Impact of in coin cell atmosphere on lithium metal battery performance
Abstract
Research on lithium metal as a high-capacity anode for future lithium metal batteries (LMBs) is currently at an all-time high. To date, the different influences of a highly pure argon glovebox (GB) and an industry-relevant ambient dry room (DR) atmosphere have received little attention in the scientific community. In this paper, we report on the impact of in coin cell atmosphere (ICCA) on the performance of an LMB as well as its interphase characteristics and properties in combination with three organic carbonate-based electrolytes with and without two well-known interphase-forming additives, namely fluoroethylene carbonate (FEC) and vinylene carbonate (VC). The results obtained from this carefully executed systematic study show a substantial impact of the ICCA on solid electrolyte interphase (SEI) resistance (RSEI) and lithium stripping/plating homogeneity. In a transition metal cathode (NMC811) containing LMBs, a DR ICCA results in an up to 50% increase in lifetime due to the improved chemical composition of the cathode electrolyte interphase (CEI). Furthermore, different impacts on electrode characteristics and cell performance were observed depending on the utilized functional additive. Since this study focuses on a largely overlooked influential factor of LMB performance, it highlights the importance of comparability and transparency in published research and the importance of taking differences between research and industrial environments into consideration in the aim of establishing and commercializing LMB cell components.
Keywords
INTRODUCTION
The application of lithium metal as the “holy grail”[1-3] electrode material has been a topic in academia and industry for more than 40 years. The high theoretical specific capacity (3,860 mAh g-1)[4,5] and the low standard reduction potential (-3.04 V vs. SHE) make lithium metal an ideal material for future high energy density batteries. To date, the main challenge for commercial liquid electrolyte-based LMBs to overcome is inhomogeneous stripping/plating coinciding with the formation of high surface area lithium (HSAL), also known as dendritic morphology[6]. There have been a variety of approaches to stabilize the lithium metal anode, the solid electrolyte interphase (SEI)[7-9], and improve lithium stripping/plating behavior. These include stabilizing functional electrolyte additives[2,10-12], lithium metal pre-treatment and artificial SEIs[13-17], lithium metal host materials[18-20], and the application of external pressure[21,22]. Multiple publications have also suggested guidelines and benchmarks for battery performance characterization to enhance research comparability[23-27]. Another external factor that can influence the performance of battery electrodes is the storage atmosphere. In particular, the degradation of cathode materials in an ambient atmosphere has been the subject of numerous publications[28,29]. However, the reactivity of lithium metal with its surrounding atmosphere, resulting in different compositions of the native passivation layer covering the lithium surface, has only recently come into focus[7,30,31]. Furthermore, the influence of different storage atmospheres on lithium metal electrode performance has - to the best of our knowledge - only been reported by
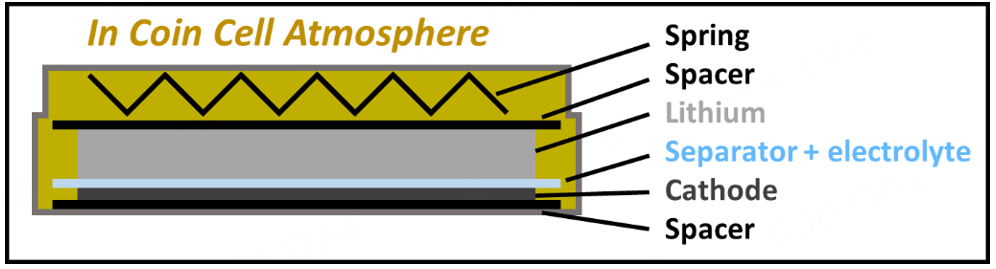
Figure 1. Schematic depiction of a coin cell 2032 setup commonly used in research, highlighting the available ICCA volume.
In an attempt to close this knowledge gap, we report on the significant impact of this remaining trapped ICCA on the chemical and electrochemical characteristics of lithium metal electrodes as well as the performance and lifetime of transition metals (NMC811) using LMBs by comparing a DR and a super-clean water-oxygen-nitrogen-free inert gas argon GB atmosphere. After precautions were taken to ensure safe storage conditions, thus ensuring equal starting conditions for each experiment, Li||Li and NMC811||Li cells were assembled in a GB and a DR. Stripping/Plating experiments, operando electrochemical impedance spectroscopy (EIS), post-mortem scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS) analysis were conducted for Li||Li symmetric cells. The impact of the ICCA on LMB full cell performance was evaluated by galvanostatic cycling in NMC811||Li cells and subsequent post-mortem attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectroscopy analysis of the CEI. In addition, based on the recorded ATR-FTIR analysis, a new decomposition mechanism of FEC on NMC811 was proposed and supported by different quantum chemistry calculations. Furthermore, the systematic analysis of two atmospheres with two additive-containing electrolytes and one baseline organic carbonate-based liquid electrolyte revealed a differently pronounced ICCA influence depending on the electrolyte formulation. The following electrolytes were chosen based on an equal molar approach: a baseline electrolyte [BE; EC:EMC 3:7 (w/w), 1.2 M LiPF6] and two additive electrolytes containing the popular additives FEC (AE-FEC; BE +6.09 wt.% FEC) and VC (AE-VC; BE +5 wt.% VC)[7].
EXPERIMENTAL
Study design
The study presented in this work required careful preparation and considerable investment in experimental equipment to ensure that factors other than the ICCA trapped inside the battery did not have an impact on the experimental result. A major challenge of the experimental preparation is the ability to conduct all experiments with equal starting materials [Supplementary Figure 1A and B]. The aforementioned degradation of cathode materials and the reactivity of lithium metal with the atmosphere cannot be completely prevented by a standard GB atmosphere (H2O and O2 content < 1 ppm). Reactions of the starting materials with liquid electrolyte aerosols or chemicals stored in the same glovebox have an influence on the conducted analysis. In particular, the degradation of lithium metal with contaminations in an argon GB atmosphere (especially N2) can result in black stains on lithium [Supplementary Figure 1C]. The native passivation layer covering battery-grade lithium[7] and the degradation of battery-grade lithium in contact with nitrogen have received little attention in the past[31]. This is despite the fact that the reaction of lithium and N2 gas has previously been reported to form an artificial Li3N-rich SEI[37]. These degradations occur slowly due to the intended low amount of reaction partners in a standard argon-filled GB and the native passivation functioning as a protective film covering the elemental lithium metal on the inside. Once the native passivation layer is removed, for example, by cutting a rod of lithium with a tungsten wire, an accelerated reaction with nitrogen in the presence of trace amounts of oxygen results in an initially rainbow-colored and ultimately black reaction product surface containing nitrogen as a main component
For this study, a two-chamber GB was designed [Supplementary Figure 2] to store cathode and anode materials safely, preventing any degradation and thus ensuring comparable experimental results. One of the two chambers is only used to store commercially purchased cathodes and anodes. The electrodes intended for cell assembly were transferred to the second chamber through an antechamber, thus preventing electrode materials from ever coming into contact with the ambient atmosphere. Commercially purchased cathodes were only taken out of the GB during the drying process in an air-tight device. The GB is equipped with an additional solvent filter, an air conditioning system (set to 25 °C), and a nitrogen purification system, preventing an increase of N2 in a GB above 5 ppm. Coin cell parts and all other components (if possible) were extensively dried before being introduced into the GB. Coin cell parts and electrolytes intended for cell assembly in a DR (dew point < -60 °C) were packed in an air-tight transport device inside the GB whenever new cells needed to be assembled. During cell assembly inside the DR, the exposure of the coin cell parts and electrodes to the DR atmosphere was kept to a minimum amount of time (< 10 min). Consequently, the ICCA trapped inside the assembled coin cells remained the only major influential difference in this study.
Materials
Lithium metal foil (Honjo Lithium GmbH., Ltd., 300 μm thickness, 99.9%) was stored in an argon-filled glovebox (MBraun Labmaster, H2O and O2 content < 0.1 ppm, N2 content < 5 ppm). NMC811 electrodes with an active mass loading of 1.03 mAh cm-2, purchased from Custom Cells, were dried at 120 °C in vacuum for at least 24 h before use and stored in an argon-filled glovebox (MBraun Labmaster, H2O and O2 content < 0.1 ppm, N2 content < 5 ppm). All lithium metal and NMC811 electrodes used in this study originated from the one respective batch/sheet of battery grade lithium and NMC811 cathode material to achieve a high level of comparability for all conducted experiments.
The study utilized three organic carbonate-based electrolyte formulations. The baseline electrolyte (BE)
Coin cell assembly
Two-electrode coin cell assembly was performed in an argon-filled glovebox (MBraun Labmaster, H2O and O2 content < 0.1 ppm, N2 content < 5 ppm) or dry room (-60 °C dew point). The exact procedure for cell assembly in the dry room is discussed in the “study design” chapter of the experimental section. In each coin cell, electrodes with a 12 mm diameter, an electrolyte volume of 30 µL with one layer of Celgard 2500 (16 mm diameter), and two 0.5 mm stainless steel spacers were used to form the battery stack shown in Figure 1.
Stripping/Plating and galvanostatic cycling experiments
Stripping/Plating experiments of Li||Li symmetric cells and galvanostatic cycling of NMC811||Li cells were conducted at 20 °C using a MACCOR battery cycler (MACCOR Series 4000).
Stripping/Plating experiments in Li||Li cells were carried out for 1,000 charge/discharge cycles at a constant current of 0.5 mA cm-2, with one-hour stripping/plating steps (0.5 mAh cm2). Galvanostatic cycling in NMC811||Li cells was carried out at a constant current density of 0.5 mA cm2 (equivalent to ~C/2, based on an areal capacity of 1.03 mAh cm-2) in the voltage range of 3 V to 4.2 V. Prior to the experiment, each cell was allowed to rest for 12 h.
Operando electrochemical impedance spectroscopy
Operando electrochemical impedance spectroscopy (EIS) measurements were carried out at a BioLogic VMP3 workstation. A Li||Li symmetric cell was cycled 50 times (charged and discharged for 1 h each at
Post-mortem sample preparation
Electrodes intended for post-mortem analysis (SEM, XPS, ATR-FTIR) were harvested from coin cells which were opened in an argon-filled glovebox (MBraun, H2O and O2 content < 0.1 ppm, N2 content < 5 ppm). The electrodes were rinsed with ethyl methyl carbonate (EMC; E-Lyte Innovations; 3 × 500 µL) and subsequently dried in an antechamber for > 15 min. They were then transported to the workstations or measurement devices using in-house-built (SEM, ATR-FTIR) or commercially available (XPS, Vacuum Transfer module by Thermo Scientific) sample transfer holders, preventing any contact with the outer atmosphere and/or moisture.
Scanning electron microscopy
Scanning electron microscopy (SEM) measurements were conducted at a Carl Zeiss Auriga Modular Crossbeam workstation utilizing a Schottky field emission gun with a Gemini column as an electron source. Images were taken at an accelerating voltage of 3 kV (SEM) using an in-lens secondary electron detector. The aperture of the lens was 20 µm and the working distance was 3.0 mm.
X-ray photoelectron spectroscopy
X-ray photoelectron spectroscopy (XPS) measurements were carried out at an angle of emission of 0° and a pass energy of 20 eV using a monochromatic Al Kα source (Ephoton = 1,486.6 eV) with a 10 mA filament current and a filament voltage source of 12 kV. The analyzed area was approximately 300 µm × 700 µm. In order to compensate for the charging of the sample, a charge neutralizer was used. The F 1s peak at 684.8 eV (LiF) was taken as an internal reference for the adjustment of the energy scale in the spectra. CasaXPS software was used for fitting and peak assignment in accordance with known literature values[38,39].
Attenuated total reflection Fourier transform infrared spectroscopy
Attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) analysis of the CEI on NMC811 electrode surfaces was conducted on a Bruker ALPHA II FT-IR spectrometer with a platinum ATR unit (diamond crystal) and a DLaTGS detector inside an argon-filled glovebox (H2O and O2 < 5 ppm). ATR-FTIR measurements were conducted at multiple spots in the center, the middle and the edge of the harvested electrode [Supplementary Figure 3]. The spectra were acquired with a spectral resolution of 4 cm-1 at an incidence angle of 45°. Each spectrum was obtained by accumulating 32 and 64 interferograms for background and sample spectra, respectively. The spectra are presented in the form of absorbance and were processed by subtracting the spectrum of pristine NMC811, ATR correction, and concave rubber band correction (10 iterations, straight lines).
Quantum chemistry calculations
Different quantum chemistry methods were used to interpret experimental results and to propose a new decomposition mechanism for FEC on NMC811 electrode. Density functional theory (DFT) calculations of putative decomposition products were performed at the B3LYP/6-311+G (3df, 2p) level of theory[40-42] using Grimme’s empirical D3 dispersion correction with Becke-Johnson damping[43]. In addition, reaction energies and free energies, as well as the corresponding barriers, were calculated using the highly accurate G4MP2 composite method[44]. All calculations were performed using the Gaussian 16 software using the SMD solvation model with acetone parameters[45].
RESULTS AND DISCUSSION
Stripping/Plating and operando EIS performed in Li||Li symmetric cell setups
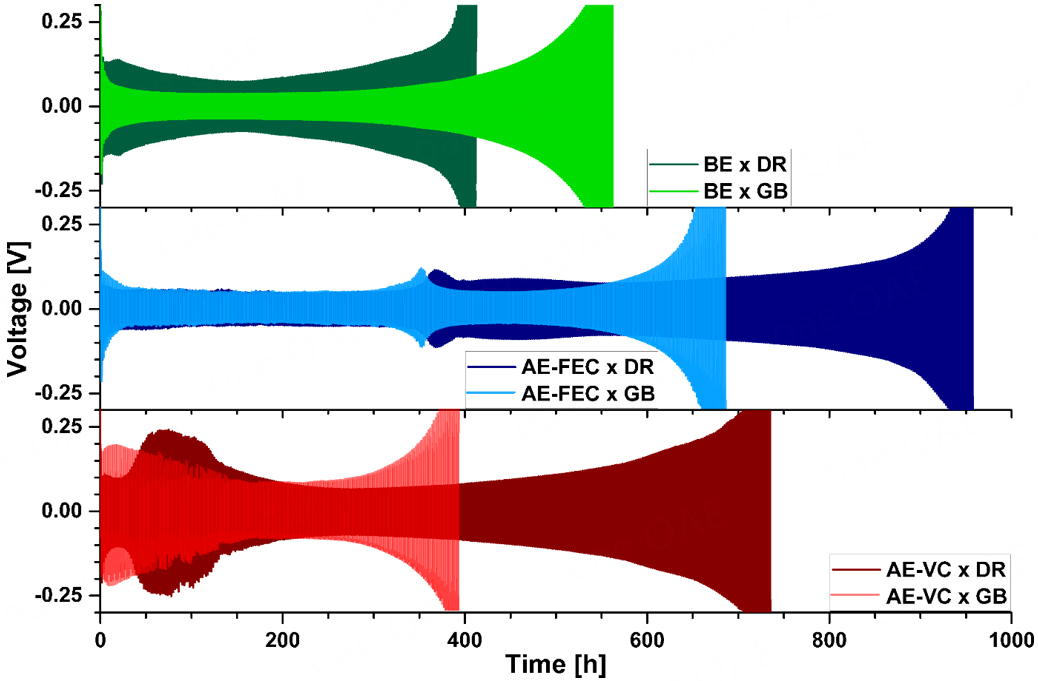
Initial electrochemical characterization to determine the influence of the ICCA on lithium metal electrodes was conducted by stripping/plating experiments [Figure 2] in Li||Li symmetric cells at a constant current density of 0.5 mA cm-2 (0.5 mAh cm-2) until the cell reached a set limit of ±0.3 V. The eventual increase in overvoltage values originates from a reduced Li transport in the electrolyte due to the consumption of Li+ ion transporting components. Utilizing a Li||Li symmetric cell setup allows the effect of the different ICCAs to be narrowed down to the impact on the lithium metal electrode and excludes the influences of intercellular crosstalk[46,47]. Six different cell setups were characterized and analyzed, each containing an individual combination of atmosphere (GB, DR) and electrolyte (BE, AE-FEC, AE-VC), and will be referred to hereafter as: electrolyte × atmosphere (e.g., BE × DR or AE-VC × GB).
Figure 2. Voltage vs. time profiles (0.5 mA cm-2, 1 h charge and discharge) of six different Li||Li cell setups. Top: BE × DR - dark green,
The different atmospheres result in different stripping/plating (voltage vs. time) profiles for all three electrolyte containing cells. The BE × DR cell setup (top) experiences increased overvoltage values
Differences in overvoltage have a strong correlation to the resistance of the SEI (RSEI)[48,51]. The first 100 h of the stripping/plating experiment were therefore investigated further utilizing operando EIS [Figure 3]. Additional stripping/plating experiments of identical cell setups were galvanostatically cycled for 100 h at 0.5 mA cm-2 and impedance spectra were recorded every 10 h. The measured, fitted, and averaged results show the characteristic drop in SEI resistance (RSEI) after the initial cycles, which is likely caused by the replacement of the native passivation layer by an in situ formed SEI[7]. After the initial drop, the evolution of the RSEI values closely mimics the overvoltage evolution of the stripping/plating experiments: The RSEI values of the BE × DR setup remain higher compared to the BE × GB setup counterparts and both AE-FEC containing cell setups show similar RSEI values over the first 100 h. The drastic difference in the overvoltage evolution of the stripping/plating experiments for AE-VC × DR and AE-VC × GB can also be seen in the evolution of the RSEI values. Initially, AE-VC × GB experiences a higher SEI resistance, but after 30 h, the resistance of the AE-VC × DR starts to rise and matches the shrinking AE-VC × GB values after 50 h. The RSEI values of AE-VC × DR subsequently reach a local maximum at 80-90 h before they gradually start to shrink, as is the case for the overvoltage values of the stripping/plating experiments.
Figure 3. RSEI measured over the initial 100 h of stripping/plating experiments (0.5 mA cm-2, 1 h stripping/plating), based on a Nyquist plot fitted by the insert RC equivalent circuit. Values depict an average of at least five measurements with the error corresponding to the standard deviation between the measured values. Top: BE × DR - dark green; BE × GB - light green; middle: AE-FEC × DR - dark blue; AE-FEC × GB - light blue; bottom: AE-VC × DR - dark red, AE-VC × GB - light red.
Overall, the stripping/plating experiments and operando EIS measurements show the notable influence of the ICCA on the electrochemical characteristics of the SEI. In the case of both BE and AE-VC, the ICCA has a strong impact on the overvoltage profiles and RSEI. However, the influence on the RSEI of FEC containing cells seems to be minimal, although different overvoltage values of
Post-mortem analysis of Li||Li symmetric cells
Electrochemical characterization revealed that the ICCA has an influence on the performance of lithium metal electrodes. In line with this, post-mortem SEM and XPS analysis after 100 h were performed to observe the differences in stripping/plating homogeneity and determine how the ICCA influences the chemical composition of the in situ formed SEI on the lithium metal.
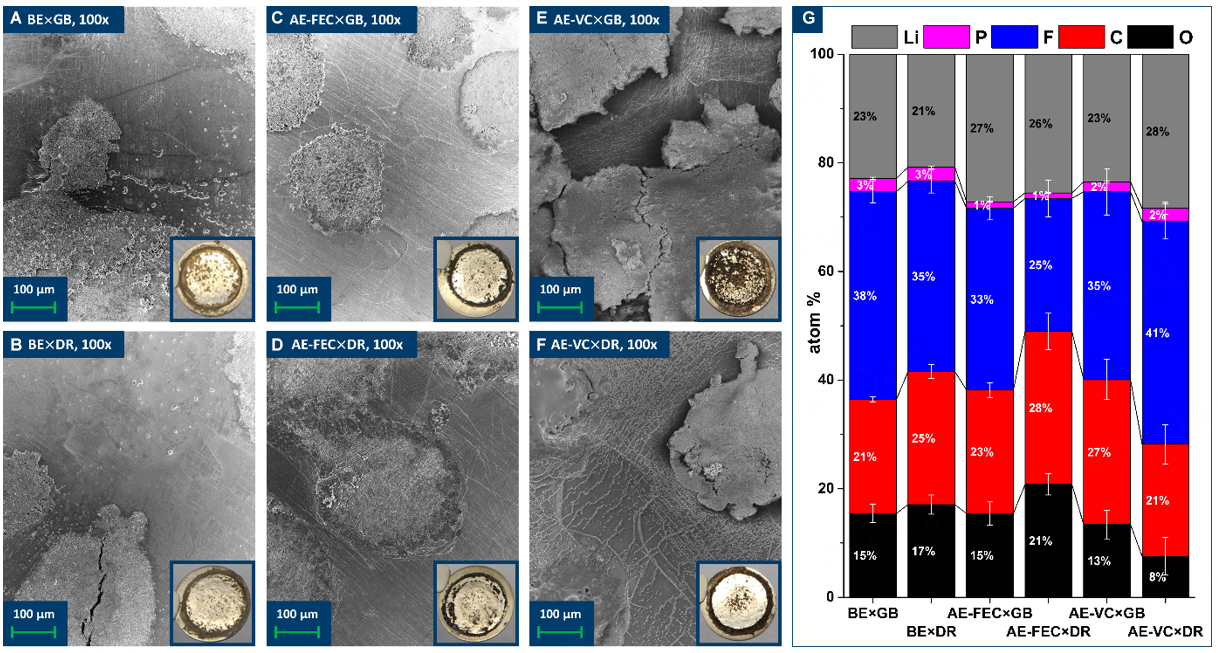
Post-mortem SEM measurements [Figure 4A-F], in combination with optical analysis of the complete harvested electrode (inlets), enable an evaluation of the stripping/plating homogeneity of lithium metal electrodes. Electrochemical characterization only revealed a small impact of different ICCAs on the first
Figure 4. Post-mortem SEM (A-F) and XPS (G) analyses of Li||Li cells after 50 cycles at 0.5 mA cm-2 (0.5 mAh cm-2). Post-mortem SEM and optical (inlets) images of electrodes harvested from (A) BE × GB, (B) BE × DR, (C) AE-FEC × GB, (D) AE-FEC × DR,
The chemical composition of the SEI formed on the lithium metal electrode after 100 h was analyzed via post-mortem XPS analysis [Figure 4G]. In an attempt to only look at the in situ formed SEI, only spots that clearly experienced stripping/plating processes (black spots, see Figure 4A-F) were analyzed and the results were subsequently averaged. For all three electrolytes, considerable differences in the atomic distributions of the in situ formed SEI were found depending on the ICCA and they all contained the expected mixture of organic, carbonate, fluoride, and phosphate components[7,39]. For BE, a higher carbon content (BE × GB: 21%; BE × DR: 25%) and a slightly lower fluorine content (BE × GB: 38%; BE × DR: 35%) were observed. In the case of AE-FEC, the in situ formed SEI contains more oxygen (AE-FEC × GB: 15%; AE-FEC × DR: 21%) and carbon (AE-FEC × GB: 23%; AE-FEC × DR: 28%), but notably less fluorine (AE-FEC × GB: 33%; AE-FEC × DR: 25%). These findings show that the similar results of the electrochemical characterizations of AE-FEC × GB and AE-FEC × DR over the first 100 h [Figures 2 and 3] do not automatically equate to an equal in situ formed SEI composition and support the hypothesis that the different long cycle life after complete FEC consumption might be caused by a different SEI composition. XPS analysis of the cells containing AE-VC shows opposite tendencies compared to AE-FEC. The in situ formed SEI in
Galvanostatic cycling performance in NMC811||Li cells
This study has so far focused on the influence of the ICCA on the lithium metal electrode. In the following section, the influence of the ICCA on NMC811||Li full cell setups will be analyzed by galvanostatic cycling at a constant current density of 0.5 mA cm-2 (~C/2, areal cathode capacity: 1.03 mAh cm-2). As with all the lithium electrodes used, all NMC811 electrodes originated from one NMC811 electrode sheet to achieve a high level of comparability. Since the functional additives considered were shown to be indispensable electrolyte components for LMB performance[7,52], only AE-FEC and AE-VC will be discussed going forward. The same nomenclature will be used in the report hereafter, although one Li electrode was exchanged with a NMC811 cathode.
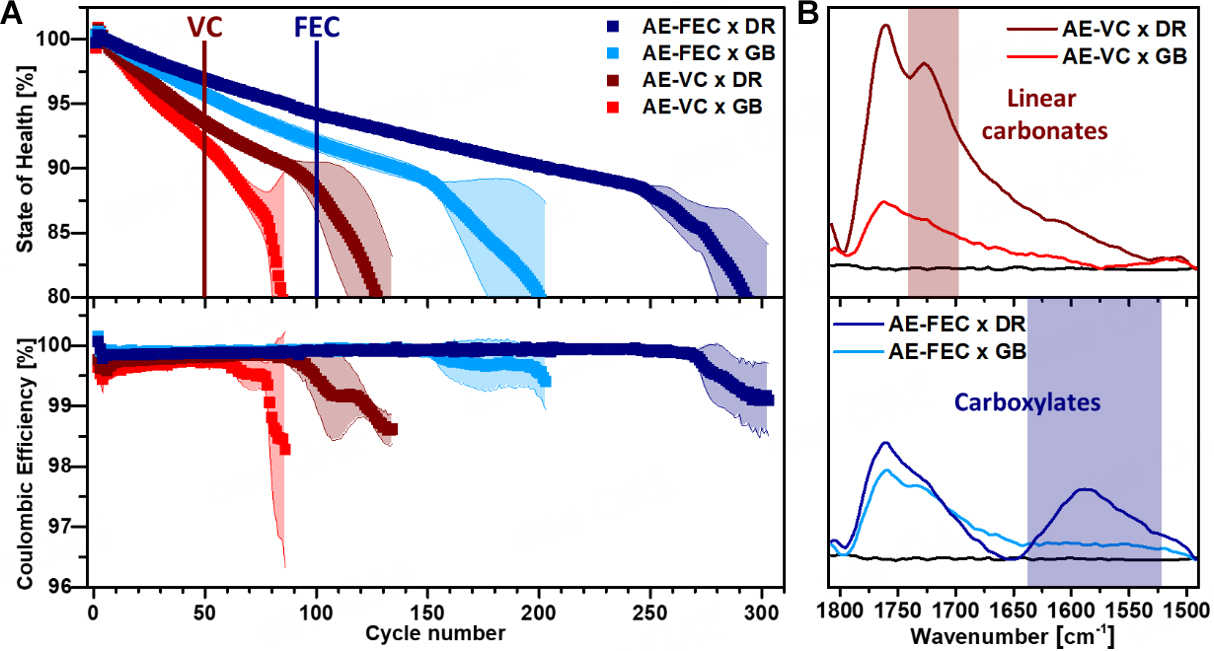
The recorded galvanostatic cycling data were investigated based on the average state of health (SOH, normalized on the 5th cycle) and the Coulombic efficiency. The advantage of FEC over VC as a film-forming additive for the analyzed cell setup has been reported previously[7]. However, to the best of our knowledge, there are no reports on the influences of the ICCA on additive comparability, especially with regard to cell lifetime (end-of-life (EOL) criteria: 80% SOH). For both additive-containing electrolytes analyzed, the lifetime of the LMB was notably enhanced by a DR ICCA [Figure 5A]. In the case of the cell setup containing the VC additive, the average lifetime increases by +50% from 85 cycles in the case of
Figure 5. (A) SOH and CE profiles of NMC811||Li cells at a constant current density of 0.5 mAh cm2 between 4.2 V and 3.0 V (~C/2, areal cathode capacity: 1.03 mAh cm-2). Top: SOH; bottom: CE. The presented results are an average of three cell setups of each kind. (B) Post-mortem IR analysis of the CEI on NMC811 cathodes after 50 cycles (AE-VC, top) and 100 cycles (AE-FEC, bottom). AE-FEC × DR - dark blue; AE-FEC × GB - light blue, AE-VC × DR - dark red, AE-VC × GB - light red, NMC811 baseline - black.
To determine the origin of the enhanced full cell performance, the CEIs chemical composition on NMC811 electrode was analyzed by means of post-mortem ATR-FTIR spectroscopy [Figure 5B] after 50 cycles
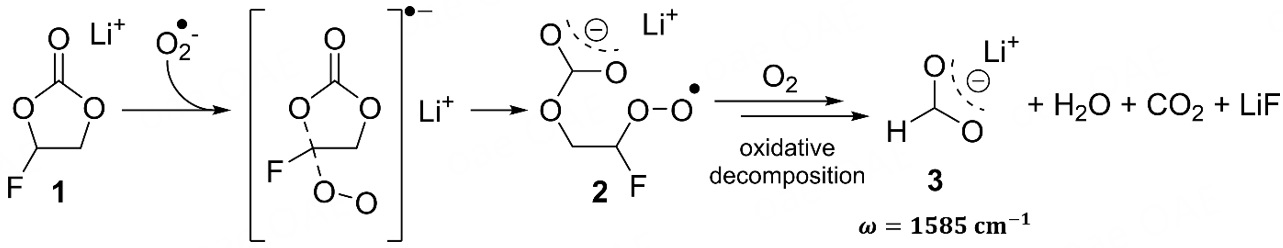
Since the new carboxylate band (1,600-1,570 cm-1) is only detected for the AE-FEC × DR cell setup, its compound origin has to be formed in a reaction involving FEC and, most likely, oxygen. For Li||O2 batteries, a decomposition mechanism of propylene carbonate to carboxylates in the presence of oxygen was proposed by Freunberger et al.[56]. A similar oxidative decomposition mechanism of FEC might be feasible and was supported by quantum chemical calculations [Scheme 1]. The energy barriers for a nucleophilic attack of a superoxide radical onto FEC (1) show a tendency towards a ring-opening reaction over a C-F bond cleavage (ring opening: 9.8 kcal mol-1; C-F bond cleavage: 11.8 kcal mol-1, see Supplementary Figure 9). This attack would result in the formation of an intermediate peroxo radical (2)[57], which could further decompose into lithium formate (3) in the presence of excess oxygen[58,59]. The calculated IR band of the proposed lithium formate (1,585 cm-1) and previously reported FTIR measurements[56] support the proposed mechanism. As an analogue product to lithium acetate of the propylene carbonate mechanism[56], lithium fluoroformate would readily decompose into LiF and CO2. A similar decomposition mechanism for VC is unlikely, because a quantum chemical calculation determined unfavorable reaction energies for the nucleophilic attack of the superoxide radical onto the electron-rich C=C double bond
Scheme 1. Proposed oxidative decomposition mechanism for FEC (1) in the presence of oxygen as part of the formation of CEI, based on FTIR analysis, quantum chemistry calculations, and known literature[56-59]. The stated IR band was determined via DFT calculations for the carbonyl bond of lithium formate (3).
Overall, the ICCA was shown to have a very high impact on the lifetime and CE of the analyzed LMBs, which is likely caused by a varying CEI composition. In both cases, the NMC811||Li cells containing DR ICCA outperform the cell lifetime of their GB analogues by an average of 46% (FEC) and 50% (VC) until 80% SOH. Post-mortem ATR-FTIR analysis of the respective cathodes revealed a more pronounced formation of linear carbonates for AE-VC × DR cell setups and the formation of carboxylates for
CONCLUSION
The two different ICCAs (GB and DR) were found to have a substantial impact on the coin cell performance of the LMB, originating from the altered interphase compositions of the SEI and CEI covering the lithium metal anode and NMC811 cathode, respectively. Furthermore, the effect of the ICCA itself was differently pronounced depending on the film-forming electrolyte additives VC or FEC. However, both additive-containing electrolytes were shown to form performance-enhancing chemical CEI compositions with a DR ICCA. Stripping/Plating experiments and operando EIS analysis also revealed that the ICCA has a considerable impact on overvoltage vs. time profiles and RSEI values for Li||Li symmetric cells containing BE and AE-VC. Differences in stripping/plating homogeneity were observed by post-mortem optical analysis (incl. SEM imaging) for different ICCAs and electrolytes, while post-mortem XPS analysis revealed different atomic SEI compositions for all three considered electrolyte formulations. In the case of
DECLARATIONS
Authors’ contributionsMethodology, investigation, formal analysis, writing (original draft): Kühn SP
Investigation, formal analysis: Weiling M, Diddens D
Formal analysis, writing (review & editing): Baghernejad M
Writing (review & editing), supervision: Winter M
Conceptualization, supervision, writing (review & editing): Cekic-Laskovic I
Availability of data and materialsData will be made available on request.
Financial support and sponsorshipThe authors are grateful for the financial support provided within the LILLINT project (13XP0225B) funded by the German Federal Ministry of Education and Research (BMBF).
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2023.
Supplementary MaterialsREFERENCES
2. Zhang JG, Xu W, Xiao J, Cao X, Liu J. Lithium metal anodes with nonaqueous electrolytes. Chem Rev 2020;120:13312-48.
5. Xu K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev 2004;104:4303-417.
6. Horstmann B, Shi J, Amine R, et al. Strategies towards enabling lithium metal in batteries: interphases and electrodes. Energy Environ Sci 2021;14:5289-314.
7. Kühn SP, Pfeiffer F, Bela M, et al. Back to the basics: advanced understanding of the as-defined solid electrolyte interphase on lithium metal electrodes. J Power Sources 2022;549:232118.
8. Peled E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems - the solid electrolyte interphase model. J Electrochem Soc 1979;126:2047-51.
10. Jie Y, Ren X, Cao R, Cai W, Jiao S. Advanced liquid electrolytes for rechargeable Li metal batteries. Adv Funct Mater 2020;30:1910777.
11. Yang H, Guo C, Naveed A, et al. Recent progress and perspective on lithium metal anode protection. Energy Stor Mater 2018;14:199-221.
12. Xia L, Miao H, Zhang C, Chen GZ, Yuan J. Review - recent advances in non-aqueous liquid electrolytes containing fluorinated compounds for high energy density lithium-ion batteries. Energy Stor Mater 2021;38:542-70.
13. Yasin G, Arif M, Mehtab T, et al. Understanding and suppression strategies toward stable Li metal anode for safe lithium batteries. Energy Stor Mater 2020;25:644-78.
14. Delaporte N, Wang Y, Zaghib K. Pre-treatments of lithium foil surface for improving the cycling life of Li metal batteries. Front Mater 2019;6:267.
15. Zhou H, Yu S, Liu H, Liu P. Protective coatings for lithium metal anodes: recent progress and future perspectives. J Power Sources 2020;450:227632.
16. Gao S, Sun F, Liu N, Yang H, Cao P. Ionic conductive polymers as artificial solid electrolyte interphase films in Li metal batteries - a review. Mater Today 2020;40:140-59.
17. Kang D, Xiao M, Lemmon JP. Artificial solid-electrolyte interphase for lithium metal batteries. Batteries Supercaps 2021;4:445-55.
18. Li Y, Wang C, Gao R, Cao F, Ye H. Recent smart lithium anode configurations for high-energy lithium metal batteries. Energy Stor Mater 2021;38:262-75.
19. Zhan Y, Shi P, Zhang X, et al. The insights of lithium metal plating/stripping in porous hosts: progress and perspectives. Energy Technol 2021;9:2000700.
20. Cheng Y, Chen J, Chen Y, et al. Lithium host: advanced architecture components for lithium metal anode. Energy Stor Mater 2021;38:276-98.
21. Zhou Y. External pressure: an overlooked metric in evaluating next-generation battery performance. Curr Opin Electrochem 2022;31:100916.
22. Fang C, Lu B, Pawar G, et al. Pressure-tailored lithium deposition and dissolution in lithium metal batteries. Nat Energy 2021;6:987-94.
23. Xiao J, Li Q, Bi Y, et al. Understanding and applying coulombic efficiency in lithium metal batteries. Nat Energy 2020;5:561-8.
24. Adams BD, Zheng J, Ren X, Xu W, Zhang J. Accurate determination of coulombic efficiency for lithium metal anodes and lithium metal batteries. Adv Energy Mater 2018;8:1702097.
25. Dai F, Cai M. Best practices in lithium battery cell preparation and evaluation. Commun Mater 2022;3:64-70.
26. Song W, Harlow J, Logan E, et al. A systematic study of electrolyte additives in single crystal and bimodal LiNi0.8Mn0.1Co0.1O2/graphite pouch cells. J Electrochem Soc 2021;168:090503.
27. Harlow JE, Ma X, Li J, et al. A wide range of testing results on an excellent lithium-ion cell chemistry to be used as benchmarks for new battery technologies. J Electrochem Soc 2019;166:A3031-44.
28. Sicklinger J, Metzger M, Beyer H, Pritzl D, Gasteiger HA. Ambient storage derived surface contamination of NCM811 and NCM111: performance implications and mitigation strategies. J Electrochem Soc 2019;166:A2322-35.
29. Li Y, Li Y, Sun Y, et al. Revealing nanoscale passivation and corrosion mechanisms of reactive battery materials in gas environments. Nano Lett 2017;17:5171-8.
30. Otto S, Moryson Y, Krauskopf T, et al. In-depth characterization of lithium-metal surfaces with XPS and ToF-SIMS: toward better understanding of the passivation layer. Chem Mater 2021;33:859-67.
31. Otto S, Fuchs T, Moryson Y, et al. Storage of lithium metal: the role of the native passivation layer for the anode interface resistance in solid state batteries. ACS Appl Energy Mater 2021;4:12798-807.
32. Momma T, Nara H, Yamagami S, Tatsumi C, Osaka T. Effect of the atmosphere on chemical composition and electrochemical properties of solid electrolyte interface on electrodeposited Li metal. J Power Sources 2011;196:6483-7.
33. Bläubaum L, Röse P, Schmidt L, Krewer U. The effects of gas saturation of electrolytes on the performance and durability of lithium-ion batteries. ChemSusChem 2021;14:2943-51.
34. Stark JK, Ding Y, Kohl PA. Role of dissolved gas in ionic liquid electrolytes for secondary lithium metal batteries. J Phys Chem C 2013;117:4980-5.
35. Wang E, Dey S, Liu T, Menkin S, Grey CP. Effects of atmospheric gases on Li metal cyclability and solid-electrolyte interphase formation. ACS Energy Lett 2020;5:1088-94.
36. Haas R, Murat M, Weiss M, Janek J, Natan A, Schröder D. Understanding the transport of atmospheric gases in liquid electrolytes for lithium - air batteries. J Electrochem Soc 2021;168:070504.
37. Zhang Y, Wang W, Tang H, et al. An
38. Ding F, Xu W, Chen X, et al. Effects of carbonate solvents and lithium salts on morphology and coulombic efficiency of lithium electrode. J Electrochem Soc 2013;160:A1894-901.
39. Becking J, Gröbmeyer A, Kolek M, et al. Lithium-metal foil surface modification: an effective method to improve the cycling performance of lithium-metal batteries. Adv Mater Interfaces 2017;4:1700166.
40. Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 1993;98:5648-52.
41. Krishnan R, Binkley JS, Seeger R, Pople JA. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 1980;72:650-4.
42. Mclean AD, Chandler GS. Contracted gaussian basis sets for molecular calculations. I. second row atoms, Z = 11-18. J Chem Phys 1980;72:5639-48.
43. Allouche R. Gabedit - a graphical user interface for computational chemistry softwares. J Comput Chem 2010;32:174-82.
44. Curtiss LA, Redfern PC, Raghavachari K. Gaussian-4 theory using reduced order perturbation theory. J Chem Phys 2007;127:124105-13.
45. Frisch MJ, Trucks GW, Schlegel HB, et al. Gaussian 16. Wallingford CT; 2016. Available from: https://gaussian.com/ [Last accessed on 4 May 2023].
46. Betz J, Brinkmann J, Nölle R, et al. Cross talk between transition metal cathode and Li metal anode: unraveling its influence on the deposition/dissolution behavior and morphology of lithium. Adv Energy Mater 2019;9:1900574.
47. Kühn SP, Edström K, Winter M, Cekic-laskovic I. Face to face at the cathode electrolyte interphase: from interface features to interphase formation and dynamics. Adv Mater Interfaces 2022;9:2102078.
48. Bieker G, Winter M, Bieker P. Electrochemical
49. Wood KN, Noked M, Dasgupta NP. Lithium metal anodes: toward an improved understanding of coupled morphological, electrochemical, and mechanical behavior. ACS Energy Lett 2017;2:664-72.
50. Jung R, Metzger M, Haering D, et al. Consumption of fluoroethylene carbonate (FEC) on Si-C composite electrodes for Li-ion batteries. J Electrochem Soc 2016;163:A1705-16.
51. Stolz L, Gaberšček M, Winter M, Kasnatscheew J. Different efforts but similar insights in battery R&D: electrochemical impedance spectroscopy
52. Hobold GM, Lopez J, Guo R, et al. Moving beyond 99.9% Coulombic efficiency for lithium anodes in liquid electrolytes. Nat Energy 2021;6:951-60.
53. Ota H, Sakata Y, Otake Y, Shima K, Ue M, Yamaki J. Structural and functional analysis of surface film on Li anode in vinylene carbonate-containing electrolyte. J Electrochem Soc 2004;151:A1778.
54. Nie M, Demeaux J, Young BT, et al. Effect of vinylene carbonate and fluoroethylene carbonate on SEI formation on graphitic anodes in Li-ion batteries. J Electrochem Soc 2015;162:A7008-14.
55. Grugeon S, Jankowski P, Cailleu D, et al. Towards a better understanding of vinylene carbonate derived SEI-layers by synthesis of reduction compounds. J Power Sources 2019;427:77-84.
56. Freunberger SA, Chen Y, Peng Z, et al. Reactions in the rechargeable lithium-O2 battery with alkyl carbonate electrolytes. J Am Chem Soc 2011;133:8040-7.
57. Bryantsev VS, Blanco M. Computational study of the mechanisms of superoxide-induced decomposition of organic carbonate-based electrolytes. J Phys Chem Lett 2011;2:379-83.
58. Sinha A, Thomson M. The chemical structures of opposed flow diffusion flames of C3 oxygenated hydrocarbons (isopropanol, dimethoxy methane, and dimethyl carbonate) and their mixtures. Combust Flame 2004;136:548-56.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Kühn SP, Weiling M, Diddens D, Baghernejad M, Winter M, Cekic-Laskovic I. Impact of in coin cell atmosphere on lithium metal battery performance. Energy Mater 2023;3:300020. http://dx.doi.org/10.20517/energymater.2023.07
AMA Style
Kühn SP, Weiling M, Diddens D, Baghernejad M, Winter M, Cekic-Laskovic I. Impact of in coin cell atmosphere on lithium metal battery performance. Energy Materials. 2023; 3(3): 300020. http://dx.doi.org/10.20517/energymater.2023.07
Chicago/Turabian Style
Kühn, Sebastian P., Matthias Weiling, Diddo Diddens, Masoud Baghernejad, Martin Winter, Isidora Cekic-Laskovic. 2023. "Impact of in coin cell atmosphere on lithium metal battery performance" Energy Materials. 3, no.3: 300020. http://dx.doi.org/10.20517/energymater.2023.07
ACS Style
Kühn, SP.; Weiling M.; Diddens D.; Baghernejad M.; Winter M.; Cekic-Laskovic I. Impact of in coin cell atmosphere on lithium metal battery performance. Energy Mater. 2023, 3, 300020. http://dx.doi.org/10.20517/energymater.2023.07
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 16 clicks
Cite This Article 16 clicks















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.