-boosted, high-performance aqueous Zn-ion battery. ACS Appl Mater Interfaces 2018;10:25446-53.
13. Xin W, Miao L, Zhang L, Peng H, Yan Z, Zhu Z. Turning the byproduct Zn4(OH)6SO4xH2O into a uniform solid electrolyte interphase to stabilize aqueous Zn anode. ACS Mater Lett 2021;3:1819-25.
14. Chen P, Yuan X, Xia Y, et al. An artificial polyacrylonitrile coating layer confining zinc dendrite growth for highly reversible aqueous zinc-based batteries. Adv Sci 2021;8:e2100309.
16. Wu J, Yuan C, Li T, Yuan Z, Zhang H, Li X. Dendrite-free zinc-based battery with high areal capacity via the region-induced deposition effect of turing membrane. J Am Chem Soc 2021;143:13135-44.
17. Dai Y, Li J, Chen L, et al. Generating H+ in catholyte and OH- in anolyte: an approach to improve the stability of aqueous zinc-ion batteries. ACS Energy Lett 2021;6:684-6.
18. Yang W, Du X, Zhao J, et al. Hydrated eutectic electrolytes with ligand-oriented solvation shells for long-cycling zinc-organic batteries. Joule 2020;4:1557-74.
19. Zeng X, Hao J, Wang Z, Mao J, Guo Z. Recent progress and perspectives on aqueous Zn-based rechargeable batteries with mild aqueous electrolytes. Energy Stor Mater 2019;20:410-37.
20. Lv Y, Xiao Y, Ma L, Zhi C, Chen S. Recent advances in electrolytes for “beyond aqueous” zinc-ion batteries. Adv Mater 2022;34:e2106409.
21. Geng Y, Pan L, Peng Z, et al. Electrolyte additive engineering for aqueous Zn ion batteries. Energy Stor Mater 2022;51:733-55.
22. Cheng H, Sun Q, Li L, et al. Emerging era of electrolyte solvation structure and interfacial model in batteries. ACS Energy Lett 2022;7:490-513.
23. Wang F, Borodin O, Gao T, et al. Highly reversible zinc metal anode for aqueous batteries. Nat Mater 2018;17:543-9.
24. Xie J, Liang Z, Lu YC. Molecular crowding electrolytes for high-voltage aqueous batteries. Nat Mater 2020;19:1006-11.
25. Cui J, Liu X, Xie Y, et al. Improved electrochemical reversibility of Zn plating/stripping: a promising approach to suppress water-induced issues through the formation of H-bonding. Mater Today Energy 2020;18:100563.
26. Du H, Wang K, Sun T, et al. Improving zinc anode reversibility by hydrogen bond in hybrid aqueous electrolyte. Chem Eng J 2022;427:131705.
27. Sun Y, Xu Z, Xu X, et al. Low-cost and long-life Zn/Prussian blue battery using a water-in-ethanol electrolyte with a normal salt concentration. Energy Stor Mater 2022;48:192-204.
28. Su Z, Chen J, Stansby J, et al. Hydrogen-bond disrupting electrolytes for fast and stable proton batteries. Small 2022;18:e2201449.
29. Miyake T, Rolandi M. Grotthuss mechanisms: from proton transport in proton wires to bioprotonic devices. J Phys Condens Matter 2016;28:023001.
30. Chen X, Li HR, Shen X, Zhang Q. The origin of the reduced reductive stability of ion-solvent complexes on alkali and alkaline earth metal anodes. Angew Chem Int Ed 2018;57:16643-7.
31. Miao L, Wang R, Di S, et al. Aqueous electrolytes with hydrophobic organic cosolvents for stabilizing zinc metal anodes. ACS Nano 2022;16:9667-78.
32. Yamada Y, Wang J, Ko S, Watanabe E, Yamada A. Advances and issues in developing salt-concentrated battery electrolytes. Nat Energy 2019;4:269-80.
33. Li C, Shyamsunder A, Hoane AG, et al. Highly reversible Zn anode with a practical areal capacity enabled by a sustainable electrolyte and superacid interfacial chemistry. Joule 2022;6:1103-20.
34. Collins KD, Washabaugh MW. The hofmeister effect and the behaviour of water at interfaces. Q Rev Biophys 1985;18:323-422.
35. Kim W, Kim H, Lee KM, et al. Demixing the miscible liquids: toward biphasic battery electrolytes based on the kosmotropic effect. Energy Environ Sci 2022;15:5217-28.
36. Zhang N, Cheng F, Liu Y, et al. Cation-deficient spinel ZnMn2O4 cathode in Zn(CF3SO3)2 electrolyte for rechargeable aqueous Zn-ion battery. J Am Chem Soc 2016;138:12894-901.
37. Geng Y, Miao L, Yan Z, et al. Super-zincophilic additive induced interphase modulation enables long-life Zn anodes at high current density and areal capacity. J Mater Chem A 2022;10:10132-8.
38. Gutmann V. Empirical parameters for donor and acceptor properties of solvents. Electrochim Acta 1976;21:661-70.
39. Cao L, Li D, Hu E, et al. Solvation structure design for aqueous Zn metal batteries. J Am Chem Soc 2020;142:21404-9.
40. Hao J, Yuan L, Ye C, et al. Boosting zinc electrode reversibility in aqueous electrolytes by using low-cost antisolvents. Angew Chem Int Ed 2021;60:7366-75.
41. Chang N, Li T, Li R, et al. An aqueous hybrid electrolyte for low-temperature zinc-based energy storage devices. Energy Environ Sci 2020;13:3527-35.
42. Shang Y, Kumar P, Musso T, et al. Long-life Zn anode enabled by low volume concentration of a benign electrolyte additive. Adv Funct Mater 2022;32:2200606.
43. Ma Q, Gao R, Liu Y, et al. Regulation of outer solvation shell toward superior low-temperature aqueous zinc-ion batteries. Adv Mater 2022;34:e2207344.
44. Wang N, Zhang Y, Yuan J, et al. A synergistic strategy of organic molecules introduced a high Zn2+ flux solid electrolyte interphase for stable aqueous zinc-ion batteries. ACS Appl Mater Interfaces 2022;14:48081-90.
45. Wu Y, Zhu Z, Shen D, et al. Electrolyte engineering enables stable Zn-ion deposition for long-cycling life aqueous Zn-ion batteries. Energy Stor Mater 2022;45:1084-91.
46. Zhao X, Zhang X, Dong N, et al. Advanced buffering acidic aqueous electrolytes for ultra-long life aqueous zinc-ion batteries. Small 2022;18:e2200742.
47. Meng C, He W, Kong Z, et al. Multifunctional water-organic hybrid electrolyte for rechargeable zinc ions batteries. Chem Eng J 2022;450:138265.
48. Yao M, Pan R, Ren Y, et al. Regulating solvation shells and interfacial chemistry in zinc-ion batteries using glutaronitrile based electrolyte. J Mater Chem A 2022;10:14345-54.
49. Reber D, Borodin O, Becker M, et al. Water/ionic liquid/succinonitrile hybrid electrolytes for aqueous batteries. Adv Funct Mater 2022;32:2112138.
50. Dong Y, Miao L, Ma G, et al. Non-concentrated aqueous electrolytes with organic solvent additives for stable zinc batteries. Chem Sci 2021;12:5843-52.
51. Kuhn A, von Eschwege KG, Conradie J. Reduction potentials of para-substituted nitrobenzenes-an infrared, nuclear magnetic resonance, and density functional theory study. J Phys Org Chem 2012;25:58-68.
52. Miao L, Liu L, Shang Z, et al. The structure-electrochemical property relationship of quinone electrodes for lithium-ion batteries. Phys Chem Chem Phys 2018;20:13478-84.
53. Di S, Miao L, Wang Y, et al. Dual-anion-coordinated solvation sheath for stable aqueous zinc batteries. J Power Sources 2022;535:231452.
54. Ji X. A perspective of ZnCl2 electrolytes: the physical and electrochemical properties. eScience 2021;1:99-107.
55. Benayad A, Diddens D, Heuer A, et al. High-throughput experimentation and computational freeway lanes for accelerated battery electrolyte and interface development research. Adv Energy Mater 2022;12:2102678.
56. Chen X, Zhang Q. Atomic insights into the fundamental interactions in lithium battery electrolytes. ACC Chem Res 2020;53:1992-2002.
57. Tian Z, Zou Y, Liu G, et al. Electrolyte solvation structure design for sodium ion batteries. Adv Sci 2022;9:e2201207.
58. Suo L, Borodin O, Wang Y, et al. “Water-in-Salt” electrolyte makes aqueous sodium-ion battery safe, green, and long-lasting. Adv Energy Mater 2017;7:1701189.
59. Wang N, Zhai S, Ma Y, et al. Tridentate citrate chelation towards stable fiber zinc-polypyrrole battery with hybrid mechanism. Energy Stor Mater 2021;43:585-94.
60. Zhang Q, Ma Y, Lu Y, et al. Designing anion-type water-free Zn2+ solvation structure for robust Zn metal anode. Angew Chem Int Ed 2021;60:23357-64.
61. Zhang Q, Ma Y, Lu Y, et al. Halogenated Zn2+ solvation structure for reversible Zn metal batteries. J Am Chem Soc 2022;144:18435-43.
62. Guan J, Shao L, Yu L, et al. Two-dimensional Mg0.2V2O5·nH2O nanobelts derived from V4C3 MXenes for highly stable aqueous zinc ion batteries. Chem Eng J 2022;443:136502.
63. Wang D, Li Q, Zhao Y, et al. Insight on organic molecules in aqueous Zn-ion batteries with an emphasis on the Zn anode regulation. Adv Energy Mater 2022;12:2102707.
64. Xing Z, Huang C, Hu Z. Advances and strategies in electrolyte regulation for aqueous zinc-based batteries. Coord Chem Rev 2022;452:214299.
65. Li C, Wang L, Zhang J, et al. Roadmap on the protective strategies of zinc anodes in aqueous electrolyte. Energy Stor Mater 2022;44:104-35.
66. Zhou M, Chen Y, Fang G, Liang S. Electrolyte/electrode interfacial electrochemical behaviors and optimization strategies in aqueous zinc-ion batteries. Energy Stor Mater 2022;45:618-46.
67. Zhang Q, Luan J, Tang Y, Ji X, Wang H. Interfacial design of dendrite-free zinc anodes for aqueous zinc-ion batteries. Angew Chem Int Ed 2020;59:13180-91.
68. Pei A, Zheng G, Shi F, Li Y, Cui Y. Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett 2017;17:1132-9.
69. Miao L, Wang R, Xin W, et al. Three-functional ether-based co-solvents for suppressing water-induced parasitic reactions in aqueous Zn-ion batteries. Energy Stor Mater 2022;49:445-53.
70. Li R, Li M, Chao Y, et al. Hexaoxacyclooctadecane induced interfacial engineering to achieve dendrite-free Zn ion batteries. Energy Stor Mater 2022;46:605-12.
71. Jin S, Zhang D, Sharma A, et al. Stabilizing zinc electrodeposition in a battery anode by controlling crystal growth. Small 2021;17:e2101798.
72. Abdulla J, Cao J, Zhang D, et al. Elimination of zinc dendrites by graphene oxide electrolyte additive for zinc-ion batteries. ACS Appl Energy Mater 2021;4:4602-9.
73. Sun C, Wu C, Gu X, Wang C, Wang Q. Interface engineering via Ti3C2Tx MXene electrolyte additive toward dendrite-free zinc deposition. Nano-Micro Lett 2021;13:89.
74. Cao J, Zhang D, Yue Y, et al. Regulating solvation structure to stabilize zinc anode by fastening the free water molecules with an inorganic colloidal electrolyte. Nano Energy 2022;93:106839.
75. Huang C, Zhao X, Hao Y, et al. Self-healing SeO2 additives enable zinc metal reversibility in aqueous ZnSO4 electrolytes. Adv Funct Mater 2022;32:2112091.
76. Zhang Y, Han X, Liu R, et al. Manipulating the zinc deposition behavior in hexagonal patterns at the preferential Zn (100) crystal plane to construct surficial dendrite-free zinc metal anode. Small 2022;18:e2105978.
77. Zheng J, Deng Y, Yin J, et al. Textured electrodes: manipulating built-in crystallographic heterogeneity of metal electrodes via severe plastic deformation. Adv Mater 2022;34:e2106867.
78. Cao J, Zhang D, Gu C, et al. Manipulating crystallographic orientation of zinc deposition for dendrite-free zinc ion batteries. Adv Energy Mater 2021;11:2101299.
79. Guo S, Qin L, Zhang T, et al. Fundamentals and perspectives of electrolyte additives for aqueous zinc-ion batteries. Energy Stor Mater 2021;34:545-62.
80. Zheng J, Zhao Q, Tang T, et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 2019;366:645-8.
81. Yuan D, Zhao J, Ren H, et al. Anion texturing towards dendrite-free Zn anode for aqueous rechargeable batteries. Angew Chem Int Ed 2021;60:7213-9.
82. Yufit V, Tariq F, Eastwood DS, et al. Operando visualization and multi-scale tomography studies of dendrite formation and dissolution in zinc batteries. Joule 2019;3:485-502.
83. Yan M, Dong N, Zhao X, Sun Y, Pan H. Tailoring the stability and kinetics of Zn anodes through trace organic polymer additives in dilute aqueous electrolyte. ACS Energy Lett 2021;6:3236-43.
84. Yan M, Xu C, Sun Y, Pan H, Li H. Manipulating Zn anode reactions through salt anion involving hydrogen bonding network in aqueous electrolytes with PEO additive. Nano Energy 2021;82:105739.
85. Wan F, Zhang L, Dai X, Wang X, Niu Z, Chen J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat Commun 2018;9:1656.
86. Bayaguud A, Luo X, Fu Y, Zhu C. Cationic surfactant-type electrolyte additive enables three-dimensional dendrite-free zinc anode for stable zinc-ion batteries. ACS Energy Lett 2020;5:3012-20.
87. Xu W, Zhao K, Huo W, et al. Diethyl ether as self-healing electrolyte additive enabled long-life rechargeable aqueous zinc ion batteries. Nano Energy 2019;62:275-81.
88. Liu X, Yang F, Xu W, Zeng Y, He J, Lu X. Zeolitic imidazolate frameworks as Zn2+ modulation layers to enable dendrite-free Zn anodes. Adv Sci 2020;7:2002173.
89. Li D, Cao L, Deng T, Liu S, Wang C. Design of a solid electrolyte interphase for aqueous Zn batteries. Angew Chem Int Ed 2021;60:13035-41.
90. An Y, Tian Y, Zhang K, et al. Stable aqueous anode-free zinc batteries enabled by interfacial engineering. Adv Funct Mater 2021;31:2101886.
91. Cao L, Li D, Soto FA, et al. Highly reversible aqueous zinc batteries enabled by zincophilic-zincophobic interfacial layers and interrupted hydrogen-bond electrolytes. Angew Chem Int Ed 2021;60:18845-51.
92. Zeng X, Mao J, Hao J, et al. Electrolyte design for
93. Wei C, Xu ZJ. The comprehensive understanding of 10 mA cmgeo-2 as an evaluation parameter for electrochemical water splitting. Small Methods 2018;2:1800168.
94. Huang J, Guo Z, Ma Y, Bin D, Wang Y, Xia Y. Recent progress of rechargeable batteries using mild aqueous electrolytes. Small Methods 2019;3:1800272.
95. Kasiri G, Trócoli R, Bani Hashemi A, La Mantia F. An electrochemical investigation of the aging of copper hexacyanoferrate during the operation in zinc-ion batteries. Electrochim Acta 2016;222:74-83.
96. Wang L, Zhang Y, Hu H, et al. A Zn(ClO4)2 electrolyte enabling long-life zinc metal electrodes for rechargeable aqueous zinc batteries. ACS Appl Mater Interfaces 2019;11:42000-5.
97. Chen Z, Chen H, Che Y, et al. Arginine cations inhibiting charge accumulation of dendrites and boosting Zn metal reversibility in aqueous rechargeable batteries. ACS Sustain Chem Eng 2021;9:6855-63.
98. Li Y, Wu P, Zhong W, et al. A progressive nucleation mechanism enables stable zinc stripping-plating behavior. Energy Environ Sci 2021;14:5563-71.
99. Guan K, Tao L, Yang R, et al. Anti-corrosion for reversible zinc anode via a hydrophobic interface in aqueous zinc batteries. Adv Energy Mater 2022;12:2103557.
100. Nie X, Miao L, Yuan W, et al. Cholinium cations enable highly compact and dendrite-free Zn metal anodes in aqueous electrolytes. Adv Funct Mater 2022;32:2203905.
101. Wang P, Xie X, Xing Z, et al. Mechanistic insights of Mg2+-electrolyte additive for high-energy and long-life zinc-ion hybrid capacitors. Adv Energy Mater 2021;11:2101158.
102. Kim M, Shin SJ, Lee J, et al. Cationic additive with a rigid solvation shell for high-performance zinc ion batteries. Angew Chem Int Ed 2022;61:e202211589.
103. Ma L, Pollard TP, Zhang Y, et al. Functionalized phosphonium cations enable zinc metal reversibility in aqueous electrolytes. Angew Chem Int Ed 2021;60:12438-45.
104. Liu S, Shang W, Yang Y, et al. Effects of I3- electrolyte additive on the electrochemical performance of Zn anodes and Zn/MnO2 batteries. Batteries Supercaps 2022;5:e202100221.
105. Chen H, Dai C, Xiao F, et al. Reunderstanding the reaction mechanism of aqueous Zn-Mn batteries with sulfate electrolytes: role of the zinc sulfate hydroxide. Adv Mater 2022;34:e2109092.
106. Yang M, Driscoll DM, Balasubramanian M, Liao C. Solvation structure and electrochemical properties of a new weakly coordinating aluminate salt as a nonaqueous electrolyte for zinc batteries. J Electrochem Soc 2020;167:160529.
107. Zhao R, Wang H, Du H, et al. Lanthanum nitrate as aqueous electrolyte additive for favourable zinc metal electrodeposition. Nat Commun 2022;13:3252.
108. Lin C, Yang X, Xiong P, et al. High-rate, large capacity, and long life dendrite-free Zn metal anode enabled by trifunctional electrolyte additive with a wide temperature range. Adv Sci 2022;9:e2201433.
109. Wang S, Li T, Yin Y, Chang N, Zhang H, Li X. High-energy-density aqueous zinc-based hybrid supercapacitor-battery with uniform zinc deposition achieved by multifunctional decoupled additive. Nano Energy 2022;96:107120.
110. Guo H, Shao Z, Zhang Y, et al. Electrolyte additives inhibit the surface reaction of aqueous sodium/zinc battery. J Colloid Interface Sci 2022;608:1481-8.
111. Zhou W, Chen M, Tian Q, et al. Stabilizing zinc deposition with sodium lignosulfonate as an electrolyte additive to improve the life span of aqueous zinc-ion batteries. J Colloid Interface Sci 2021;601:486-94.
112. Cao H, Huang X, Liu Y, et al. An efficient electrolyte additive of tetramethylammonium sulfate hydrate for dendritic-free zinc anode for aqueous zinc-ion batteries. J Colloid Interface Sci 2022;627:367-74.
113. Han J, Mariani A, Varzi A, Passerini S. Green and low-cost acetate-based electrolytes for the highly reversible zinc anode. J Power Sources 2021;485:229329.
114. Song X, He H, Aboonasr Shiraz MH, Zhu H, Khosrozadeh A, Liu J. Enhanced reversibility and electrochemical window of Zn-ion batteries with an acetonitrile/water-in-salt electrolyte. Chem Commun 2021;57:1246-9.
115. Zhong X, Wang F, Ding Y, Duan L, Shi F, Wang C. Water-in-salt electrolyte Zn/LiFePO4 batteries. J Electroanal Chem 2020;867:114193.
116. Zhu Y, Yin J, Zheng X, et al. Concentrated dual-cation electrolyte strategy for aqueous zinc-ion batteries. Energy Environ Sci 2021;14:4463-73.
117. Clarisza A, Bezabh HK, Jiang SK, et al. Highly concentrated salt electrolyte for a highly stable aqueous dual-ion zinc battery. ACS Appl Mater Interfaces 2022;14:36644-55.
118. Wang W, Yang C, Chi X, Liu J, Wen B, Liu Y. Ultralow-water-activity electrolyte endows vanadium-based zinc-ion batteries with durable lifespan exceeding 30,000 cycles. Energy Stor Mater 2022;53:774-82.
119. Chen S, Nian Q, Zheng L, et al. Highly reversible aqueous zinc metal batteries enabled by fluorinated interphases in localized high concentration electrolytes. J Mater Chem A 2021;9:22347-52.
120. Hou Z, Lu Z, Chen Q, Zhang B. Realizing wide-temperature Zn metal anodes through concurrent interface stability regulation and solvation structure modulation. Energy Stor Mater 2021;42:517-25.
121. Feng R, Chi X, Qiu Q, et al. Cyclic ether-water hybrid electrolyte-guided dendrite-free lamellar zinc deposition by tuning the solvation structure for high-performance aqueous zinc-ion batteries. ACS Appl Mater Interfaces 2021;13:40638-47.
122. Ming F, Zhu Y, Huang G, et al. Co-solvent electrolyte engineering for stable anode-free zinc metal batteries. J Am Chem Soc 2022;144:7160-70.
123. Di S, Nie X, Ma G, et al. Zinc anode stabilized by an organic-inorganic hybrid solid electrolyte interphase. Energy Stor Mater 2021;43:375-82.
124. Ma G, Miao L, Yuan W, et al. Non-flammable, dilute, and hydrous organic electrolytes for reversible Zn batteries. Chem Sci 2022;13:11320-9.
125. Ma Y, Zhang Q, Liu L, et al. N,N-dimethylformamide tailors solvent effect to boost Zn anode reversibility in aqueous electrolyte. Natl Sci Rev 2022;9:nwac051.
126. Wu F, Chen Y, Chen Y, et al. Achieving highly reversible zinc anodes via N, N-dimethylacetamide enabled Zn-ion solvation regulation. Small 2022;18:e2202363.
127. Geng L, Meng J, Wang X, et al. Eutectic electrolyte with unique solvation structure for high-performance zinc-ion batteries. Angew Chem Int Ed 2022;61:e202206717.
128. Qiu H, Du X, Zhao J, et al. Zinc anode-compatible in-situ solid electrolyte interphase via cation solvation modulation. Nat Commun 2019;10:5374.
129. Thorat GM, Ho VC, Mun J. Zn-based deep eutectic solvent as the stabilizing electrolyte for Zn metal anode in rechargeable aqueous batteries. Front Chem 2021;9:825807.
130. Han M, Huang J, Xie X, et al. Hydrated eutectic electrolyte with ligand-oriented solvation shell to boost the stability of zinc battery. Adv Funct Mater 2022;32:2110957.
131. Lin X, Zhou G, Robson MJ, Yu J, Kwok SCT, Ciucci F. Hydrated deep eutectic electrolytes for high-performance Zn-ion batteries capable of low-temperature operation. Adv Funct Mater 2022;32:2109322.
132. Shi J, Sun T, Bao J, et al. “Water-in-deep eutectic solvent” electrolytes for high-performance aqueous Zn-ion batteries. Adv Funct Mater 2021;31:2102035.
133. Su K, Chen J, Zhang X, et al. Inhibition of zinc dendrites by dopamine modified hexagonal boron nitride electrolyte additive for zinc-ion batteries. J Power Sources 2022;548:232074.
134. Zhou T, Mu Y, Chen L, et al. Toward stable zinc aqueous rechargeable batteries by anode morphology modulation via polyaspartic acid additive. Energy Stor Mater 2022;45:777-85.
135. Zhang L, Miao L, Xin W, Peng H, Yan Z, Zhu Z. Engineering zincophilic sites on Zn surface via plant extract additives for dendrite-free Zn anode. Energy Stor Mater 2022;44:408-15.
136. Qiu M, Sun P, Qin A, Cui G, Mai W. Metal-coordination chemistry guiding preferred crystallographic orientation for reversible zinc anode. Energy Stor Mater 2022;49:463-70.
137. Luo M, Wang C, Lu H, et al. Dendrite-free zinc anode enabled by zinc-chelating chemistry. Energy Stor Mater 2021;41:515-21.
138. Lin Y, Hu Y, Zhang S, et al. Highly reversible aqueous zinc-ion battery using the chelating agent triethanolamine as an electrolyte additive. CrystEngComm 2022;24:7950-61.
139. Han D, Sun T, Du H, et al. Controlling horizontal growth of zinc platelet by OP-10 additive for dendrite-free aqueous zinc-ion batteries. Batteries Supercaps 2022;5:e202200219.
140. Qiu M, Sun P, Wang Y, Ma L, Zhi C, Mai W. Anion-trap engineering toward remarkable crystallographic reorientation and efficient cation migration of Zn Ion batteries. Angew Chem Int Ed 2022;61:e202210979.
141. Yang J, Zhang Y, Li Z, et al. Three birds with one stone: tetramethylurea as electrolyte additive for highly reversible Zn-metal anode. Adv Funct Mater 2022;32:2209642.
142. Qian L, Yao W, Yao R, et al. Cations coordination-regulated reversibility enhancement for aqueous Zn-ion battery. Adv Funct Mater 2021;31:2105736.
143. Huang H, Xie D, Zhao J, et al. Boosting reversibility and stability of Zn anodes via manipulation of electrolyte structure and interface with addition of trace organic molecules. Adv Energy Mater 2022;12:2202419.
144. Zhao K, Liu F, Fan G, et al. Stabilizing zinc electrodes with a vanillin additive in mild aqueous electrolytes. ACS Appl Mater Interfaces 2021;13:47650-8.
145. Meng Q, Zhao R, Cao P, et al. Stabilization of Zn anode via a multifunctional cysteine additive. Chem Eng J 2022;447:137471.
146. Wang H, Li H, Tang Y, et al. Stabilizing Zn anode interface by simultaneously manipulating the thermodynamics of Zn nucleation and overpotential of hydrogen evolution. Adv Funct Mater 2022;32:2207898.
147. Wang B, Zheng R, Yang W, et al. Synergistic solvation and interface regulations of eco-friendly silk peptide additive enabling stable aqueous zinc-ion batteries. Adv Funct Mater 2022;32:2112693.
148. Ren H, Li S, Wang B, et al. Molecular-crowding effect mimicking cold-resistant plants to stabilize the zinc anode with wider service temperature range. Adv Mater 2023;35:e2208237.
149. Wang K, Qiu T, Lin L, Liu X, Sun X. A low fraction electrolyte additive as interface stabilizer for Zn electrode in aqueous batteries. Energy Stor Mater 2023;54:366-73.
150. Xie K, Ren K, Sun C, et al. Toward stable zinc-ion batteries: use of a chelate electrolyte additive for uniform zinc deposition. ACS Appl Energy Mater 2022;5:4170-8.
151. Zhang Y, Peng C, Zeng Z, et al. Sustainable phytic acid-zinc anticorrosion interface for highly reversible zinc metal anodes. ACS Appl Mater Interfaces 2022;14:10419-27.
152. Zhao F, Jing Z, Guo X, et al. Trace amounts of fluorinated surfactant additives enable high performance zinc-ion batteries. Energy Stor Mater 2022;53:638-45.
153. Liu B, Wei C, Zhu Z, et al. Regulating surface reaction kinetics through ligand field effects for fast and reversible aqueous zinc batteries. Angew Chem Int Ed 2022;61:e202212780.
154. Lu H, Zhang X, Luo M, et al. Amino acid-induced interface charge engineering enables highly reversible Zn anode. Adv Funct Mater 2021;31:2103514.
155. Huang C, Zhao X, Liu S, et al. Stabilizing zinc anodes by regulating the electrical double layer with saccharin anions. Adv Mater 2021;33:e2100445.
156. Qiu M, Ma L, Sun P, Wang Z, Cui G, Mai W. Manipulating interfacial stability via absorption-competition mechanism for long-lifespan Zn anode. Nanomicro Lett 2021;14:31.
157. Hashemi A, Kasiri G, La Mantia F. The effect of polyethyleneimine as an electrolyte additive on zinc electrodeposition mechanism in aqueous zinc-ion batteries. Electrochim Acta 2017;258:703-8.
158. Zhang Y, Zheng X, Wu K, et al. Nonionic surfactant-assisted in situ generation of stable passivation protective layer for highly stable aqueous Zn metal anodes. Nano Lett 2022;22:8574-83.
159. Yuan W, Ma G, Nie X, et al. In-situ construction of a hydroxide-based solid electrolyte interphase for robust zinc anodes. Chem Eng J 2022;431:134076.
160. Yang M, Zhu J, Bi S, Wang R, Niu Z. A binary hydrate-melt electrolyte with acetate-oriented cross-linking solvation shells for stable zinc anodes. Adv Mater 2022;34:e2201744.
161. Wang C, Sun L, Li M, et al. Aqueous Zn2+/Na+ dual-salt batteries with stable discharge voltage and high columbic efficiency by systematic electrolyte regulation. Sci China Chem 2022;65:399-407.
162. Guo X, Zhang Z, Li J, et al. Alleviation of dendrite formation on zinc anodes via electrolyte additives. ACS Energy Lett 2021;6:395-403.
163. Li H, Firby CJ, Elezzabi AY. Rechargeable aqueous hybrid Zn2+/Al3+ electrochromic batteries. Joule 2019;3:2268-78.
164. Cao J, Zhang D, Chanajaree R, et al. Stabilizing zinc anode via a chelation and desolvation electrolyte additive. Adv Powder Mater 2022;1:100007.
165. Zhang S, Hao J, Luo D, et al. Dual-function electrolyte additive for highly reversible Zn anode. Adv Energy Mater 2021;11:2102010.
166. Xi M, Liu Z, Ding J, Cheng W, Jia D, Lin H. Saccharin anion acts as a “traffic assistant” of Zn2+ to achieve a long-life and dendritic-free zinc plate anode. ACS Appl Mater Interfaces 2021;13:29631-40.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Miao L, Guo Z, Jiao L. Insights into the design of mildly acidic aqueous electrolytes for improved stability of Zn anode performance in
AMA Style
Miao L, Guo Z, Jiao L. Insights into the design of mildly acidic aqueous electrolytes for improved stability of Zn anode performance in
Chicago/Turabian Style
Miao, Licheng, Zaiping Guo, Lifang Jiao. 2023. "Insights into the design of mildly acidic aqueous electrolytes for improved stability of Zn anode performance in
ACS Style
Miao, L.; Guo Z.; Jiao L. Insights into the design of mildly acidic aqueous electrolytes for improved stability of Zn anode performance in
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 38 clicks
Cite This Article 38 clicks




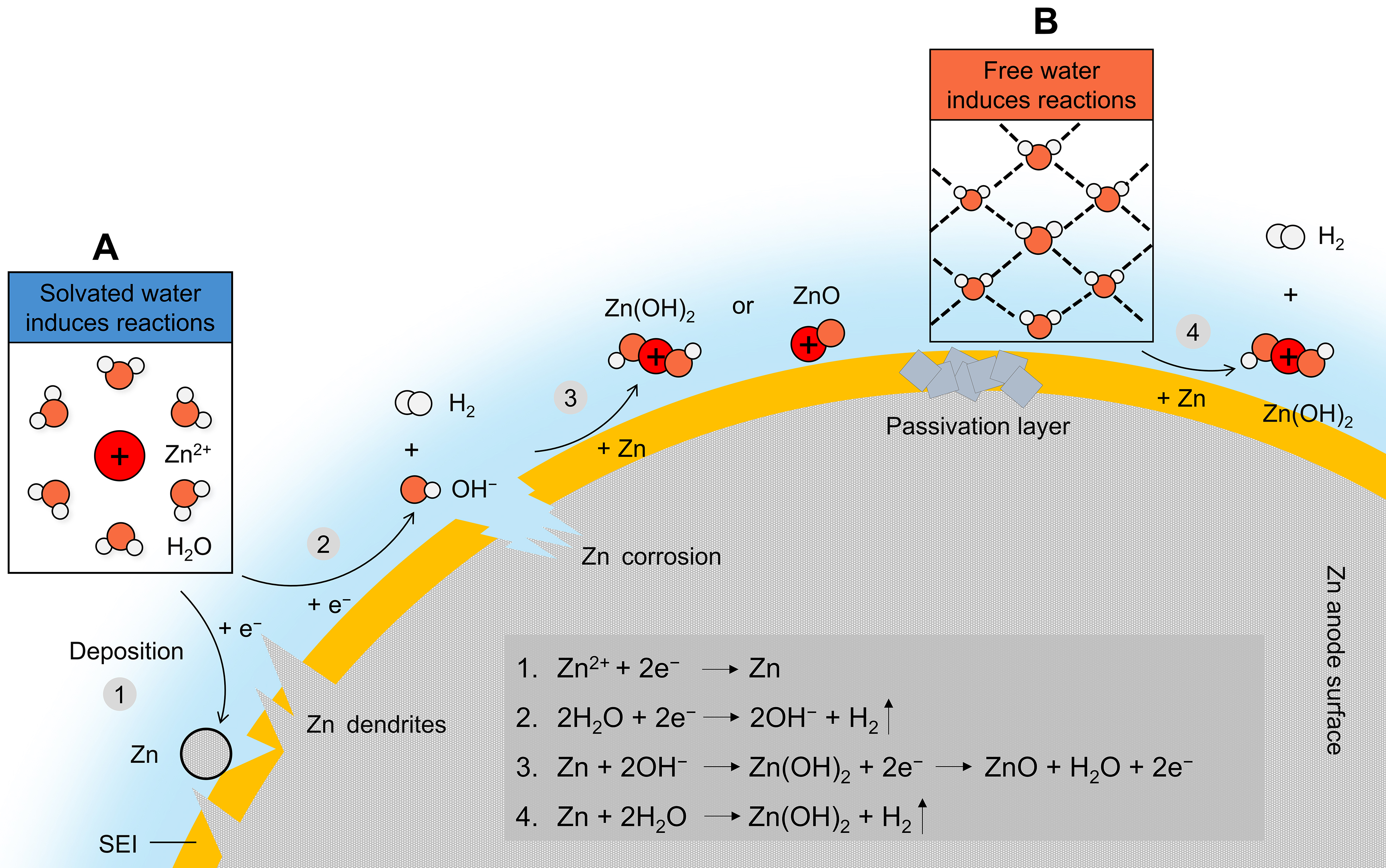
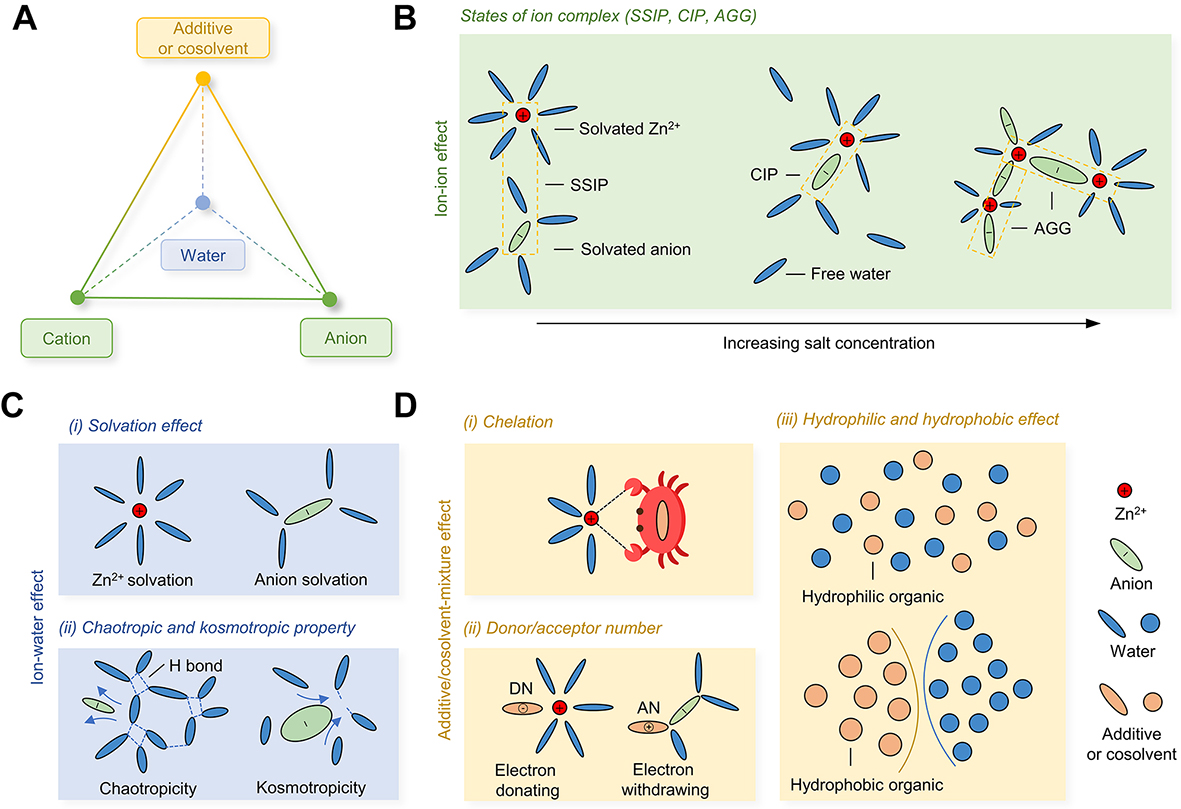



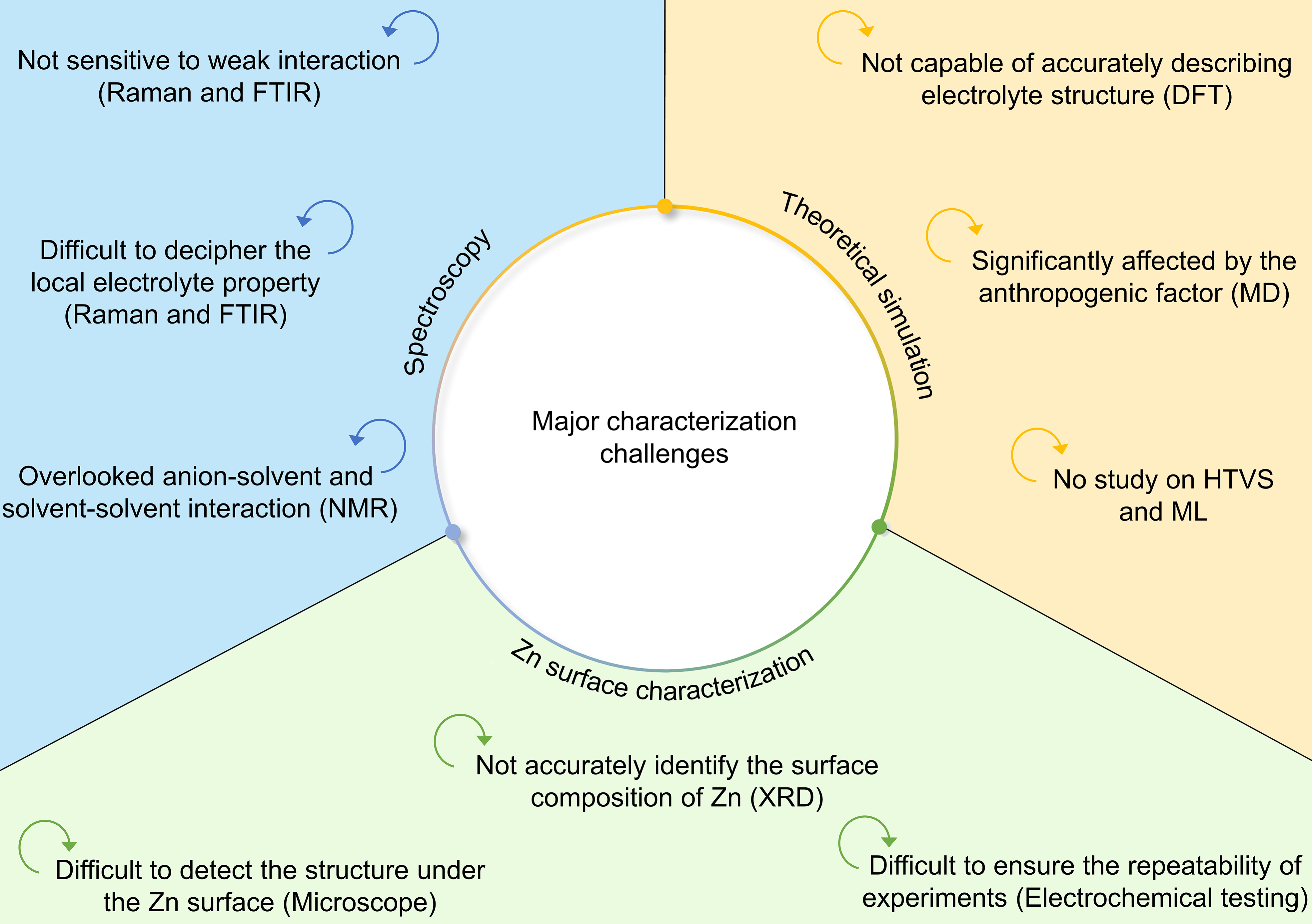



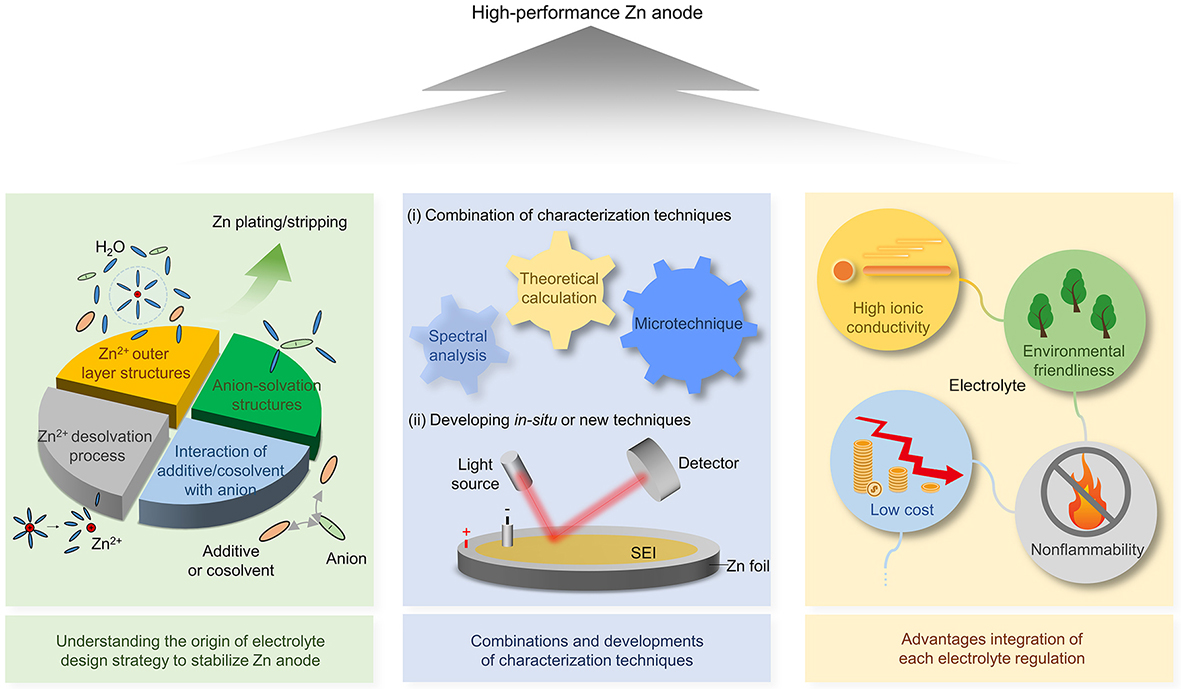











Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.